Reoxygenation Modulates the Adverse Effects of Hypoxia on Wound Repair
Abstract
1. Introduction
2. Results
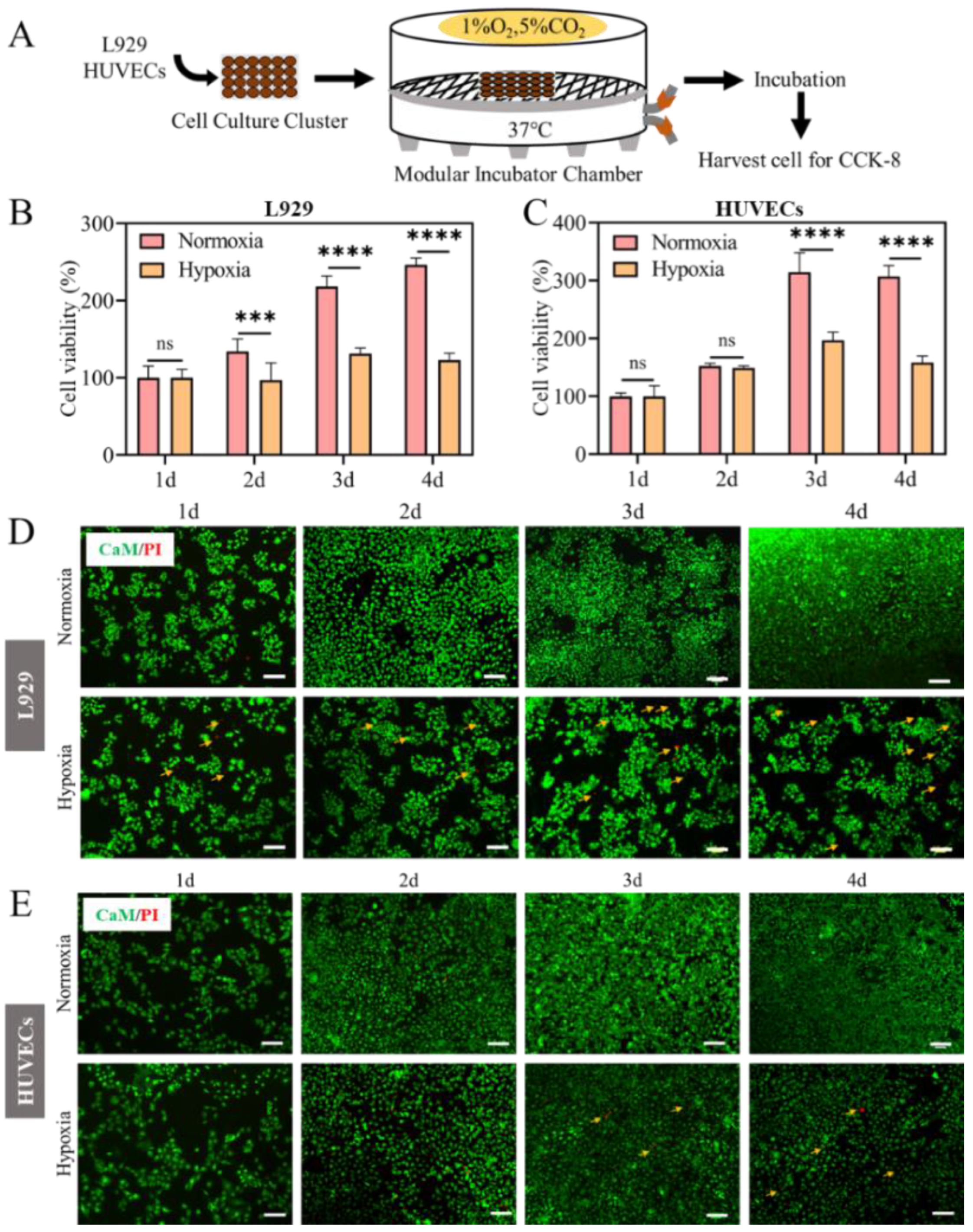
2.1. Cell Proliferation Inhibited in Hypoxia
2.2. ROS and MMP Changed in Hypoxia
2.3. mRNA Expression of Collagen and Anti-Inflammatory Factors Decreased in Hypoxia
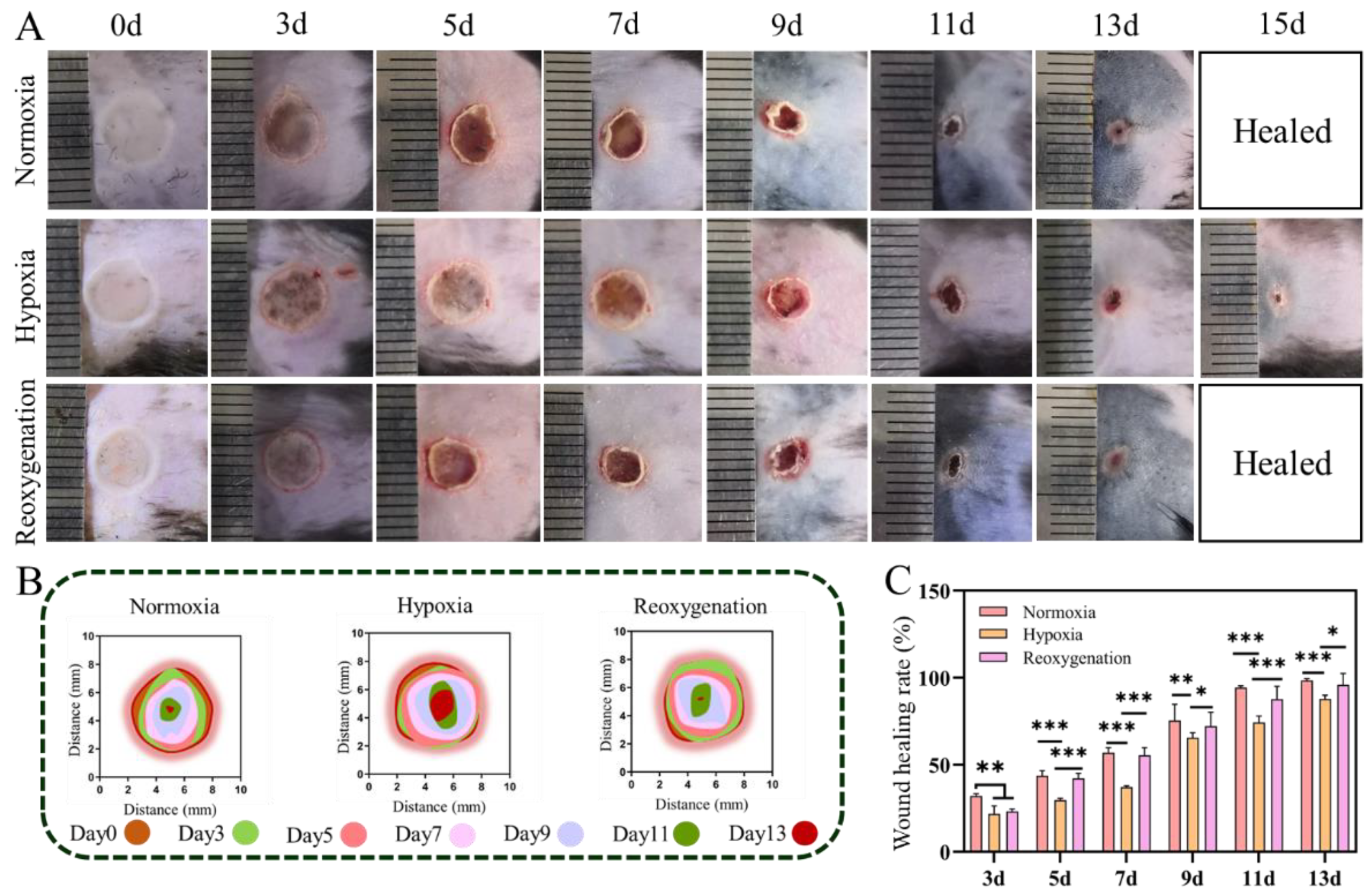
2.4. Acute Wound Healing under Hypoxia In Vivo
2.5. Histological Evaluation of the Regenerated Tissue in Acute Wound
2.6. Burn Wound Healing under Hypoxia In Vivo
2.7. Histological Evaluation of Burn
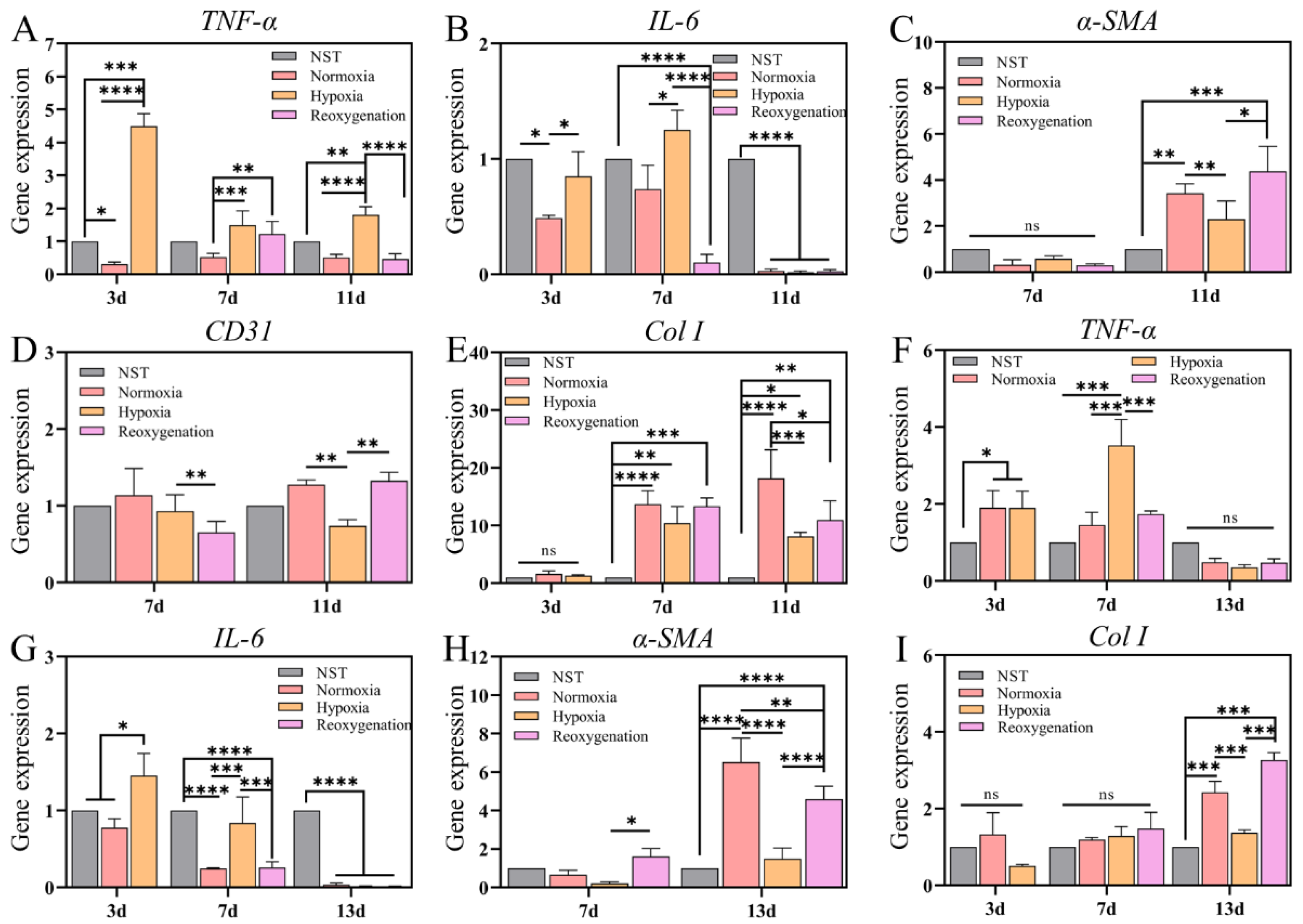
2.8. Gene Expression Analysis
2.9. Immunohistochemical Analysis
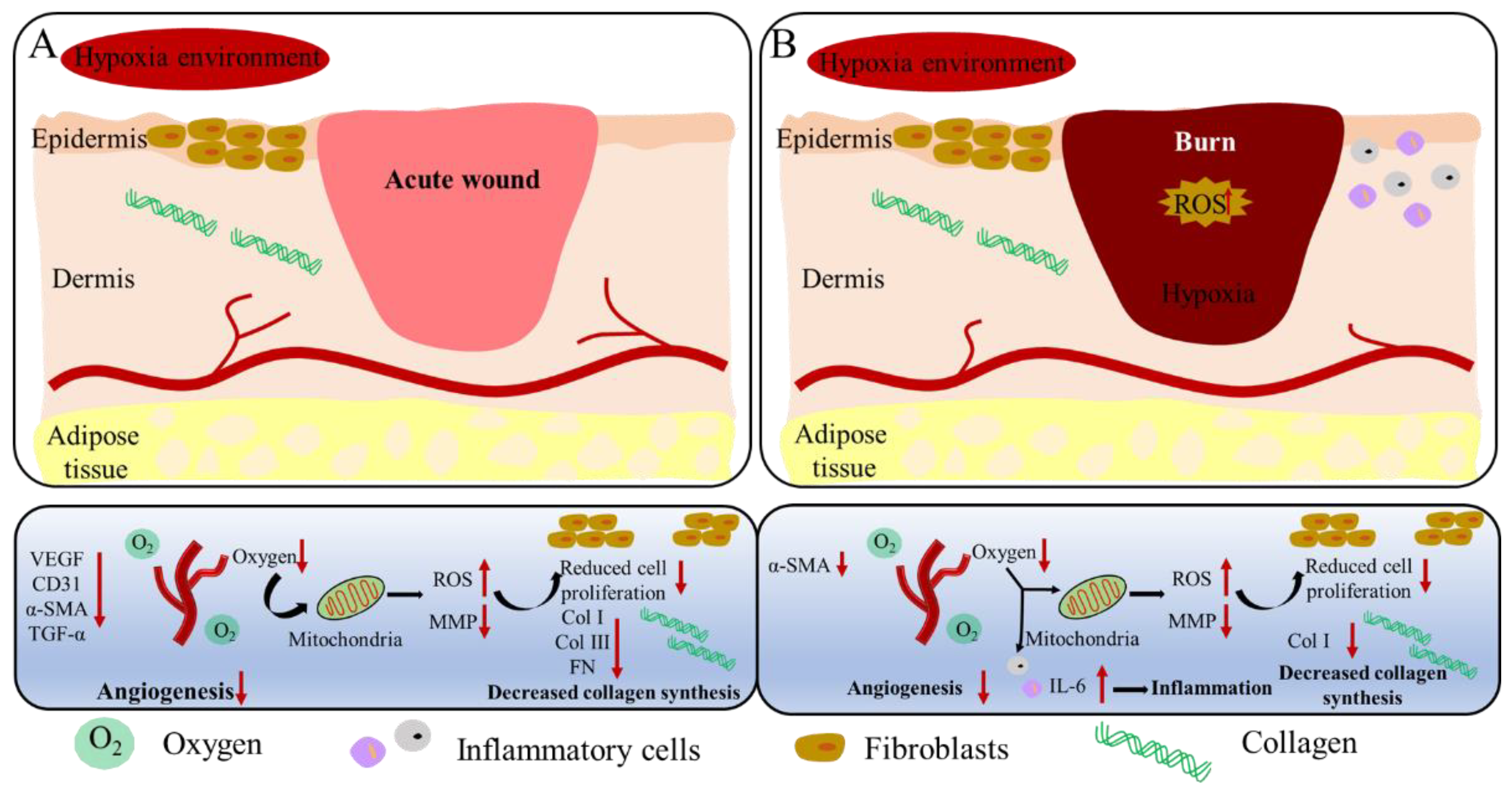
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Hypoxia Chamber
4.3. Cell Culture and Activity Assay
4.4. Reactive Oxygen Species Measurement
4.5. Mitochondrial Membrane Potential (MMP) Measurement
4.6. Annexin V/PI Measurement
4.7. RNA Extraction and Quantitative Real-Time PCR
4.8. Hypoxia Wound Models in Mice
4.9. Histological and Immunofluorescence Staining Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willemen, N.G.A.; Hassan, S.; Gurian, M.; Li, J.H.; Allijn, I.E.; Shin, S.R.; Leijten, J. Oxygen-releasing biomaterials: Current challenges and future applications. Trends Biotechnol. 2021, 39, 1144–1159. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Sanz-Ros, J.; Roman-Dominguez, A.; Ingles, M.; Gimeno-Mallench, L.; El Alami, M.; Vina-Almunia, J.; Gambini, J.; Vina, J.; Borras, C. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int. J. Mol. Sci. 2019, 20, 1195. [Google Scholar] [CrossRef]
- Otsu, K.; Ida, H.Y.; Ikezaki, S.; Ema, M.; Hitomi, J.; Ohshima, H.; Harada, H. Oxygen regulates epithelial stem cell proliferation via RhoA-actomyosin-YAP/TAZ signal in mouse incisor. Development 2021, 148, dev194787. [Google Scholar] [CrossRef]
- Liu, B.H.; Wen, H.S.; Li, X.H.; Yang, J.; Li, G.L.; Zhang, M.Z.; Li, J.F.; He, F. Acute hypoxia effects on Keap1/Nrf2 (Mafs)-GST pathway related oxidative metabolism in muscle of Japanese flounder (Paralichthys olivaceus). Sci. Total Environ. 2021, 795, 148646. [Google Scholar] [CrossRef]
- Kubota, Y.; Takubo, K.; Suda, T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem. Biophys. Res. Commun. 2008, 366, 335–339. [Google Scholar] [CrossRef]
- Motealleh, A.; Kehr, N.S. Injectable oxygen-generating nanocomposite hydrogels with prolonged oxygen delivery for enhanced cell proliferation under hypoxic and normoxic conditions. J. Mater. Chem. B 2020, 8, 4195–4201. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Guan, Y.; Niu, H.; Liu, Z.T.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J.J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Magnavacca, A.; Perego, F.; Fumagalli, M.; Sangiovanni, E.; Prato, M.; Dell’Agli, M.; Basilico, N. Effect of hypoxia on gene expression in cell populations involved in wound healing. Biomed. Res. Int. 2019, 2626374. [Google Scholar] [CrossRef]
- Grose, R.; Werner, S. Wound-healing studies in transgenic and knockout mice. Mol. Biotechnol. 2004, 28, 147–166. [Google Scholar] [CrossRef]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.Y.; Zhang, Y.N.; Long, Q.F.; Lu, T.L. Potential applications of nanomaterials and technology for diabetic wound healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef] [PubMed]
- Ruthenborg, R.J.; Ban, J.J.; Wazir, A.; Takeda, N.; Kim, J.W. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol. Cells 2014, 37, 637–643. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Zhao, R.L.; Liang, H.L.N.; Clarke, E.; Jackson, C.; Xue, M.L. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Wanderer, A.A. Hypoxia and inflammation. New Engl. J. Med. 2011, 364, 1976. [Google Scholar] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef]
- Eltzschig, H.K. Targeting hypoxia-induced inflammation. Anesthesiology 2011, 114, 239–242. [Google Scholar] [CrossRef]
- Colgan, S.P.; Campbell, E.L.; Kominsky, D.J. Hypoxia and mucosal inflammation. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.H.; Huangpu, H.Q.; Yao, F.Z. Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 2019, 109, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Apostolova, N.; Victor, V.M. Molecular strategies for targeting antioxidants to mitochondria: Therapeutic implications. Antioxid. Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Terraneo, L.; Paroni, R.; Bianciardi, P.; Giallongo, T.; Carelli, S.; Gorio, A.; Samaja, M. Brain adaptation to hypoxia and hyperoxia in mice. Redox Biol. 2017, 11, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.L.; Lai, U.H.; Zhu, L.L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia inducible factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef]
- Rybnikova, E.; Nalivaeva, N. Glucocorticoid-dependent mechanisms of brain tolerance to hypoxia. Int. J. Mol. Sci. 2021, 22, 7982. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, H.; Chen, Y.; Chen, X.; Shi, J.Z.; Chen, O.; Li, J.S.; Sun, X.J. Hyperbaric oxygen preconditioning ameliorates hypoxia-ischemia brain damage by activating Nrf2 expression in vivo and in vitro. Free Radic. Res. 2016, 50, 454–466. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.W.; Xiang, F.; Tan, J.L.; Chen, Z.; Zhang, X.R.; Wu, C.Z.; Mao, Z.W.; Luo, G.X.; Chen, X.Y.; et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef]
- Gojkovic, M.; Cunha, P.P.; Darmasaputra, G.S.; Barbieri, L.; Rundqvist, H.; Velica, P.; Johnson, R.S. Oxygen-mediated suppression of CD8+T cell proliferation by macrophages: Role of pharmacological inhibitors of HIF degradation. Front. Immunol. 2021, 12, 633586. [Google Scholar] [CrossRef]
- Watts, E.R.; Walmsley, S.R. Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol. Med. 2019, 25, 33–46. [Google Scholar] [CrossRef]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF transcription factors, inflammation, and immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wu, T.Y.; Liu, G.W.; Cheng, R.Y.; Fei, J.; Song, X.P.; Chai, Y.M.; Fan, C.Y.; Liu, X.D.; Cui, W.G.; et al. Promoting coagulation and activating SMAD3 phosphorylation in wound healing via a dual -release thrombin-hydrogel. Chem. Eng. J. 2020, 397, 125414. [Google Scholar] [CrossRef]
- Wang, L.; Wu, T.Y.; Liu, G.W.; Cheng, R.Y.; Fei, J.; Song, X.P.; Chai, Y.M.; Fan, C.Y.; Liu, X.D.; Cui, W.G.; et al. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem. Eng. J. 2021, 424, 130563. [Google Scholar] [CrossRef]
- He, J.H.; Shi, M.T.; Liang, Y.P.; Guo, B.L. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Befani, C.; Liakos, P. Hypoxia upregulates integrin gene expression in microvascular endothelial cells and promotes their migration and capillary-like tube formation. Cell Biol. Int. 2017, 41, 769–778. [Google Scholar] [CrossRef]
- Liu, K.; Fang, C.C.; Shen, Y.W.; Liu, Z.Q.; Zhang, M.; Ma, B.B.; Pang, X.Y. Hypoxia-inducible factor 1a induces phenotype switch of human aortic vascular smooth muscle cell through PI3K/AKT/AEG-1 signaling. Oncotarget 2017, 8, 33343–33352. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.P.; Zhang, Q.Y. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials 2020, 260, 120314. [Google Scholar] [CrossRef]
- Hu, B.; Gao, M.Z.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.Y.; Zhang, X.Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Mcgarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Winning, S.; Fandrey, J. Dendritic cells under hypoxia: How oxygen shortage affects the linkage between innate and adaptive immunity. J. Immunol. Res. 2016, 2016, 5134329. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Corrales, N.; Galvez, N.M.S.; Bueno, S.M.; Kalergis, A.M.; Gonzalez, P.A. Contribution of hypoxia inducible factor-1 during viral infections. Virulence 2020, 11, 1482–1500. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Fearon, U.; Canavan, M.; Biniecka, M.; Veale, D.J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Tan, J.; Miao, Y.Y.; Lei, P.; Zhang, Q. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Lian, G.J. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Kang, J.I.; Park, K.M.; Park, K.D. In situ forming and reactive oxygen species-scavenging gelatin hydrogels for enhancing wound healing efficacy. Acta Biomater. 2020, 103, 142–152. [Google Scholar] [CrossRef]
- Schito, L.; Rey, S. Cell-autonomous metabolic reprogramming in hypoxia. Trends Cell Biol. 2018, 28, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Lim, H.; Seo, J.Y.; Kang, K.R.; Kim, D.; Oh, J.S.; Seo, Y.S.; Lee, G.J.; Kim, J.S.; Kim, H.J.; et al. 25-Hydroxycholesterol-induced oxiapoptophagy in L929 mouse fibroblast cell line. Molecules 2022, 27, 199. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Wu, W.D.; Kuca, K. From hypoxia and hypoxia-inducible factors (HIF) to oxidative stress: A new understanding of the toxic mechanism of mycotoxins. Food Chem. Toxicol. 2020, 135, 110968. [Google Scholar] [CrossRef]
- Stuart, J.A.; Aibueku, O.; Bagshaw, O.; Moradi, F. Hypoxia inducible factors as mediators of reactive oxygen/nitrogen species homeostasis in physiological normoxia. Med. Hypotheses 2019, 129, 109249. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Miyake, T.; Ehsanipour, E.A.; Kelly, P.J.; Becker, L.M.; McGrail, D.J.; Sugimoto, H.; LeBleu, V.S.; Ge, Y.; Kalluri, R. Dermal αSMA myofibroblasts orchestrate skin wound repair via β1 integrin and independent of type I collagen production. EMBO J. 2022, 41, e109470. [Google Scholar]
- Wang, X.L.; Qu, M.L.; Li, J.; Danielson, P.; Yang, L.L.; Zhou, Q.J. Induction of fibroblast senescence during mouse corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3669–3679. [Google Scholar] [CrossRef]
- Rafii, S.; Butler, J.M.; Ding, B.S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kawamoto, A. Stem cell-based peripheral vascular regeneration. Adv. Drug Deliv. Rev. 2017, 120, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Kellermair, J.; Redwan, B.; Alias, S.; Jabkowski, J.; Panzenboeck, A.; Kellermair, L.; Winter, M.P.; Weltermann, A.; Lang, I.M. Platelet endothelial cell adhesion molecule 1 deficiency misguides venous thrombus resolution. Blood 2013, 122, 3376–3384. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.J.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.S.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Q.; Gao, Q.; Hu, F.; Zheng, C.; Sun, N.; Chen, W.; Liu, J.; Zhang, Y.; Wu, X.; Lu, T. Reoxygenation Modulates the Adverse Effects of Hypoxia on Wound Repair. Int. J. Mol. Sci. 2022, 23, 15832. https://doi.org/10.3390/ijms232415832
Bai Q, Gao Q, Hu F, Zheng C, Sun N, Chen W, Liu J, Zhang Y, Wu X, Lu T. Reoxygenation Modulates the Adverse Effects of Hypoxia on Wound Repair. International Journal of Molecular Sciences. 2022; 23(24):15832. https://doi.org/10.3390/ijms232415832
Chicago/Turabian StyleBai, Que, Qian Gao, Fangfang Hu, Caiyun Zheng, Na Sun, Wenting Chen, Jinxi Liu, Yanni Zhang, Xianglong Wu, and Tingli Lu. 2022. "Reoxygenation Modulates the Adverse Effects of Hypoxia on Wound Repair" International Journal of Molecular Sciences 23, no. 24: 15832. https://doi.org/10.3390/ijms232415832
APA StyleBai, Q., Gao, Q., Hu, F., Zheng, C., Sun, N., Chen, W., Liu, J., Zhang, Y., Wu, X., & Lu, T. (2022). Reoxygenation Modulates the Adverse Effects of Hypoxia on Wound Repair. International Journal of Molecular Sciences, 23(24), 15832. https://doi.org/10.3390/ijms232415832





