Effects of Streamer Discharge on PM2.5 Containing Endotoxins and Polyaromatic Hydrocarbons and Their Biological Responses In Vitro
Abstract
1. Introduction
2. Results
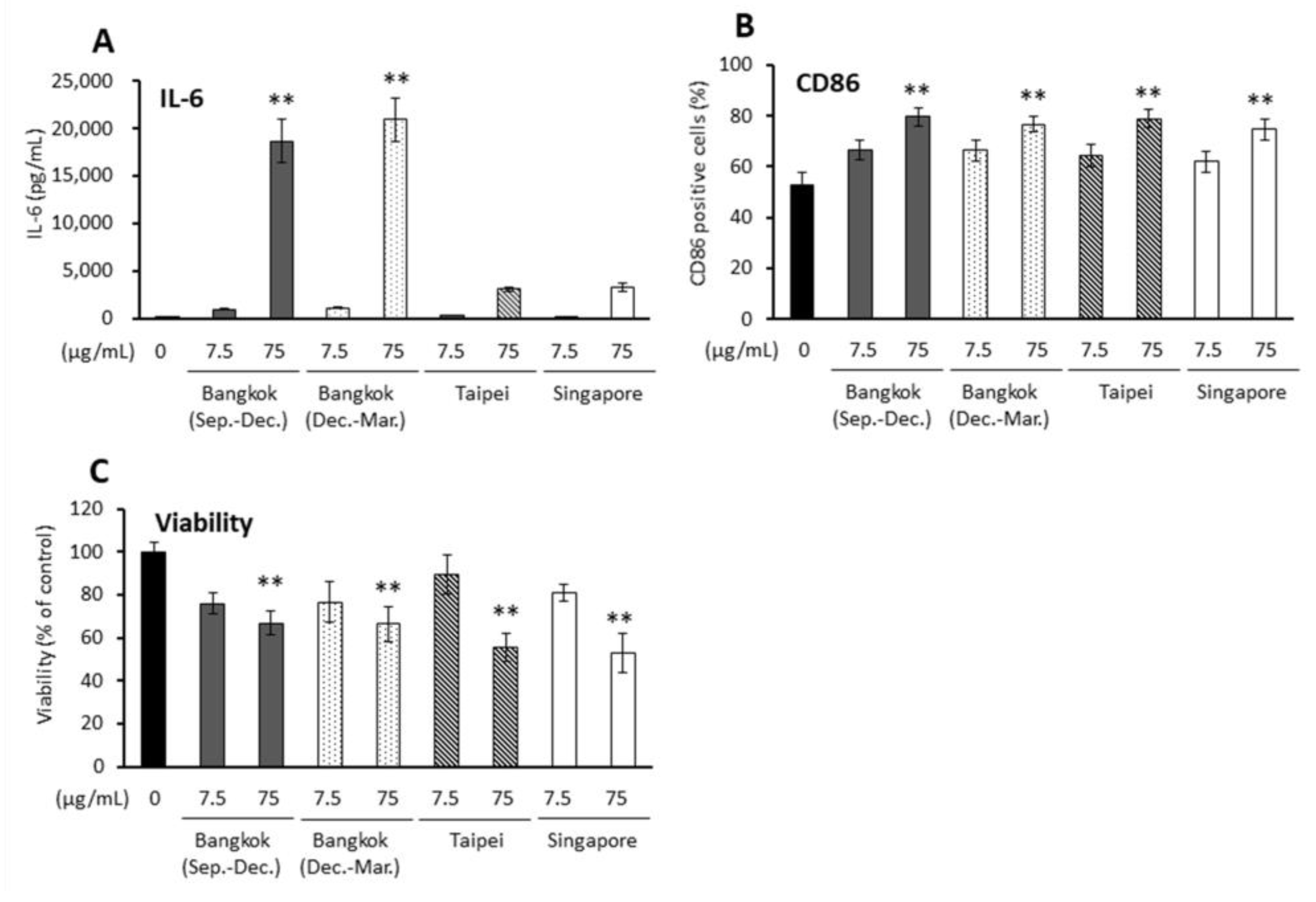
2.1. Various PM2.5 Samples Caused Proinflammatory Responses or Oxidative Stress in BEAS-2B Cells
2.2. Effects of Several PM2.5 Samples on Activation of Bone Marrow-Derived Antigen Presenting Cells (APCs)
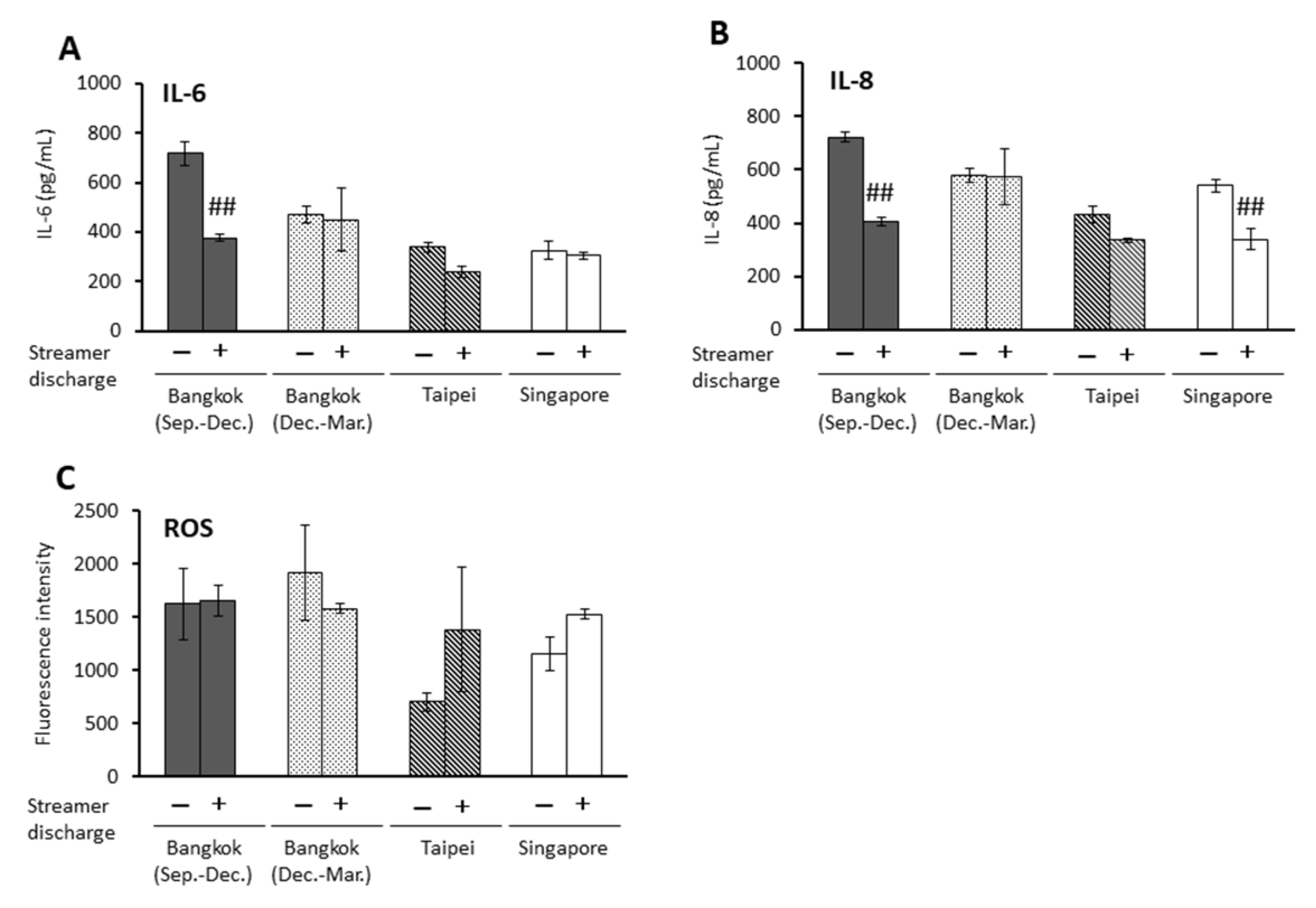
2.3. Effects of Streamer-Discharged PM2.5 on Proinflammatory Parameters and ROS Production in BEAS-2B Cells
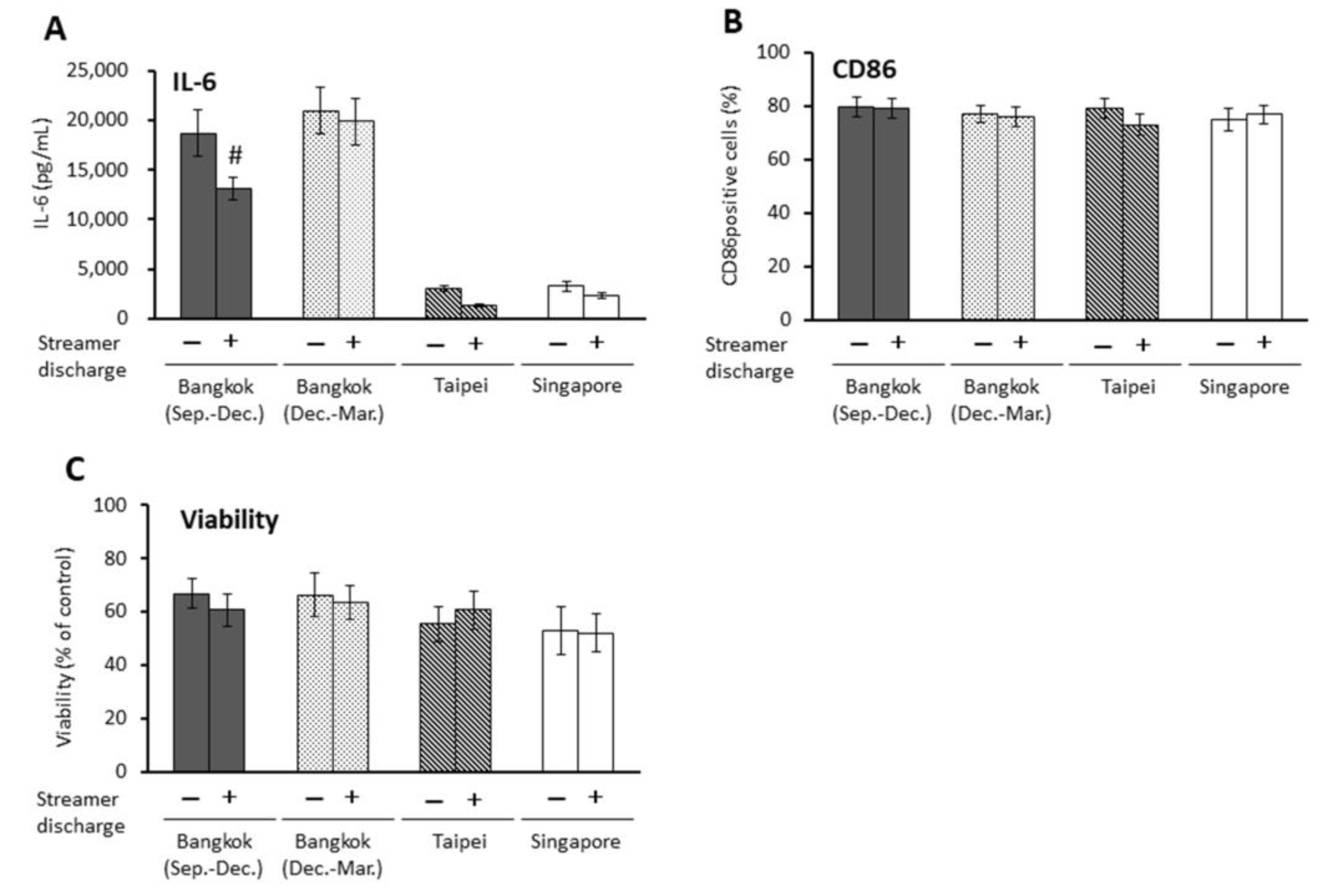
2.4. Effects of Streamer-Discharged PM2.5 on APCs
2.5. Components in Non-Treated PM2.5 and Streamer-Discharged PM2.5
3. Discussion
4. Materials and Methods
4.1. PM2.5 Collection
4.2. Cell Culture
4.3. Treatment of PM2.5 with Streamer Discharge
4.4. Experimental Protocol
4.5. Cell Viability
4.6. Cytokines in the Culture Supernatants
4.7. Reactive oxygen Species Generation
4.8. Fluorescence-Activated Cell Sorting
4.9. Characterization of PM2.5
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, S.; Yadav, A.; Tsai, C.-J.; Kumar, P. A review on recent progress in observations, sources, classification and regulations of PM2.5 in Asian environments. Environ. Sci. Pollut. Res. 2016, 23, 21165–21175. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent insights into particulate matter (PM(2.5))-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.C.; Hsieh, W.Y.; Tsai, C.H.; Chen, C.Y.; Chang, H.F.; Lin, C.S. In vitro and in vivo experimental studies of PM(2.5) on disease progression. Int. J. Environ. Res. Public Health 2018, 15, 1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, D.; Wu, W.; Huo, X. PM2.5 exposure inducing ATP alteration links with NLRP3 inflammasome activation. Environ. Sci. Pollut. Res. 2022, 29, 24445–24456. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, H.; Janssen, N.A.; Fischer, P.H.; Kos, G.P.; Weijers, E.P.; Cassee, F.R.; Van Der Zee, S.C.; De Hartog, J.J.; Brunekreef, B.; Hoek, G. Contrasts in Oxidative Potential and Other Particulate Matter Characteristics Collected Near Major Streets and Background Locations. Environ. Health Perspect. 2012, 120, 185–191. [Google Scholar] [CrossRef]
- Lippmann, M.; Chen, L.-C.; Gordon, T.; Ito, K.; Thurston, G.D. National Particle Component Toxicity (NPACT) Initiative: Integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res. Rep. (Health Eff. Inst.) 2013, 177, 5–13. [Google Scholar]
- Perrone, M.; Gualtieri, M.; Consonni, V.; Ferrero, L.; Sangiorgi, G.; Longhin, E.; Ballabio, D.; Bolzacchini, E.; Camatini, M. Particle size, chemical composition, seasons of the year and urban, rural or remote site origins as determinants of biological effects of particulate matter on pulmonary cells. Environ. Pollut. 2013, 176, 215–227. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, J.; Sun, J.; Xin, L. PM2.5 induces inflammatory responses via oxidative stress-mediated mitophagy in human bronchial epithelial cells. Toxicol. Res. 2022, 11, 195–205. [Google Scholar] [CrossRef]
- Hung, N.T.; Li, C.T.; Wang, S.H.; Ou-Yang, C.-F.; Lin, C.-Y.; Lee, C.-T.; Lin, N.-H.; Chi, K.H. Long-term monitoring of atmospheric PCDD/Fs at Mount Lulin during spring season: PCDD/F source apportionment through a simultaneous measurement in Southeast Asia. Chemosphere 2017, 185, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Borgie, M.; Ledoux, F.; Verdin, A.; Cazier, F.; Greige, H.; Shirali, P.; Courcot, D.; Dagher, Z. Genotoxic and epigenotoxic effects of fine particulate matter from rural and urban sites in Lebanon on human bronchial epithelial cells. Environ. Res. 2015, 136, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Murayama, R.; Tsuji, K.; Matsuda, Y.; Koike, E.; Okamoto, Y.; Shirasawa, N.; Takano, H. Streamer discharge reduces pollen-induced inflammatory responses and injury in human airway epithelial cells. Exp. Biol. Med. 2013, 238, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Okamoto, Y.; Enokida, T.; Tanaka, T.; Kagawa, K.; Nozaki, A. Decomposition performance of air purifier using Streamer discharge technology for chemical substances adhering to PM2.5. In Proceedings of the 13th International Conference on Indoor Air Quality and Climate, Hong Kong, China, 7–12 July 2014. [Google Scholar]
- Okamoto, Y. New deodorization & antibacterial technique using the interaction with photocatalytic materials & streamer discharge. In Proceedings of the symposium on Society of indoor, Tokyo, Japan, 20 September 2007. Abstract Book. [Google Scholar]
- Kagawa, K. Removal of indoor air pollutant by streamer discharge technique. In Proceedings of the International Symposium on Refrigeration Technology, Zhunhai, China, 6–7 August 2010. Abstract Book. [Google Scholar]
- Lung, S.C.; Thi Hien, T.; Cambaliza, M.O.L.; Hlaing, O.M.T.; Oanh, N.T.K.; Latif, M.T.; Lestari, P.; Salam, A.; Lee, S.-Y.; Wang, W.-C.V.; et al. Research priorities of applying low-cost PM(2.5) sensors in Southeast Asian countries. Int. J. Environ. Res. Public Health 2022, 19, 1522. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-S.; Chiu, Y.-W.; Weng, Y.-H.; Yang, C.-Y. Association between fine particulate air pollution and the risk of death from lung cancer in Taiwan. J. Toxicol. Environ. Health Part A 2022, 85, 431–438. [Google Scholar] [CrossRef]
- Chew, F.T.; Goh, D.Y.; Ooi, B.C.; Saharom, R.; Hui, J.K.; Lee, B.W. Association of ambient air-pollution levels with acute asthma exacerbation among children in Singapore. Allergy 1999, 54, 320–329. [Google Scholar] [CrossRef]
- Onishi, T.; Honda, A.; Tanaka, M.; Chowdhury, P.H.; Okano, H.; Okuda, T.; Shishido, D.; Terui, Y.; Hasegawa, S.; Kameda, T.; et al. Ambient fine and coarse particles in Japan affect nasal and bronchial epithelial cells differently and elicit varying immune response. Environ. Pollut. 2018, 242, 1693–1701. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, J.; Lu, L.; Bold, T.; Li, X.; Wang, S.; Chang, Z.; Chen, J.; Kong, X.; Zheng, Y.; et al. Intracellular GSH/GST antioxidants system change as an earlier biomarker for toxicity evaluation of iron oxide nanoparticles. Nanoimpact 2021, 23, 100338. [Google Scholar] [CrossRef]
- Honda, A.; Inoue, K.-I.; Takai, S.; Kameda, T.; Ueda, K.; Takano, H. Effects of Oxidized Pyrenes on the Biological Responses in the Human Bronchial Epithelial Cells. Appl. Sci. 2022, 12, 9664. [Google Scholar] [CrossRef]
- Schulz, C.; Farkas, L.; Wolf, K.; Krätzel, K.; Eissner, G.; Pfeifer, M. Differences in LPS-Induced Activation of Bronchial Epithelial Cells (BEAS-2B) and Type II-Like Pneumocytes (A-549). Scand. J. Immunol. 2002, 56, 294–302. [Google Scholar] [CrossRef]
- Schins, R.P.; Lightbody, J.H.; Borm, P.J.; Shi, T.; Donaldson, K.; Stone, V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol. Appl. Pharmacol. 2004, 195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Long, C.M.; Suh, H.H.; Kobzik, L.; Catalano, P.J.; Ning, Y.Y.; Koutrakis, P. A pilot investigation of the relative toxicity of indoor and outdoor fine particles: In vitro effects of endotoxin and other particulate properties. Environ. Health Perspect. 2001, 109, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Monn, C.; Becker, S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol. Appl. Pharmacol. 1999, 155, 245–252. [Google Scholar] [CrossRef]
- Kawasaki, S.; Takizawa, H.; Takami, K.; Desaki, M.; Okazaki, H.; Kasama, T.; Kobayashi, K.; Yamamoto, K.; Nakahara, K.; Tanaka, M.; et al. Benzene-extracted components are important for the major activity of diesel exhaust particles: Effect on interleukin-8 gene expression in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 419–426. [Google Scholar] [CrossRef]
- Farina, F.; Sancini, G.; Mantecca, P.; Gallinotti, D.; Camatini, M.; Palestini, P. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol. Lett. 2011, 202, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Happo, M.S.; Huttunen, K.; Sillanpää, M.; Hillamo, R.; Salonen, R.O.; Hirvonen, M.R. Chemical and microbial components of urban air PM cause seasonal variation of toxicological activity. Environ. Toxicol. Pharmacol. 2015, 40, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Lin, Z.; Wang, Y.; He, H.; Liu, T.; Kamp, D.W. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ. Toxicol. 2016, 31, 923–936. [Google Scholar] [CrossRef]
- Chowdhury, P.H.; Honda, A.; Ito, S.; Okano, H.; Onishi, T.; Higashihara, M.; Okuda, T.; Tanaka, T.; Hirai, S.; Takano, H. Effects of Ambient PM2.5 Collected Using Cyclonic Separator from Asian Cities on Human Airway Epithelial Cells. Aerosol Air Qual. Res. 2019, 19, 1808–1819. [Google Scholar] [CrossRef]
- Figliuzzi, M.; Tironi, M.; Longaretti, L.; Mancini, A.; Teoldi, F.; Sangalli, F.; Remuzzi, A. Copper-dependent biological effects of particulate matter produced by brake systems on lung alveolar cells. Arch. Toxicol. 2020, 94, 2965–2979. [Google Scholar] [CrossRef]
- Fu, X.; Hong, W.; Li, S.; Chen, Z.; Zhou, W.; Dai, J.; Deng, X.; Zhou, H.; Li, B.; Ran, P. Wood smoke particulate matter (WSPM2.5) induces pyroptosis through both Caspase-1/IL-1β/IL-18 and ATP/P2Y-dependent mechanisms in human bronchial epithelial cells. Chemosphere 2022, 307, 135726. [Google Scholar] [CrossRef]
- Okuda, T.; Isobe, R.; Nagai, Y.; Okahisa, S.; Funato, K.; Inoue, K. Development of a High-Volume PM2.5 Particle Sampler Using Impactor and Cyclone Techniques. Aerosol Air Qual. Res. 2015, 15, 759–767. [Google Scholar] [CrossRef]

| Before Streamer Discharge | After Streamer Discharge | Rate of Change (%) | |
|---|---|---|---|
| Endotoxin (EU/mg) | 7.560 | 4.448 | −41.2 |
| PAH (ng/mg) | |||
| Pyrene | 0.74 | 0.37 | −50.0 |
| Fluoranthene | 0.58 | 0.29 | −50.0 |
| Benz[a]anthracene | 0.21 | 0.1 | −52.4 |
| Chrysene | 1.1 | 0.5 | −54.5 |
| Benzo[b]fluoranthene | 1.2 | 0.45 | −62.5 |
| Benzo[k]fluoranthene | 0.33 | 0.15 | −54.5 |
| Benzo[a]pyrene | 0.33 | 0.18 | −45.5 |
| Benzo[g,h,i]perylene | 1.3 | 0.61 | −53.1 |
| Indeno[1,2,3-c,d]pyrene | 0.87 | 0.37 | −57.5 |
| Dibenz[a,h]anthracene | 0.08 | 0.04 | −50.0 |
| Carbon (ngC/mg) | |||
| OC1 | N.D. | N.D. | N.D. |
| OC2 | 20,000 | 16,000 | −20.0 |
| OC3 | 65,000 | 66,000 | 1.5 |
| OC4 | 23,000 | 37,000 | 60.9 |
| EC1 | 73,000 | 57,000 | −21.9 |
| EC2 | 93,000 | 79,000 | −15.1 |
| EC3 | 6000 | 6200 | 3.3 |
| Metals and Other Inorganic Components (ng/mg) | |||
| Na | 28,000 | 27,000 | −3.6 |
| Al | 42,000 | 38,000 | −9.5 |
| K | 12,000 | 12,000 | 0.0 |
| Ca | 33,000 | 33,000 | 0.0 |
| Sc | 21 | 20 | −4.8 |
| Ti | 1300 | 1300 | 0.0 |
| V | 140 | 140 | 0.0 |
| Cr | 3400 | 3500 | 2.9 |
| Mn | 720 | 690 | −4.2 |
| Fe | 28,000 | 27,000 | −3.6 |
| Co | 29 | 30 | 3.4 |
| Ni | 1000 | 1100 | 10.0 |
| Cu | 350 | 360 | 2.9 |
| Zn | 2700 | 2500 | −7.4 |
| As | 69 | 71 | 2.9 |
| Se | 48 | 44 | −8.3 |
| Rb | 48 | 46 | −4.2 |
| Mo | 42 | 41 | −2.4 |
| Sb | 66 | 68 | 3.0 |
| Cs | 3.3 | 3.2 | −3.0 |
| Ba | 410 | 410 | 0.0 |
| La | 12 | 12 | 0.0 |
| Ce | 19 | 18 | −5.3 |
| Sm | 0.99 | N.D. | N.D. |
| Hf | 1.9 | 1.3 | −31.6 |
| W | 77 | 69 | −10.4 |
| Ta | 1 | 0.93 | −7.0 |
| Th | 2.8 | 2.4 | −14.3 |
| Pb | 420 | 440 | 4.8 |
| Cd | 12 | 11 | −8.3 |
| Cl− | 4200 | 3900 | −7.1 |
| NO3− | 37,000 | 36,000 | −2.7 |
| SO42− | 140,000 | 140,000 | 0.0 |
| Na+ | 26,000 | 25,000 | −3.8 |
| NH4+ | 7600 | 7800 | 2.6 |
| K+ | 7500 | 7600 | 1.3 |
| Mg2+ | 4000 | 3800 | −5.0 |
| Ca2+ | 32,000 | 33,000 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, A.; Inoue, K.-i.; Tamura, S.; Tanaka, M.; Wang, Z.; Tanaka, T.; Hirai, S.; Okuda, T.; Ueda, K.; Takano, H. Effects of Streamer Discharge on PM2.5 Containing Endotoxins and Polyaromatic Hydrocarbons and Their Biological Responses In Vitro. Int. J. Mol. Sci. 2022, 23, 15891. https://doi.org/10.3390/ijms232415891
Honda A, Inoue K-i, Tamura S, Tanaka M, Wang Z, Tanaka T, Hirai S, Okuda T, Ueda K, Takano H. Effects of Streamer Discharge on PM2.5 Containing Endotoxins and Polyaromatic Hydrocarbons and Their Biological Responses In Vitro. International Journal of Molecular Sciences. 2022; 23(24):15891. https://doi.org/10.3390/ijms232415891
Chicago/Turabian StyleHonda, Akiko, Ken-ichiro Inoue, Shin Tamura, Michitaka Tanaka, Zaoshi Wang, Toshio Tanaka, Seitarou Hirai, Tomoaki Okuda, Kayo Ueda, and Hirohisa Takano. 2022. "Effects of Streamer Discharge on PM2.5 Containing Endotoxins and Polyaromatic Hydrocarbons and Their Biological Responses In Vitro" International Journal of Molecular Sciences 23, no. 24: 15891. https://doi.org/10.3390/ijms232415891
APA StyleHonda, A., Inoue, K.-i., Tamura, S., Tanaka, M., Wang, Z., Tanaka, T., Hirai, S., Okuda, T., Ueda, K., & Takano, H. (2022). Effects of Streamer Discharge on PM2.5 Containing Endotoxins and Polyaromatic Hydrocarbons and Their Biological Responses In Vitro. International Journal of Molecular Sciences, 23(24), 15891. https://doi.org/10.3390/ijms232415891







