Genome-Wide Identification of Wheat KNOX Gene Family and Functional Characterization of TaKNOX14-D in Plants
Abstract
1. Introduction
2. Results
2.1. Identification of KNOX Genes in Wheat and Prediction of Encoded Proteins
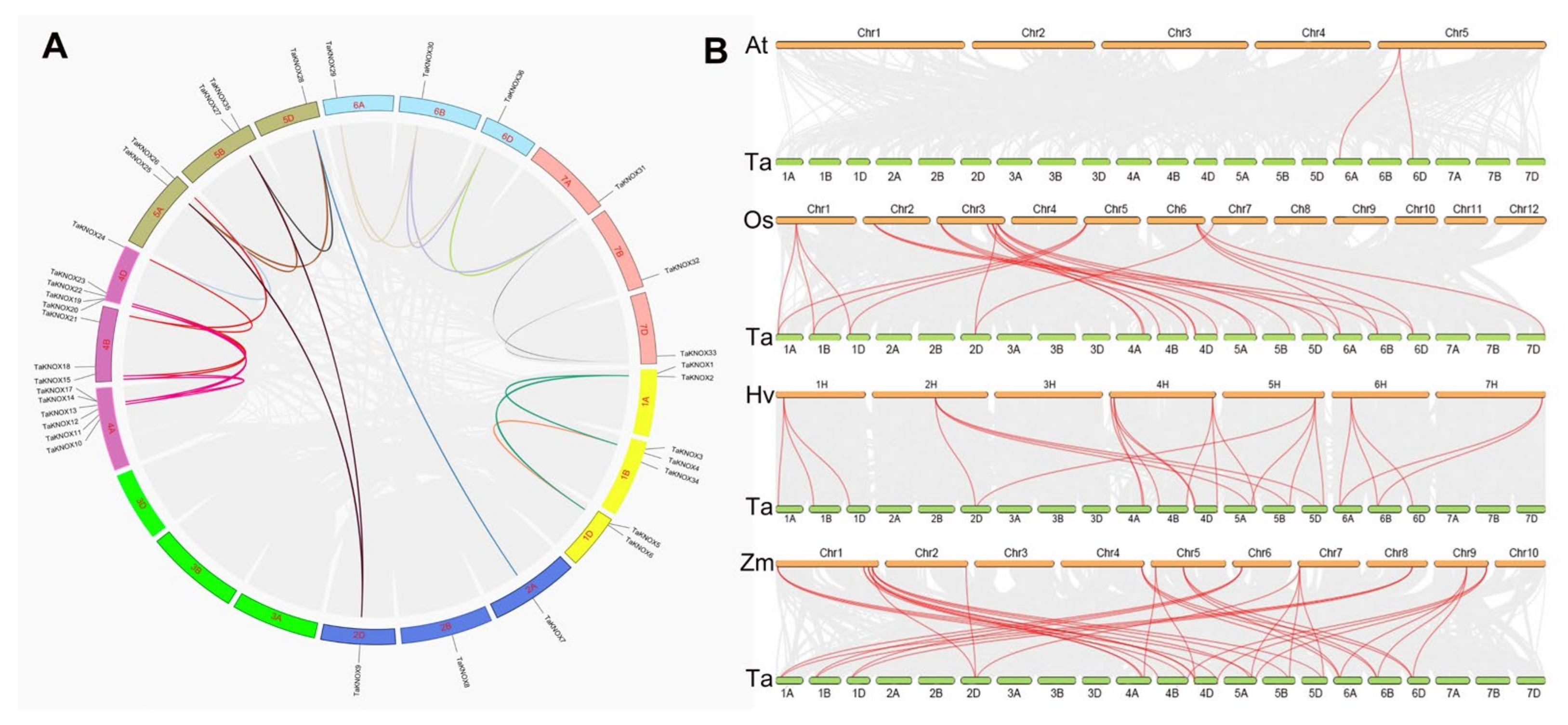
2.2. Gene Duplication and Homology Analysis of the KNOX Gene Family
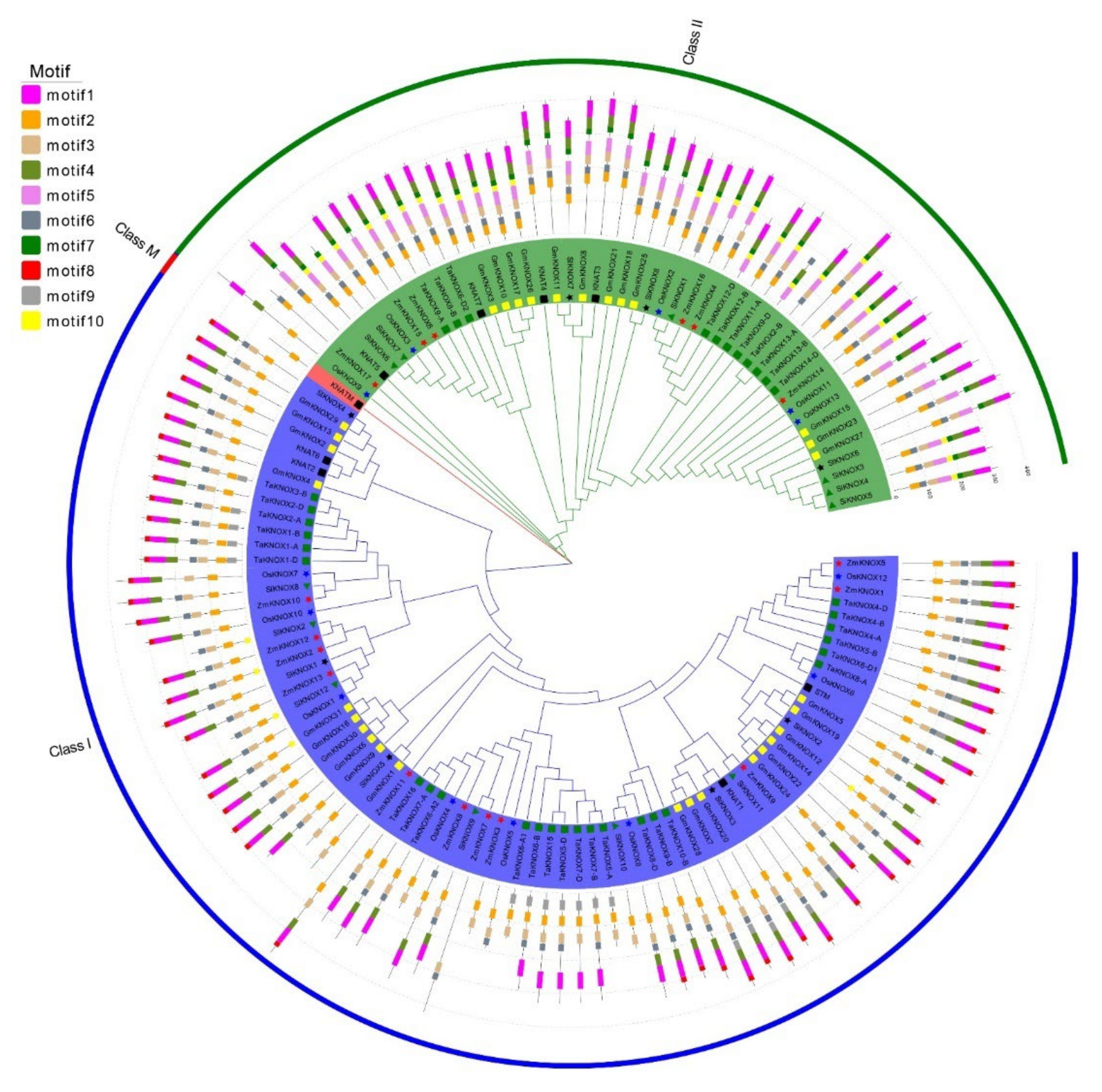
2.3. Phylogenetic Tree and Motif Analysis of KNOX Genes in Multiple Species
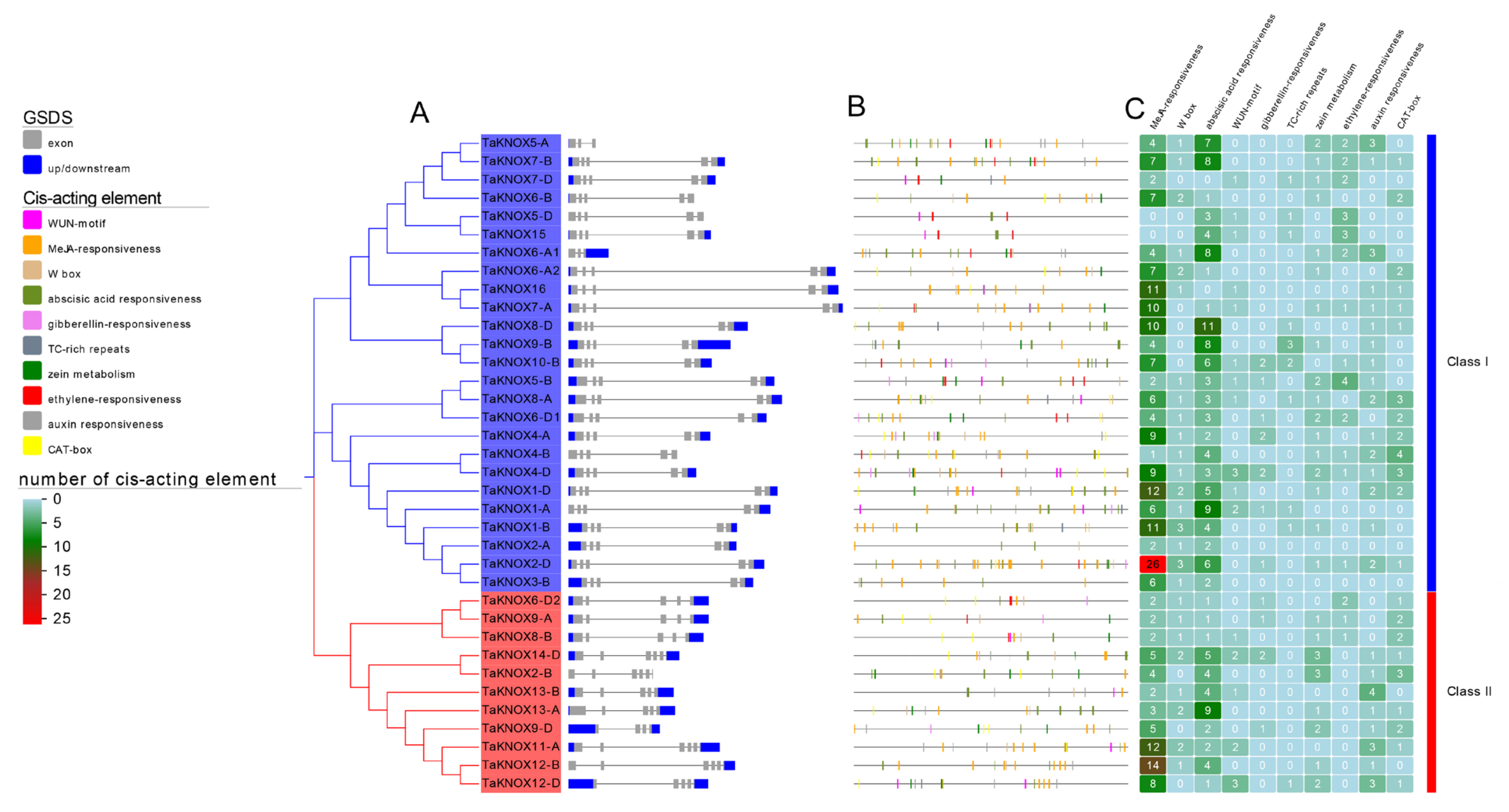
2.4. Gene Structure and Cis-Acting Elements of TaKNOX Genes
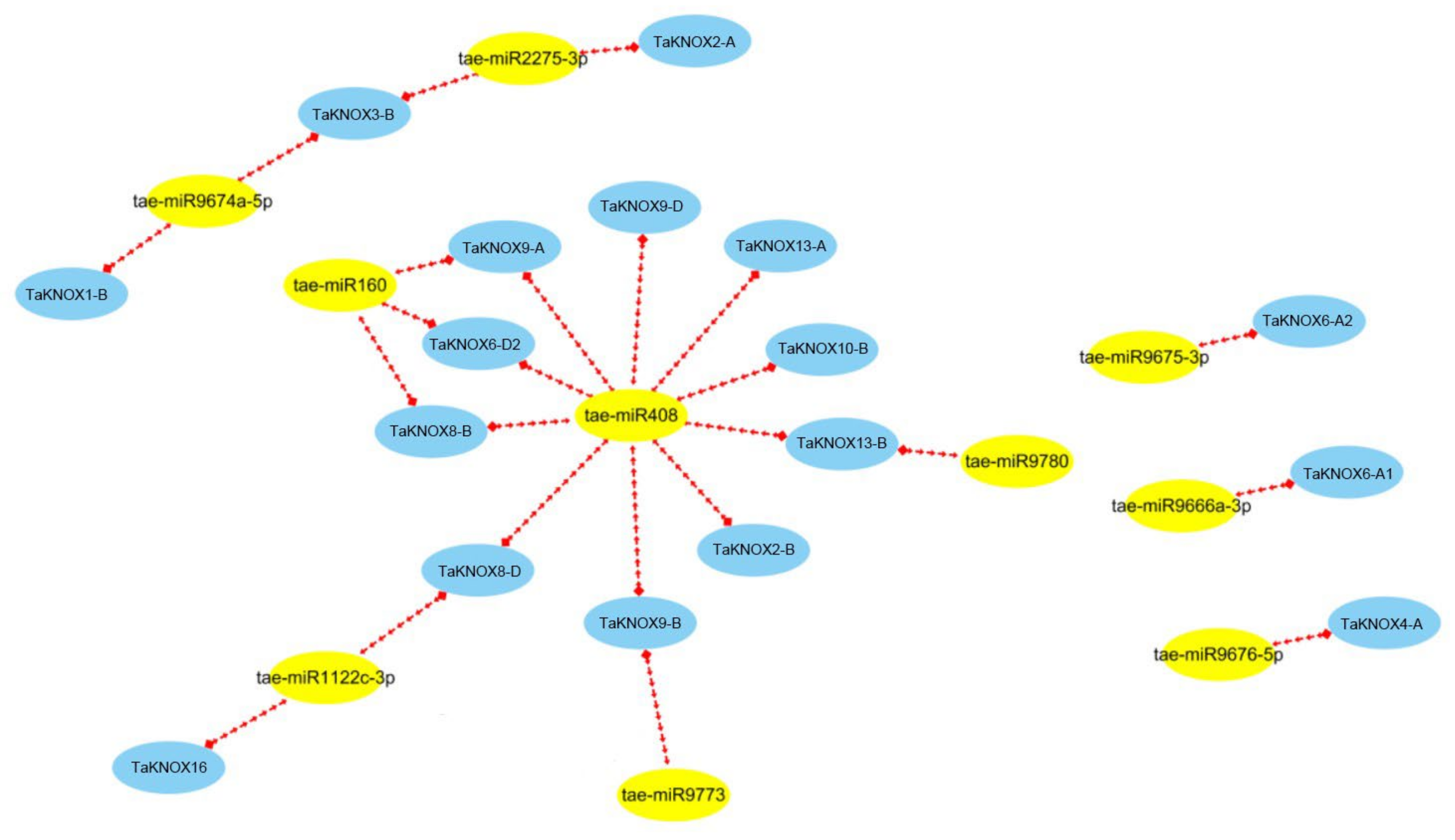
2.5. Network Construction of TaKNOX Cascade Genes
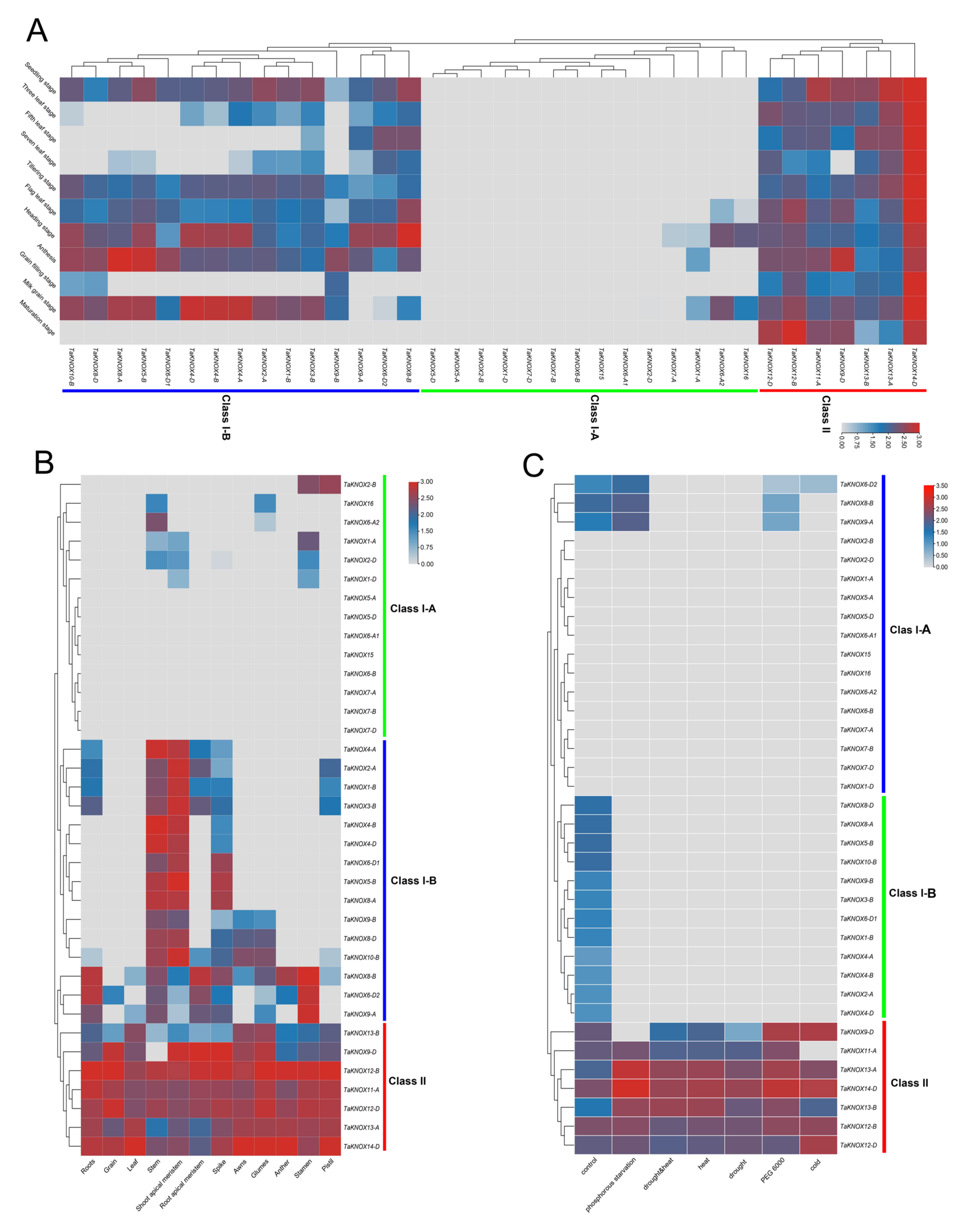
2.6. Expression Patterns of KNOX Genes in Different Growth Stages and Tissues and under Different Abiotic Stresses
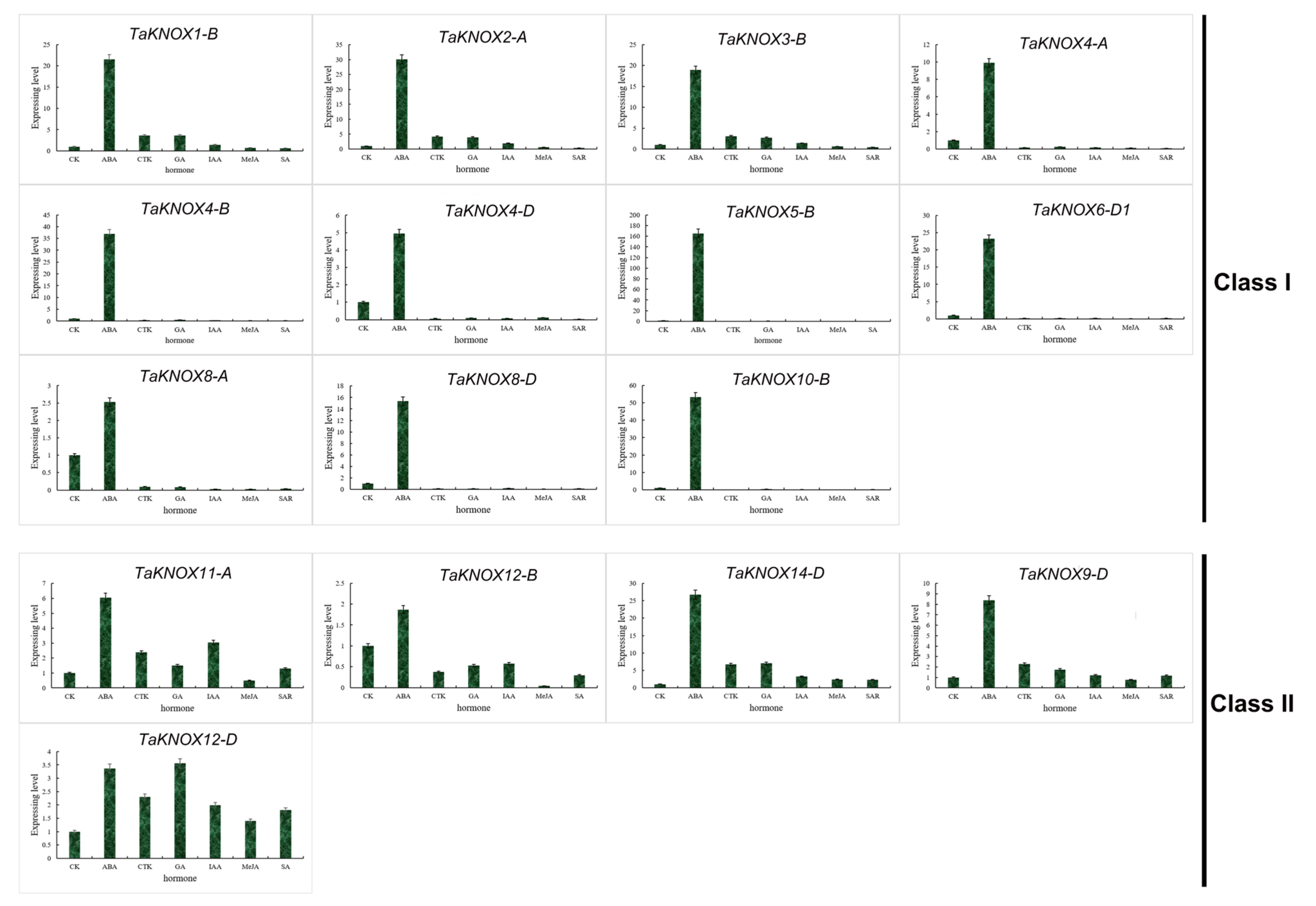
2.7. Expression Patterns of KNOX Genes under Different Hormone Treatments
2.8. Expression Pattern of KNOX Genes after Mowing
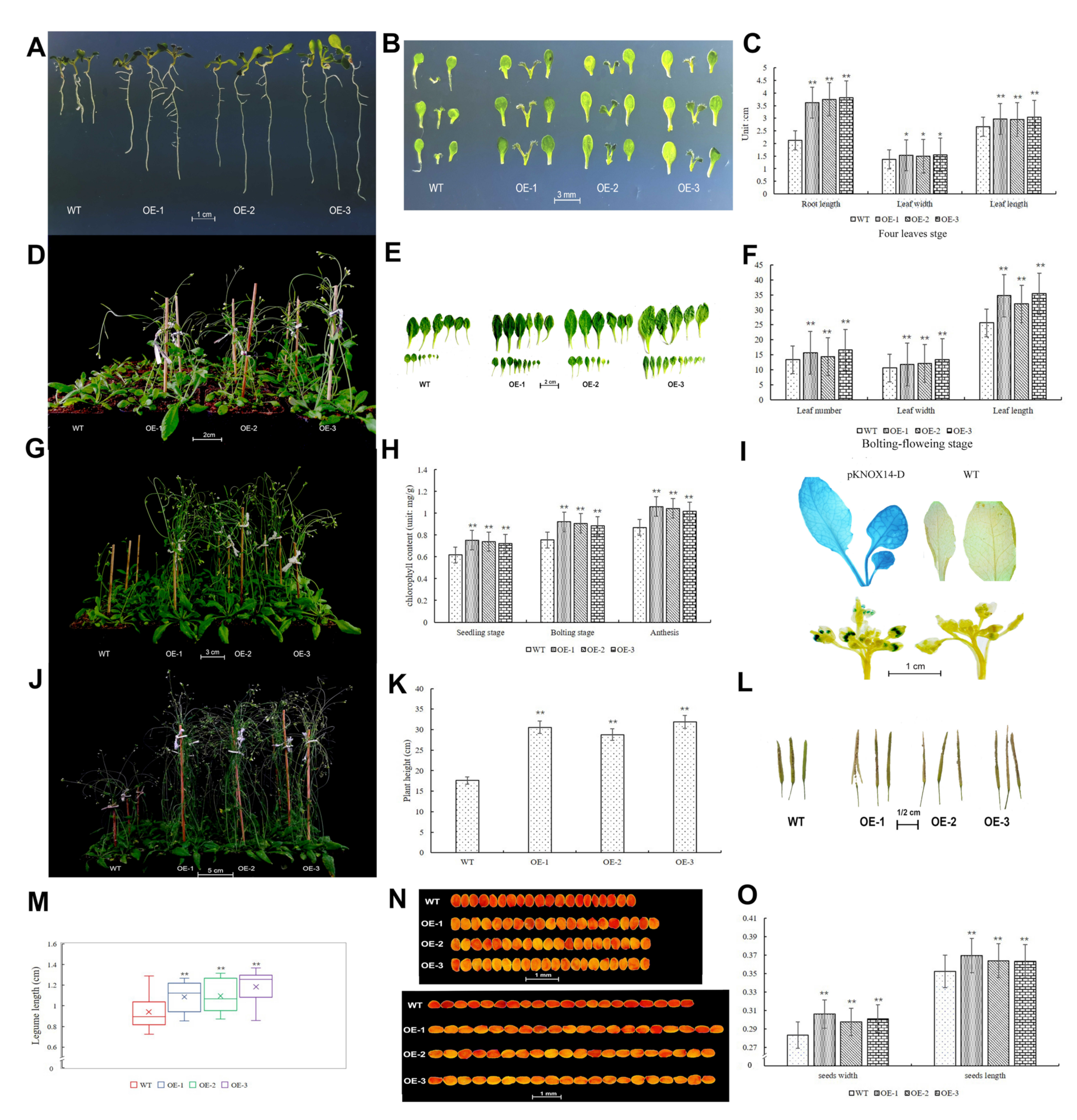
2.9. Gene Function Verification in Arabidopsis thaliana
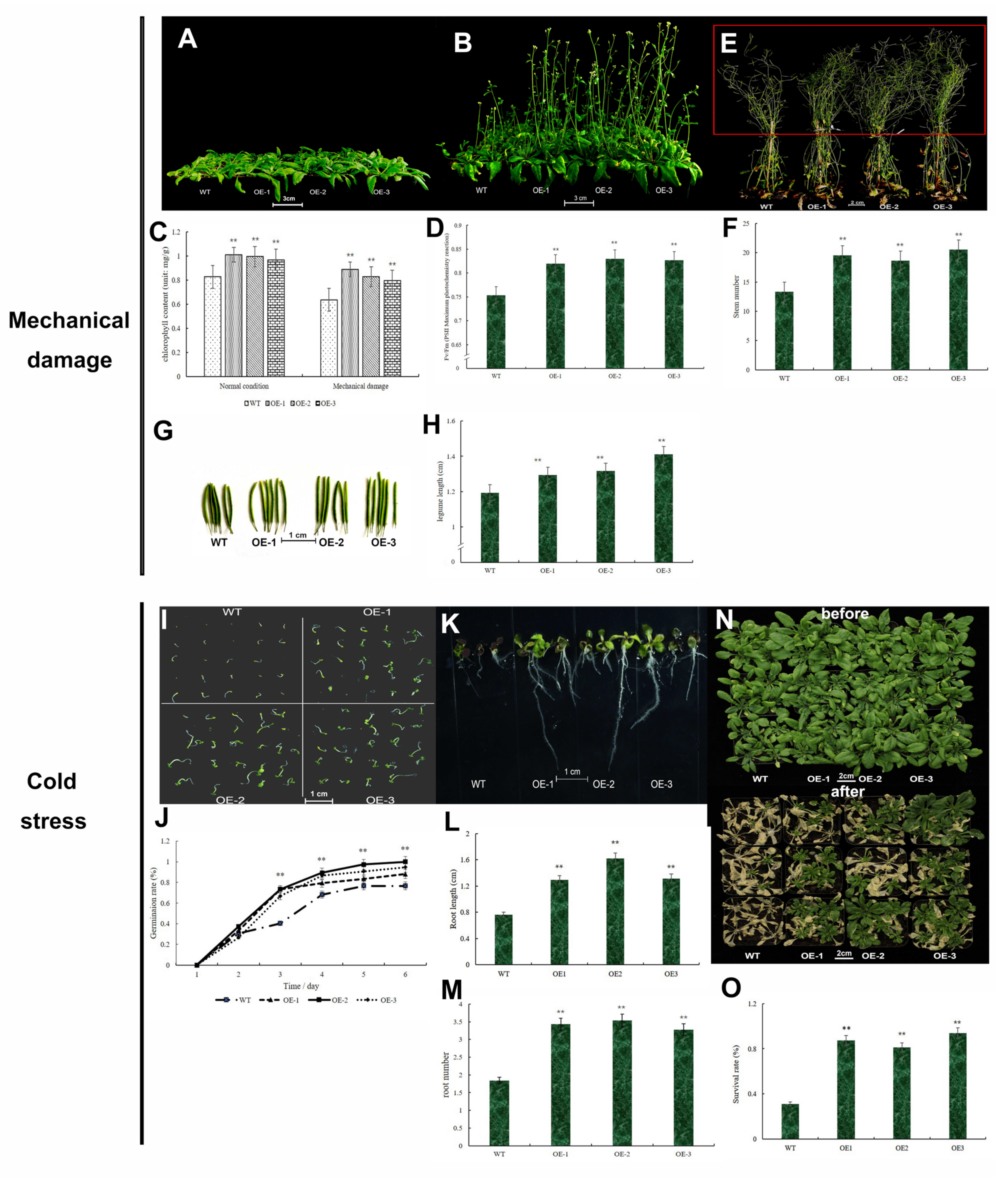
2.10. Gene Function Analysis by Arabidopsis thaliana under Mechanical Damage and Cold Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Identification and Bioinformatics Analyses of TaKNOX Genes
4.3. TaKNOX Gene Duplication and Homology Analysis
4.4. Gene Structure, Conserved Motif and Cis-Acting Element Analyses
4.5. TaKNOX Genes and miRNA Co-Expression Networks Construction
4.6. Gene Expression Pattern of Tissues, Age Expression and under Different Abiotic Stresses
4.7. RNA Isolated and qRT-PCR
4.8. Target Gene and Promoter Cloned
4.9. Phonotype Analysis in Arabidopsis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, Y.; Yan, C.J. Molecular mechanism of leaf development. Hereditas 2008, 30, 1127–1135. [Google Scholar]
- Bar, M.; Ori, N. Leaf development and morphogenesis. Development 2014, 141, 4219–4230. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, N.; Tyers, R.G.; Freeling, M.; Hake, S. Unequal redundancy in maize knotted1 homeobox genes. Plant Physiol. 2014, 164, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Mandel, T.; Kuhlemeier, C. Stem cell activation by light guides plant organogenesis. Genes Dev. 2011, 25, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Scarpella, E.; Marcos, D.; Friml, J.; Berleth, T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006, 20, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Gusti, A.; Cheminant, S.; Alioua, M.; Dhondt, S.; Coppens, F.; Beemster, G.T.; Genschik, P. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr. Biol. 2009, 19, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Barkoulas, M.; Galinha, C.; Grigg, S.P.; Tsiantis, M. From genes to shape: Regulatory interactions in leaf development. Curr. Opin. Plant Biol. 2007, 10, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, F.; Mentink, R.A.; Hajheidari, M.; Bailey, C.D.; Filatov, D.A.; Tsiantis, M. Coupled enhancer and coding sequence evolution of a homeobox gene shaped leaf diversity. Genes Dev. 2016, 30, 2370–2375. [Google Scholar] [CrossRef]
- Scofield, S.; Dewitte, W.; Murray, J.A. A model for Arabidopsis class-1 KNOX gene function. Plant Signal Behav. 2008, 3, 257–259. [Google Scholar] [CrossRef]
- Hay, A.; Tsiantis, M. KNOX genes: Versatile regulators of plant development and diversity. Development 2010, 137, 3153–3165. [Google Scholar] [CrossRef]
- Bolduc, N.; Yilmaz, A.; Mejia-Guerra, M.K.; Morohashi, K.; O’Connor, D.; Grotewold, E.; Hake, S. Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 2012, 26, 1685–1690. [Google Scholar] [CrossRef]
- Ramirez, J.; Bolduc, N.; Lisch, D.; Hake, S. Distal expression of knotted1 in maize leaves leads to reestablishment of proximal/distal patterning and leaf dissection. Plant Physiol. 2009, 151, 1878–1888. [Google Scholar] [CrossRef]
- Byrne, M.E.; Simorowski, J.; Martienssen, R.A. ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 2002, 129, 1957–1965. [Google Scholar] [CrossRef]
- Hay, A.; Tsiantis, M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat. Genet. 2006, 38, 942–947. [Google Scholar] [CrossRef]
- Shani, E.; Burko, Y.; Ben-Yaakov, L.; Berger, Y.; Amsellem, Z.; Goldshmidt, A.; Sharon, E.; Ori, N. Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell 2009, 21, 3078–3092. [Google Scholar] [CrossRef]
- Piazza, P.; Bailey, C.D.; Cartolano, M.; Krieger, J.; Cao, J.; Ossowski, S.; Schneeberger, K.; He, F.; de Meaux, J.; Hall, N.; et al. Arabidopsis thaliana leaf form evolved via loss of KNOX expression in leaves in association with a selective sweep. Curr. Biol. 2010, 20, 2223–2228. [Google Scholar] [CrossRef]
- Magnani, E.; Hake, S. KNOX lost the OX: The Arabidopsis KNATM gene defines a novel class of KNOX transcriptional regulators missing the homeodomain. Plant Cell 2008, 20, 875–887. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Zhao, W.; Lang, T.; Samuelsson, T. Evolution, diversification, and expression of KNOX proteins in plants. Front. Plant Sci. 2015, 6, 882. [Google Scholar] [CrossRef]
- Xiong, H.; Shi, A.; Wu, D.; Weng, Y.; Qin, J.; Ravelombola, W.S.; Shu, X.; Zhou, W. Genome-Wide Identification, Classification and Evolutionary Expansion of KNOX Gene Family in Rice (Oryza sativa) and Populus(Populustrichocarpa). Am. J. Plant Sci. 2018, 9, 1071–1092. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, N.; Hao, P.; Sun, H.; Wang, C.; Ma, L.; Wang, H.; Zhang, X.; Wei, H.; Yu, S. Genome-wide identification and characterization of TALE superfamily genes in cotton reveals their functions in regulating secondary cell wall biosynthesis. BMC Plant Biol. 2019, 19, 432. [Google Scholar] [CrossRef]
- Tsuda, K.; Ito, Y.; Sato, Y.; Kurata, N. Positive autoregulation of a KNOX gene is essential for shoot apical meristem maintenance in rice. Plant Cell 2011, 23, 4368–4381. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhou, C.; Li, Y.J.; Yu, Y.; Tang, L.P.; Zhang, W.J.; Yao, W.J.; Huang, R.; Laux, T.; Zhang, X.S. Integration of pluripotency pathways regulates stem cell maintenance in the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 2020, 117, 22561–22571. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.U.; Sun, Y.M.; Jia, Z.C. Effects of Overexpression of IPT and KN1 on Development and Growth in Transgenic Tobacco Plants. Plant Physiol. Commun. 2009, 45, 537–543. [Google Scholar]
- Tsuda, K.; Hake, S. Diverse functions of KNOX transcription factors in the diploid body plan of plants. Curr. Opin. Plant Biol. 2015, 27, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Furumizu, C.; Alvarez, J.P.; Sakakibara, K.; Bowman, J.L. Antagonistic roles for KNOX1 and KNOX2 genes in patterning the land plant body plan following an ancient gene duplication. PLoS Genet. 2015, 11, e1004980. [Google Scholar] [CrossRef]
- Chai, M.; Zhou, C.; Molina, I.; Fu, C.; Nakashima, J.; Li, G.; Zhang, W.; Park, J.; Tang, Y.; Jiang, Q.; et al. A class II KNOX gene, KNOX4, controls seed physical dormancy. Proc. Natl. Acad. Sci. USA 2016, 113, 6997–7002. [Google Scholar] [CrossRef]
- Wang, S.; Yamaguchi, M.; Grienenberger, E.; Martone, P.T.; Samuels, A.L.; Mansfield, S.D. The Class II KNOX genes KNAT3 and KNAT7 work cooperatively to influence deposition of secondary cell walls that provide mechanical support to Arabidopsis stems. Plant J. Cell Mol. Biol. 2020, 101, 293–309. [Google Scholar] [CrossRef]
- Hamant, O.; Pautot, V. Plant development: A TALE story. C. R. Biol. 2010, 333, 371–381. [Google Scholar] [CrossRef]
- Peng, Z.S.; Li, X.; Yang, Z.J.; Liao, M.L. A new reduced height gene found in the tetraploid semi-dwarf wheat landrace Aiganfanmai. Genet Mol. Res. 2011, 10, 2349–2357. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yan, L.; Xiong, X.; Wang, W.; Zhang, X.H.; Min, D.H. Genome-wide analysis of TALE superfamily in Triticum aestivum reveals TaKNOX11-A is involved in abiotic stress response. BMC Genom. 2022, 23, 89. [Google Scholar] [CrossRef]
- Zhang, J. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Geng, X.; Chen, Y.; Zhang, S.; Gao, Z.; Liu, S.; Yang, Q.; Wu, J.; Chen, X. Genome-Wide Analysis of Type-III Polyketide Synthases in Wheat and Possible Roles in Wheat Sheath-Blight Resistance. Int. J. Mol. Sci. 2022, 23, 7187. [Google Scholar] [CrossRef]
- Hay, A.; Tsiantis, M. A KNOX family TALE. Curr. Opin. Plant Biol. 2009, 12, 593–598. [Google Scholar] [CrossRef]
- Bueno, N.; Alvarez, J.M.; Ordás, R.J. Characterization of the KNOTTED1-LIKE HOMEOBOX (KNOX) gene family in Pinus pinaster Ait. Plant Sci. 2020, 301, 110691. [Google Scholar] [CrossRef]
- Sano, R.; Juárez, C.M.; Hass, B.; Sakakibara, K.; Ito, M.; Banks, J.A.; Hasebe, M. KNOX homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems. Evol. Dev. 2005, 7, 69–78. [Google Scholar] [CrossRef]
- Cheng, X.; Li, M.; Abdullah, M.; Li, G.; Zhang, J.; Manzoor, M.A.; Wang, H.; Jin, Q.; Jiang, T.; Cai, Y.; et al. In Silico Genome-Wide Analysis of the Pear (Pyrus bretschneideri) KNOX Family and the Functional Characterization of PbKNOX1, an Arabidopsis BREVIPEDICELLUS Orthologue Gene, Involved in Cell Wall and Lignin Biosynthesis. Front Genet. 2019, 10, 632. [Google Scholar] [CrossRef]
- Chatterjee, M.; Bermudez-Lozano, C.L.; Clancy, M.A.; Davis, T.M.; Folta, K.M. A strawberry KNOX gene regulates leaf, flower and meristem architecture. PLoS ONE 2011, 6, e24752. [Google Scholar] [CrossRef]
- Takumi, S.; Kosugi, T.; Murai, K.; Mori, N.; Nakamura, C. Molecular cloning of three homoeologous cDNAs encoding orthologs of the maize KNOTTED1 homeobox protein from young spikes of hexaploid wheat. Gene 2000, 249, 171–181. [Google Scholar] [CrossRef]
- Yao, F.Q.; Li, X.H.; Wang, H.; Song, Y.N.; Li, Z.Q.; Li, X.G.; Gao, X.Q.; Zhang, X.S.; Bie, X.M. Down-expression of TaPIN1s Increases the Tiller Number and Grain Yield in Wheat. BMC Plant Biol. 2021, 21, 443. [Google Scholar] [CrossRef]
- Bolduc, N.; Hake, S. The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell 2009, 21, 1647–1658. [Google Scholar] [CrossRef]
- Mukherjee, K.; Brocchieri, L.; Bürglin, T.R. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef] [PubMed]
- Nadakuduti, S.S.; Holdsworth, W.L.; Klein, C.L.; Barry, C.S. KNOX genes influence a gradient of fruit chloroplast development through regulation of GOLDEN2-LIKE expression in tomato. Plant J. Cell Mol. Biol. 2014, 78, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Sabzehzari, M.; Naghavi, M.R. Phyto-miRNA: A molecule with beneficial abilities for plant biotechnology. Gene 2019, 683, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L. SQUAMOSA promoter binding protein-like7 regulated microRNA408 is required for vegetative development in Arabidopsis. Plant J. Cell Mol. Biol. 2013, 74, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Mutum, R.D.; Balyan, S.C.; Kansal, S.; Agarwal, P.; Kumar, S.; Kumar, M.; Raghuvanshi, S. Evolution of variety-specific regulatory schema for expression of osa-miR408 in indica rice varieties under drought stress. Febs J. 2013, 280, 1717–1730. [Google Scholar] [CrossRef]
- Hajyzadeh, M.; Turktas, M.; Khawar, K.M.; Unver, T. miR408 overexpression causes increased drought tolerance in chickpea. Gene 2015, 555, 186–193. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Hong, P.; Wu, J.Y.; Chen, X.B.; Ye, X.G.; Pan, Y.Y.; Wang, J.; Zhang, X.S. The tae-miR408-Mediated Control of TaTOC1 Genes Transcription Is Required for the Regulation of Heading Time in Wheat. Plant Physiol. 2016, 170, 1578–1594. [Google Scholar] [CrossRef]
- Ma, C.; Burd, S.; Lers, A. miR408 is involved in abiotic stress responses in Arabidopsis. Plant J. Cell Mol. Biol. 2015, 84, 169–187. [Google Scholar] [CrossRef]
- Scofield, S.; Murray, J.A. KNOX gene function in plant stem cell niches. Plant Mol. Biol. 2006, 60, 929–946. [Google Scholar] [CrossRef]
- Qin, W.; Yin, Q.; Chen, J.; Zhao, X.; Yue, F.; He, J.; Yang, L.; Liu, L.; Zeng, Q.; Lu, F.; et al. The class II KNOX transcription factors KNAT3 and KNAT7 synergistically regulate monolignol biosynthesis in Arabidopsis. J. Exp. Bot. 2020, 71, 5469–5483. [Google Scholar] [CrossRef]
- Roy, S.W.; Gilbert, W. The evolution of spliceosomal introns: Patterns, puzzles and progress. Nat. Rev. Genet. 2006, 7, 211–221. [Google Scholar] [CrossRef]
- Roy, S.W.; Penny, D. A very high fraction of unique intron positions in the intron-rich diatom Thalassiosira pseudonana indicates widespread intron gain. Mol. Biol. Evol. 2007, 24, 1447–1457. [Google Scholar] [CrossRef]
- Meng, L.; Liu, X.; He, C.; Xu, B.; Li, Y.; Hu, Y. Functional divergence and adaptive selection of KNOX gene family in plants. Open Life Sci. 2020, 15, 346–363. [Google Scholar] [CrossRef]
- Hake, S.; Smith, H.M.; Holtan, H.; Magnani, E.; Mele, G.; Ramirez, J. The role of knox genes in plant development. Annu. Rev. Cell Dev. Biol. 2004, 20, 125–151. [Google Scholar] [CrossRef]
- Du, F.; Guan, C.; Jiao, Y. Molecular Mechanisms of Leaf Morphogenesis. Mol. Plant 2018, 11, 1117–1134. [Google Scholar] [CrossRef]
- Moon, J.; Hake, S. How a leaf gets its shape. Curr. Opin. Plant Biol. 2011, 14, 24–30. [Google Scholar] [CrossRef]
- Coudert, Y.; Novák, O.; Harrison, C.J. A KNOX-Cytokinin Regulatory Module Predates the Origin of Indeterminate Vascular Plants. Curr. Biol. 2019, 29, 2743–2750.e5. [Google Scholar] [CrossRef]
- Jasinski, S.; Piazza, P.; Craft, J.; Hay, A.; Woolley, L.; Rieu, I.; Phillips, A.; Hedden, P.; Tsiantis, M. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. CB 2005, 15, 1560–1565. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Guo, J.C.; Duan, R.J.; Hu, X.W.; Li, K.M.; Fu, S.P. Isopentenyl transferase gene (ipt) downstream transcriptionally fused with gene expression improves the growth of transgenic plants. Transgenic Res. 2010, 19, 197–209. [Google Scholar] [CrossRef]
- Ori, N.; Juarez, M.T.; Jackson, D.; Yamaguchi, J.; Banowetz, G.M.; Hake, S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell 1999, 11, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Rathee, S.; Sharma, P.; Ahmad, M.; Batish, D.R.; Singh, H.P.; Kaur, S.; Yadav, S.S. Seed size dimorphism in Hyptis suaveolens aids in differentiation of the germination niche. Plant Biol. 2022, 24, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Gordon, S.P.; Meyerowitz, E.M. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011, 21, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Grafi, G. How cells dedifferentiate: A lesson from plants. Dev. Biol. 2004, 268, 1–6. [Google Scholar] [CrossRef]
- Niu, X.; Guan, Y.; Chen, S.; Li, H. Genome-wide analysis of basic helix-loop-helix (bHLH) transcription factors in Brachypodium distachyon. BMC Genom. 2017, 18, 619. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Gu, Z.; Cavalcanti, A.; Chen, F.C.; Bouman, P.; Li, W.H. Extent of gene duplication in the genomes of Drosophila, nematode, and yeast. Mol. Biol. Evol. 2002, 19, 256–262. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2011, 39, W155–W159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yao, Y.; Ye, W.; Wang, S.; Zhang, C.; Liu, S.; Sun, F.; Xi, Y. Genome-Wide Identification of Wheat KNOX Gene Family and Functional Characterization of TaKNOX14-D in Plants. Int. J. Mol. Sci. 2022, 23, 15918. https://doi.org/10.3390/ijms232415918
Li S, Yao Y, Ye W, Wang S, Zhang C, Liu S, Sun F, Xi Y. Genome-Wide Identification of Wheat KNOX Gene Family and Functional Characterization of TaKNOX14-D in Plants. International Journal of Molecular Sciences. 2022; 23(24):15918. https://doi.org/10.3390/ijms232415918
Chicago/Turabian StyleLi, Song, Yaxin Yao, Wenjie Ye, Shaoyu Wang, Chao Zhang, Shudong Liu, Fengli Sun, and Yajun Xi. 2022. "Genome-Wide Identification of Wheat KNOX Gene Family and Functional Characterization of TaKNOX14-D in Plants" International Journal of Molecular Sciences 23, no. 24: 15918. https://doi.org/10.3390/ijms232415918
APA StyleLi, S., Yao, Y., Ye, W., Wang, S., Zhang, C., Liu, S., Sun, F., & Xi, Y. (2022). Genome-Wide Identification of Wheat KNOX Gene Family and Functional Characterization of TaKNOX14-D in Plants. International Journal of Molecular Sciences, 23(24), 15918. https://doi.org/10.3390/ijms232415918





