Changes in the Phytochemical and Bioactive Compounds and the Antioxidant Properties of Wolfberry during Vinegar Fermentation Processes
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Parameters of Wolfberry Fruit Vinegar during Fermentation
2.2. Nutritional Components of Wolfberry Fruit Vinegar during Fermentation
2.3. Bioactive Components of Wolfberry Fruit Vinegar during Fermentation
2.4. Polyphenolic Compounds of Wolfberry Fruit Vinegar during Fermentation
2.5. Antioxidant Activities of Wolfberry Fruit Vinegar during Fermentation
3. Materials and Methods
3.1. Raw Materials and Chemicals
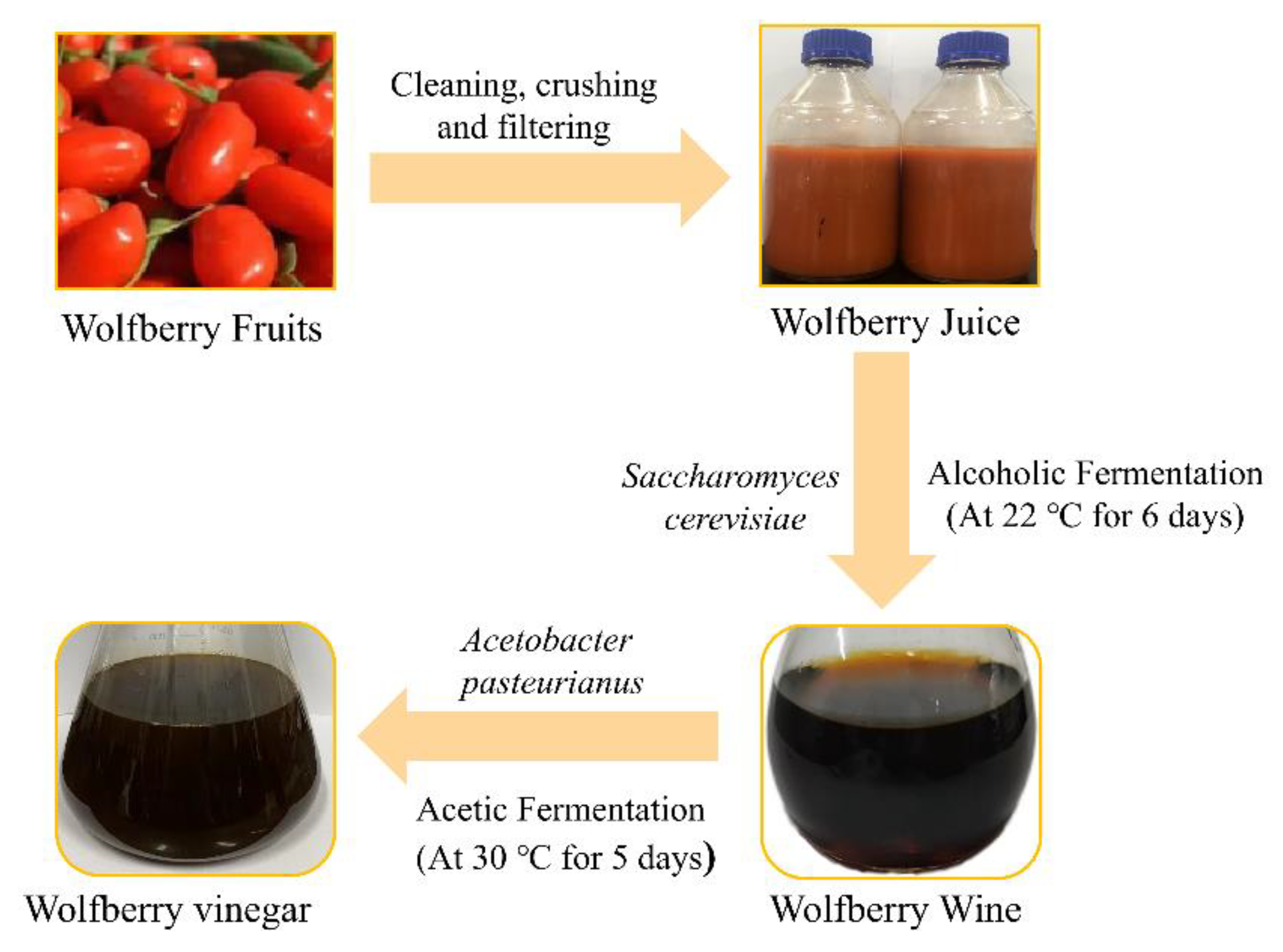
3.2. Production of Wolfberry Fruit Vinegar
3.3. Determination of the Physicochemical and Nutritional Components
3.4. Determination of the Amino Acids
3.5. Determination of the Functional Components
3.5.1. Determination of the TPC
3.5.2. Determination of the TFC
3.5.3. Determination of the Polysaccharide
3.5.4. Determination of the Betaine
3.5.5. Determination of the Carotenoids
3.6. Determination of the Polyphenolic Compounds
3.7. Antioxidant Activity Analyses
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, Z.W.; Sheng, H.P.; He, L.J.; Fan, X.W.; He, Z.X.; Sun, T.; Zhang, X.; Zhao, R.J.; Gu, L.; et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Dev. Ther. 2014, 9, 33–78. [Google Scholar]
- Liu, W.; Xu, J.; Zhu, R.; Zhu, Y.; Zhao, Y.; Chen, P.; Pan, C.; Yao, W.; Gao, X. Fingerprinting profile of polysaccharides from Lycium barbarum using multiplex approaches and chemometrics. Int. J. Biol. Macromol. 2015, 78, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, L. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). LWT—Food Sci. Technol. 2021, 154, 112716. [Google Scholar] [CrossRef]
- Yuan, G.; Ren, J.; Ouyang, X.; Wang, L.; Wang, M.; Shen, X.; Zhang, B.; Zhu, B. Effect of raw material, pressing and glycosidase on the volatile compound composition of wine made from goji berries. Molecules 2016, 21, 1324. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Dong, B.T.; Chen, J.J.; Zhao, B.; Wang, X.D.; Wang, L.W.; Zha, S.H.; Wang, Y.C.; Zhang, J.H.; Wang, Y.L. Effect of drying methods on physicochemical properties and antioxidant activities of wolfberry (Lycium barbarum) polysaccharide. Carbohyd. Polym. 2015, 127, 176–181. [Google Scholar] [CrossRef]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of physicochemical properties, functional compounds and antioxidant capacity during spontaneous fermentation of Lycium ruthenicum murr. (Qinghai-Tibet Plateau) natural vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Duan, W.H.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic Acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Xia, T.; Duan, W.; Zhang, Z.; Li, S.; Zhao, Y.; Geng, B.; Zheng, Y.; Yu, J.; Wang, M. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res. Int. 2021, 140, 110064. [Google Scholar] [CrossRef] [PubMed]
- Cantadori, E.; Brugnoli, M.; Centola, M.; Uffredi, E.; Colonello, A.; Gullo, M. Date fruits as raw material for vinegar and non-alcoholic fermented beverages. Foods 2022, 11, 1972. [Google Scholar] [CrossRef] [PubMed]
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Giovanni, M. Balsamic vinegar of modena: From product to market value: Competitive strategy of a typical Italian product. Brit. Food J. 2004, 106, 722–745. [Google Scholar]
- Durán-Guerrero, E.; Castro, R.; García-Moreno, M.V.; Rodríguez-Dodero, M.D.C.; Schwarz, M.; Guillén-Sánchez, D. Aroma of sherry products: A review. Foods 2021, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Wang, M.Y.; Cao, L.W.; Wang, X.T.; Sun, J.R.; Yuan, J.F.; Gu, S.B. Changes and correlation of microorganism and flavor substances during persimmon vinegar fermentation. Food Biosci. 2022, 46, 101565. [Google Scholar] [CrossRef]
- Xu, S.; Ma, Z.; Chen, Y.; Li, J.; Jiang, H.; Qu, T.; Zhang, W.; Li, C.; Liu, S. Characterization of the flavor and nutritional value of coconut water vinegar based on metabolomics. Food Chem. 2022, 369, 130872. [Google Scholar] [CrossRef]
- Burdon, J.; Pidakala, P.; Martin, P.; Billing, D.; Boldingh, H. Fruit maturation and the soluble solids harvest index for ‘Hayward’ kiwifruit. Sci. Hortic. 2016, 213, 193–198. [Google Scholar] [CrossRef]
- Crozier, A.; Borges, G. (Poly) phenolic constituents and the beneficial effects of moderate red wine consumption. J. Wine Res. 2011, 22, 131–134. [Google Scholar] [CrossRef]
- Akyüz, E.; Başkan, K.S.; Tütem, E.; Apak, R. High performance liquid chromatographic method with post-column detection for quantification of reducing sugars in foods. J. Chromatogr. A 2021, 1660, 462664. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem. 2018, 266, 262–274. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, F.; Durán-Guerrero, E.; Riponi, C. Discrimination of some European vinegars with protected denomination of origin as a function of their amino acid and biogenic amine content. J. Sci. Food Agric. 2016, 96, 3762–3771. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.O. Quality characteristics of fermented vinegars using pear. KJFP 2016, 23, 778–787. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of wort amino acids on beer flavour: A review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Carlavilla, D.; Moreno-Arribas, M.V.; Fanali, S.; Cifuentes, A. Chiral MEKC-LIF of amino acids in foods: Analysis of vinegars. Electrophoresis 2006, 27, 2551–2557. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Kamal, G.M.; Jiang, B.; Sun, P.; Zhang, X.; Liu, M. Characterization and comparison of commercial Chinese cereal and European grape vinegars using 1H-NMR spectroscopy combined with multivariate analysis. Chin. J. Chem. 2016, 34, 1183–1193. [Google Scholar] [CrossRef]
- Geng, J.Y.; Zhao, L.; Zhang, H.L. Formation mechanism of isoprene compounds degraded from carotenoids during fermentation of goji wine. Food Qual. Saf. 2021, 5, fyaa033. [Google Scholar] [CrossRef]
- Sun, Y.; Rukeya, J.; Tao, W.; Sun, P.; Ye, X. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci. Rep. 2017, 7, 40605. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Yang, Y.Y.; Xu, M.D.; Wang, J.H. Change analysis of polyphenols and flavonoids in the process of jujube vinegar brewing. China Condiment. 2012, 37, 56–59. [Google Scholar]
- Özdemir, G.B.; Özdemir, N.; Ertekin-Filiz, B.; Gökırmaklı, Ç.; Kök-Taş, T.; Budak, N.H. Volatile aroma compounds and bioactive compounds of hawthorn vinegar produced from hawthorn fruit (Crataegus tanacetifolia (lam.) pers.). J. Food Biochem. 2022, 46, e13676. [Google Scholar] [CrossRef] [PubMed]
- Kharchoufi, S.; Gomez, J.; Lasanta, C.; Castro, R.; Sainz, F.; Hamdi, M. Benchmarking laboratory-scale pomegranate vinegar against commercial wine vinegars: Antioxidant activity and chemical composition. J. Sci. Food Agric. 2018, 98, 4749–4758. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Gewers, F.L.; Ferreira, G.R.; De-Arruda, H.F.; Silva, F.N.; Comin, C.H.; Amancio, D.R.; Costa, L.F. Principal component analysis: A natural approach to data exploration. ACM Comput. Surv. 2018, 54, 9–22. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef]
- Ching, T.K.; Chin, W.H.; Ling, J.W.A.; Lazim, A.; Fazry, S.; Lim, S.J. Chemical changes and optimisation of acetous fermentation time and mother of vinegar concentration in the production of vinegar-like fermented papaya beverage. Sains Malays. 2018, 47, 2017–2026. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Eezymol. 1999, 299, 152–178. [Google Scholar]


| Products | pH Value | Total Acids (g/100 mL) | Non-Volatile Acid (g/100 mL) | Soluble Solids Content (g/100 mL) | Reducing Sugar (g/100 mL) |
|---|---|---|---|---|---|
| WFJ | 4.48 ± 0.16 a | 0.56 ± 0.04 b | 0.42 ± 0.05 c | 16.26 ± 0.24 a | 12.08 ± 0.32 a |
| WFW | 4.20 ± 0.12 a | 0.60 ± 0.07 b | 0.56 ± 0.02 b | 8.42 ± 0.26 b | 1.31 ± 0.11 c |
| WFV | 3.38 ± 0.08 b | 6.72 ± 0.12 a | 0.84 ± 0.13 a | 8.66 ± 0.28 b | 1.42 ± 0.14 b |
| Products | Sugar (g/100 mL) | Protein (g/100 mL) | Fat (g/100 mL) |
|---|---|---|---|
| WFJ | 15.63 ± 0.24 a | 0.49 ± 0.08 a | 0.10 ± 0.02 a |
| WFW | 1.98 ± 0.15 b | 0.38 ± 0.06 a | 0.09 ± 0.04 a |
| WFV | 2.46 ± 0.12 b | 0.46 ± 0.04 a | 0.12 ± 0.02 a |
| Compound | Amino Acid Content (mg/L) | ||
|---|---|---|---|
| WFJ | WFW | WFV | |
| Proline | 722.10 ± 18.72 a | 552.36 ± 26.88 b | 439.51 ± 18.34 c |
| Serine | 505.62 ± 18.92 a | 40.28 ± 5.42 b | 38.60 ± 3.53 b |
| Histidine | 353.41 ± 5.78 c | 433.79 ± 5.25 b | 543.65 ± 4.38 a |
| Alanine | 312.36 ± 16.97 a | 79.15 ± 12.65 b | 75.62 ± 8.97 b |
| Aspartic acid | 135.14 ± 4.38 a | 12.46 ± 2.45 b | 11.74 ± 2.50 b |
| Arginine | 107.59 ± 27.80 a | 68.87 ± 3.33 a | 66.54 ± 4.51 a |
| Glutamic acid | 73.59 ± 8.58 a | 50.53 ± 8.48 b | 43.38 ± 0.41 b |
| Cysteine | 54.78 ± 3.38 a | 42.04 ± 3.13 b | 45.03 ± 1.40 b |
| Phenyllalanine | 46.31 ± 14.73 a | 51.08 ± 15.29 a | 50.33 ± 9.82 a |
| Lysine | 40.80 ± 7.10 a | 6.83 ± 1.43 b | 4.89 ± 1.12 b |
| Valine | 36.91 ± 0.59 a | - | - |
| Leucine | 31.66 ± 2.81 c | 24.28 ± 0.36 b | 42.46 ± 1.75 a |
| Threonine | 29.50 ± 2.45 a | 21.85 ± 2.32 b | 25.41 ± 4.29 a b |
| Isoleucine | 8.70 ± 2.74 a | - | 11.51 ± 0.58 a |
| Glycine | 5.31 ± 0.41 b | 18.16 ± 4.15 a | 10.47 ± 2.53 a b |
| Products | Polysaccharide (mg/mL) | Betaine (mg/mL) | Carotenoids (mg/mL) | TPC (mg GAE/mL) | TFC (mg RE/mL) |
|---|---|---|---|---|---|
| WFJ | 8.58 ± 0.15 a | 2.94 ± 0.12 a | 0.58 ± 0.08 a | 1.87 ± 0.01 b | 1.02 ± 0.05 b |
| WFW | 7.26 ± 0.26 b | 2.68 ± 0.14 a | 0.46 ± 0.04 b | 1.88 ± 0.02 b | 1.63 ± 0.01 a |
| WFV | 8.94 ± 0.27 a | 2.88 ± 0.22 a | 0.42 ± 0.02 b | 2.42 ± 0.05 a | 1.69 ± 0.02 a |
| No | Polyphenolic Compounds | Contents (mg/mL) | ||
|---|---|---|---|---|
| WFJ | WFW | WFV | ||
| 1 | 4-Hydroxy cinnamic acid | 0.408 ± 0.017 a | 0.116 ± 0.010 b | 0.131 ± 0.005 b |
| 2 | 4-Hydroxyphenethyl alcohol | 0.004 ± 0.001 c | 0.337 ± 0.015 a | 0.216 ± 0.024 b |
| 3 | Phloroglucinol | 0.349 ± 0.005 a | 0.329 ± 0.008 a | 0.105 ± 0.004 b |
| 4 | 4-Hydroxy benzoic acid | 0.100 ± 0.005 b | 0.079 ± 0.008 b | 0.317 ± 0.011 a |
| 5 | 3-Hydroxy cinnamic acid | 0.339 ± 0.008 a | 0.195 ± 0.012 b | 0.311 ± 0.011 a |
| 6 | Chlorogenic acid | 0.026 ± 0.005 c | 0.096 ± 0.020 b | 0.222 ± 0.008 a |
| 7 | 3-(4-Hydroxy-3-methoxyphenyl) propionic acid | - | 0.159 ± 0.007 b | 0.222 ± 0.008 a |
| 8 | 4-Hydroxy benzaldehyde | 0.005 ± 0.001 c | 0.015 ± 0.002 b | 0.022 ± 0.001 a |
| 9 | 2-Hydroxybenzoic Acid | 0.098 ± 0.008 a | 0.044 ± 0.003 c | 0.059 ± 0.002 b |
| 10 | Ferulic acid | 0.212 ± 0.001 a | 0.094 ± 0.004 c | 0.134 ± 0.005 b |
| 11 | 3-(4-Hydroxymethyl) propionic acid | 0.141 ± 0.010 a b | 0.104 ± 0.009 b | 0.151 ± 0.005 a |
| 12 | Caffeic acid | 0.063 ± 0.009 b | 0.077 ± 0.005 b | 0.143 ± 0.005 a |
| 13 | vanillic acid | 0.042 ± 0.002 b | 0.051 ± 0.006 b | 0.097 ± 0.003 a |
| 14 | 3-(4-Hydroxyphenyl)-2-hydroxypropionic acid | 0.034 ± 0.003 a | 0.018 ± 0.007 a | 0.038 ± 0.001 a |
| 15 | 4-Hydroxyphenylacetic acid | 0.030 ± 0.002 a | 0.015 ± 0.002 b | 0.028 ± 0.001 a |
| 16 | 3,4-Dihydroxyphenylpropionic acid | 0.014 ± 0.001 b | 0.014 ± 0.002 b | 0.027 ± 0.001 a |
| 17 | 3-Hydroxy-4-methoxycinnamic acid | 0.006 ± 0.001 c | 0.037 ± 0.001 b | 0.062 ± 0.002 a |
| 18 | Protocatechuic acid | 0.005 ± 0.001 b | 0.007 ± 0.001 b | 0.022 ± 0.001 a |
| Products | DPPH (mM Trolox/L) | ABTS (mM Trolox/L) | FRAP (mM Trolox/L) |
|---|---|---|---|
| WFJ | 25.71 ± 0.52 b | 20.59 ± 0.46 b | 8.26 ± 0.16 b |
| WFW | 24.84 ± 0.06 c | 19.57 ± 0.33 c | 7.82 ± 0.18 c |
| WFV | 27.87 ± 0.37 a | 21.92 ± 0.28 a | 8.76 ± 0.23 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, T.; Qiang, X.; Geng, B.; Zhang, X.; Wang, Y.; Li, S.; Meng, Y.; Zheng, Y.; Wang, M. Changes in the Phytochemical and Bioactive Compounds and the Antioxidant Properties of Wolfberry during Vinegar Fermentation Processes. Int. J. Mol. Sci. 2022, 23, 15839. https://doi.org/10.3390/ijms232415839
Xia T, Qiang X, Geng B, Zhang X, Wang Y, Li S, Meng Y, Zheng Y, Wang M. Changes in the Phytochemical and Bioactive Compounds and the Antioxidant Properties of Wolfberry during Vinegar Fermentation Processes. International Journal of Molecular Sciences. 2022; 23(24):15839. https://doi.org/10.3390/ijms232415839
Chicago/Turabian StyleXia, Ting, Xiao Qiang, Beibei Geng, Xiaodong Zhang, Yiming Wang, Shaopeng Li, Yuan Meng, Yu Zheng, and Min Wang. 2022. "Changes in the Phytochemical and Bioactive Compounds and the Antioxidant Properties of Wolfberry during Vinegar Fermentation Processes" International Journal of Molecular Sciences 23, no. 24: 15839. https://doi.org/10.3390/ijms232415839
APA StyleXia, T., Qiang, X., Geng, B., Zhang, X., Wang, Y., Li, S., Meng, Y., Zheng, Y., & Wang, M. (2022). Changes in the Phytochemical and Bioactive Compounds and the Antioxidant Properties of Wolfberry during Vinegar Fermentation Processes. International Journal of Molecular Sciences, 23(24), 15839. https://doi.org/10.3390/ijms232415839







