Nanocellulose-Based Polymer Composites Functionalized with New Gemini Ionic Liquids
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Nanofillers
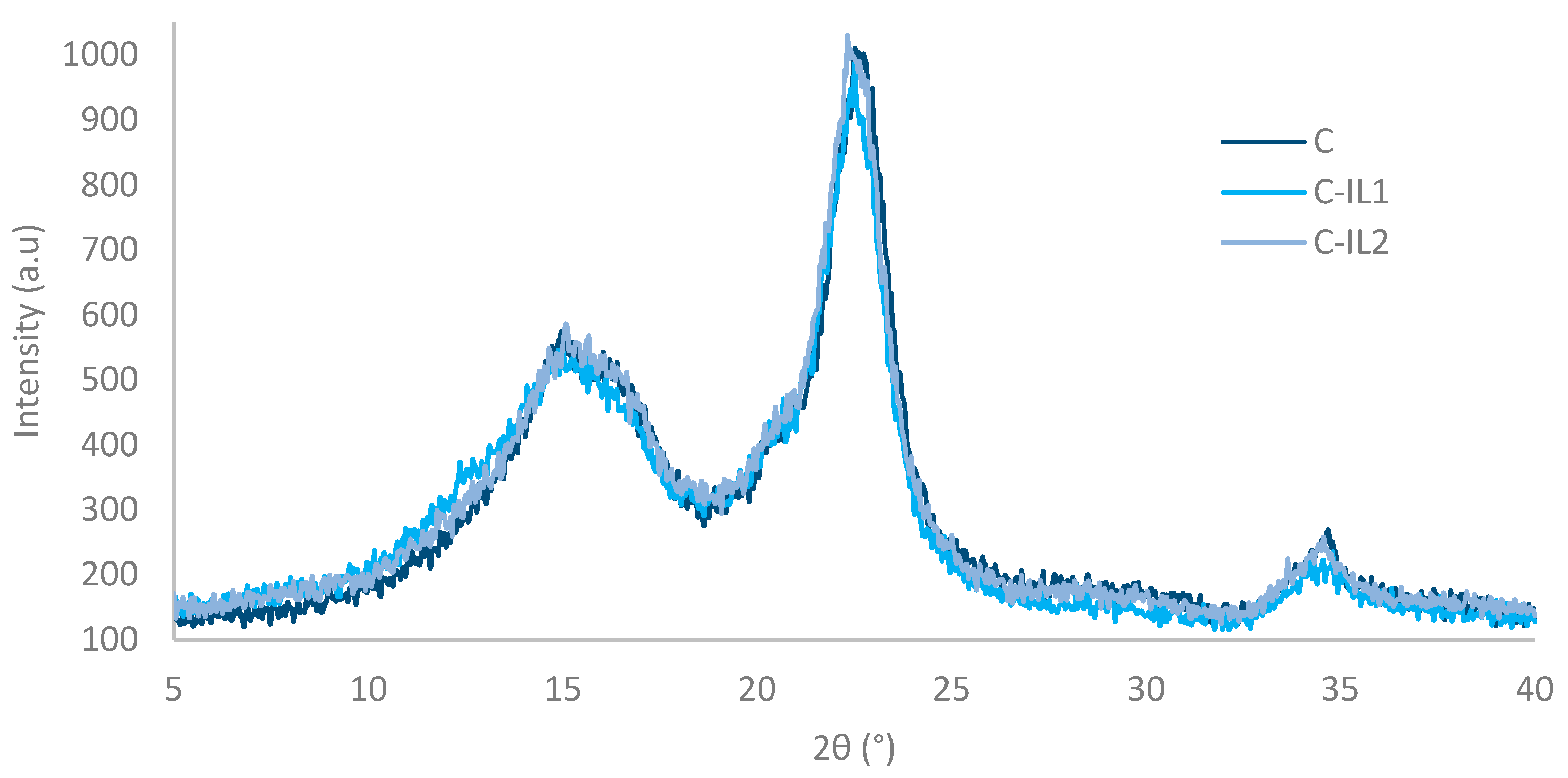
2.1.1. Supermolecular Structure of Obtained Nanocellulosic Fillers
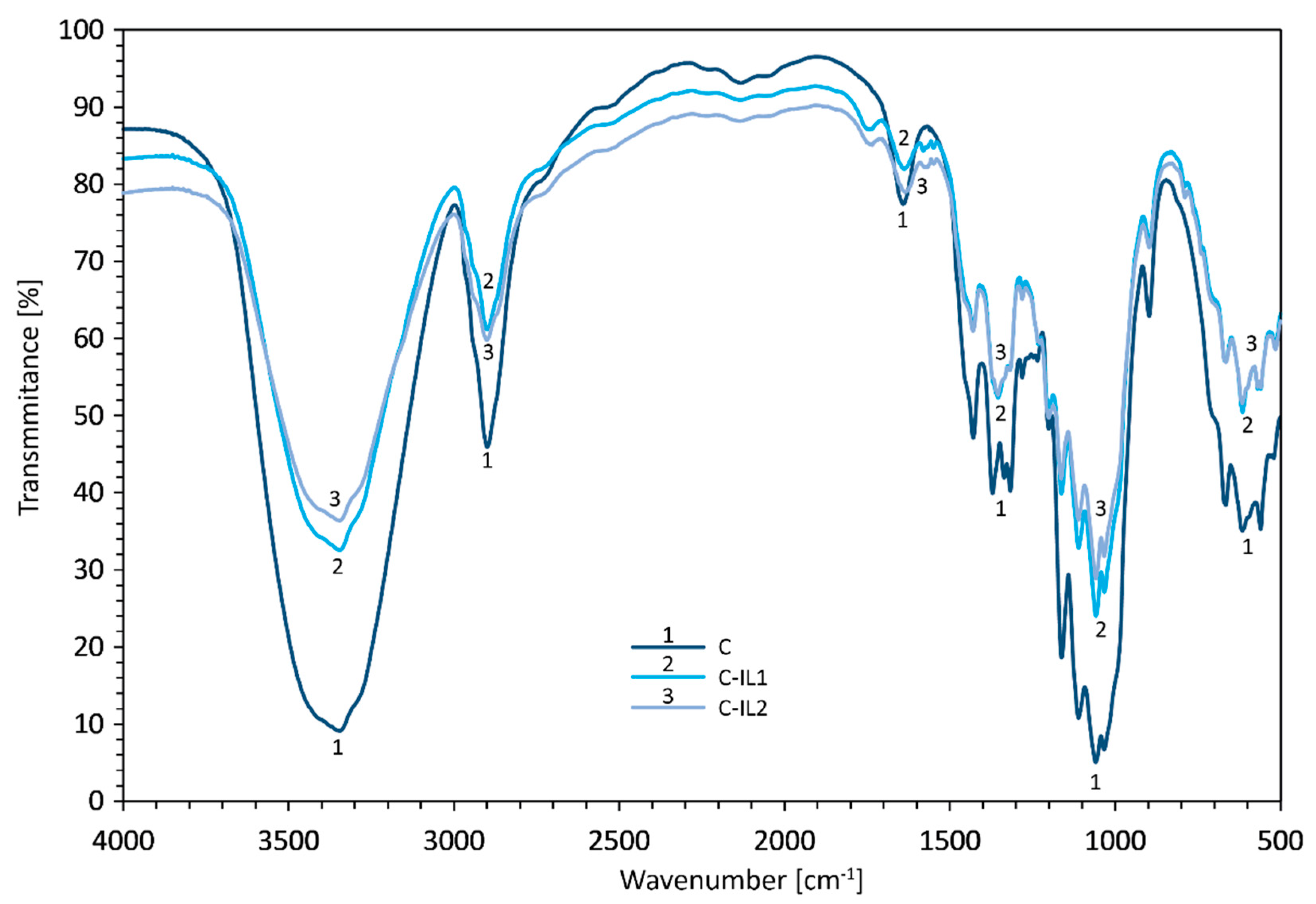
2.1.2. FTIR of Fillers
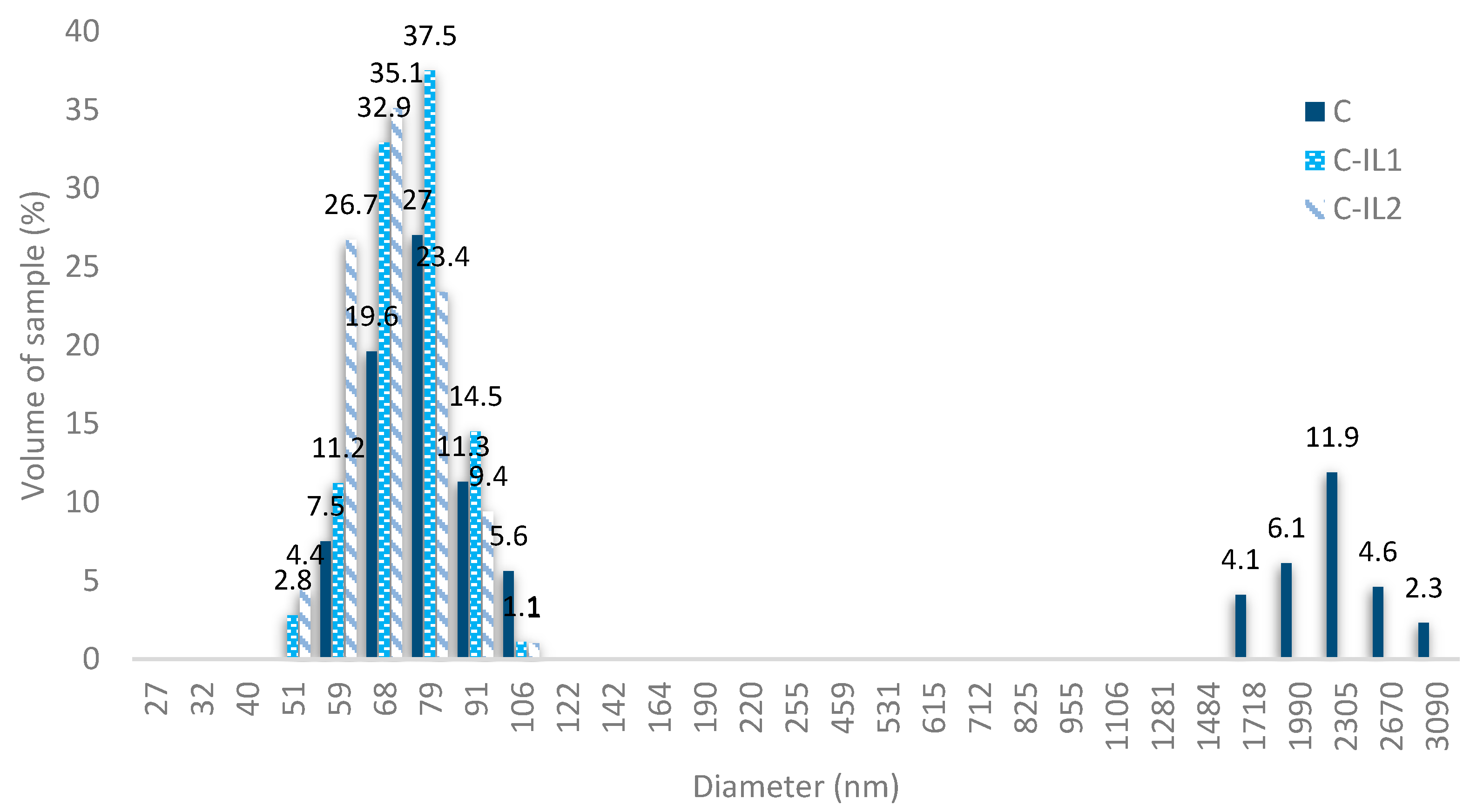
2.1.3. Dispersive Properties of Obtained Fillers
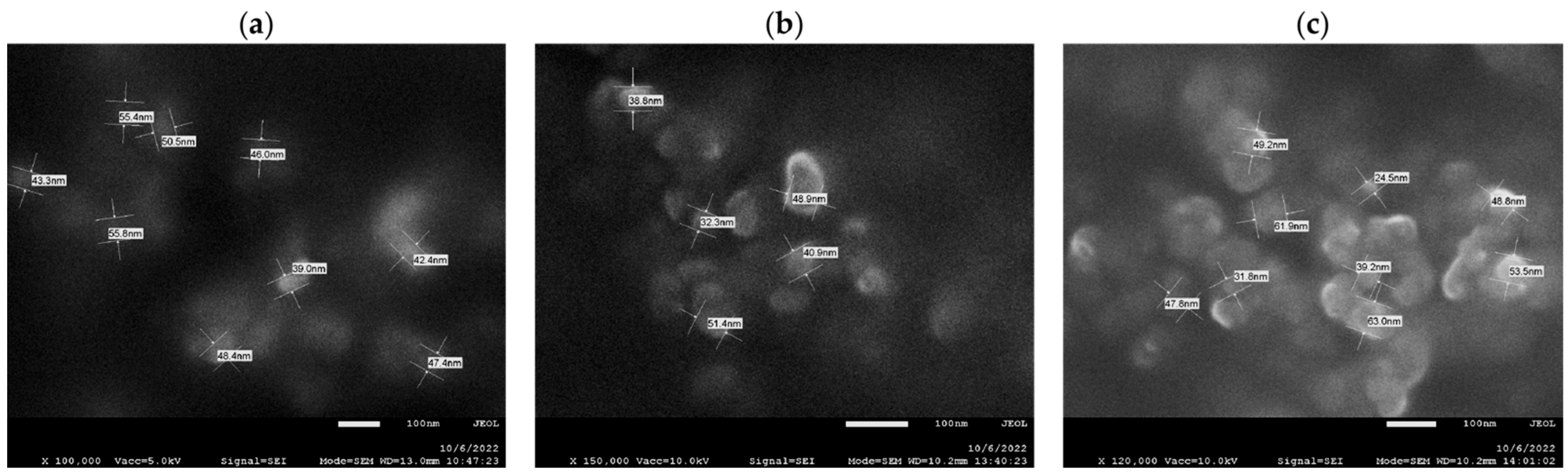
2.1.4. Morphological Characteristics of Analyzed Nanomaterials
2.2. Characterization of Nanocomposites
2.2.1. Analysis of Phase Transition
2.2.2. Analysis of Crystallization Process
2.2.3. Supermolecular Structure of Nanocomposite Materials
2.2.4. Mechanical Properties
3. Materials and Methods
3.1. Controlled Enzymatic Bioconversion of Cellulose
3.2. Synthesis of Ionic Liquids
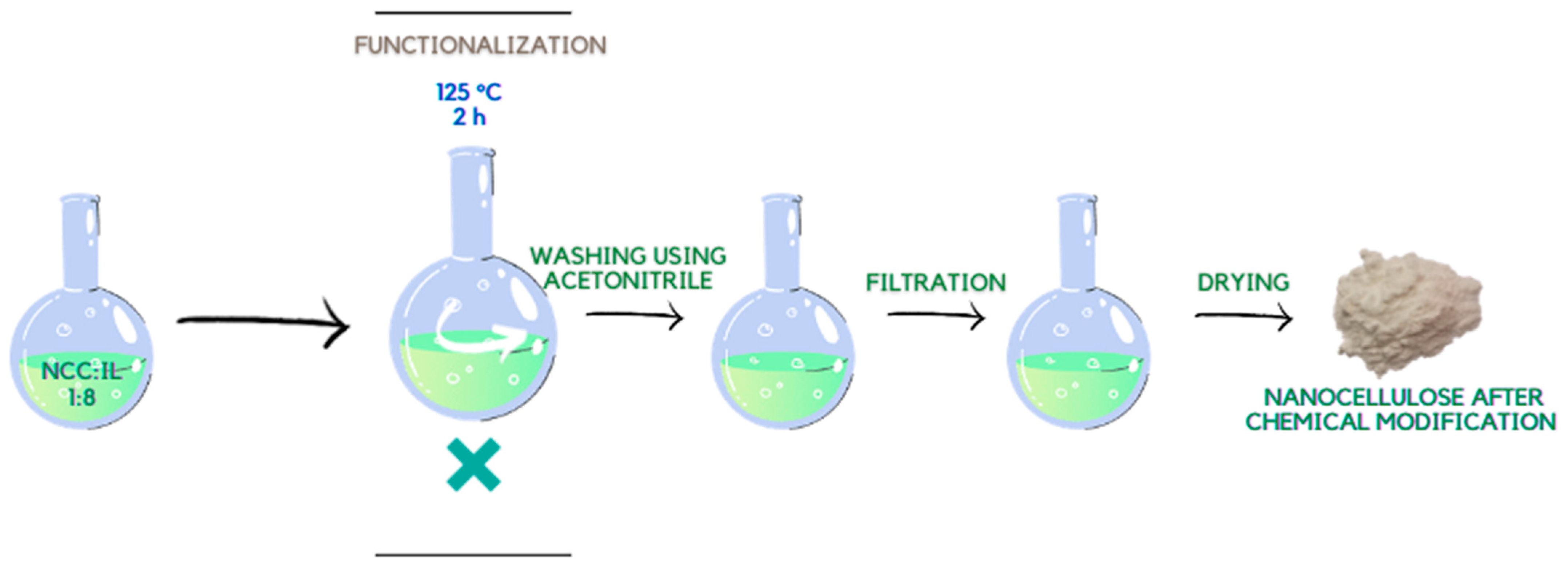
3.3. Functionalization of Cellulose Using Ionic Liquids (Chemical Modification)
3.4. Characterization of Synthesized Nanofillers
3.5. Production of Polypropylene Nanocomposites
3.6. Characterization of Nanocomposites with Functionalized Nanofillers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Lowe, A.; Kalyanasundaram, S. Effect of Chemical Treatments on Flax Fibre Reinforced Polypropylene Composites on Tensile and Dome Forming Behaviour. Int. J. Mol. Sci. 2015, 16, 6202–6216. [Google Scholar] [CrossRef]
- Martins, G.; Antunes, F.; Mateus, A.; Malça, C. Optimization of a Wood Plastic Composite for Architectural Applications. Procedia Manuf. 2017, 12, 203–220. [Google Scholar] [CrossRef]
- Hejna, A.; Sulyman, M.; Przybysz, M.; Saeb, M.R.; Klein, M.; Formela, K. On the Correlation of Lignocellulosic Filler Composition with the Performance Properties of Poly(ε-Caprolactone) Based Biocomposites. Waste Biomass Valorization 2020, 11, 1467–1479. [Google Scholar] [CrossRef]
- Borysiak, S.; Grząbka-Zasadzińska, A.; Odalanowska, M.; Skrzypczak, A.; Ratajczak, I. The effect of chemical modification of wood in ionic liquids on the supermolecular structure and mechanical properties of wood/polypropylene composites. Cellulose 2018, 25, 4639–4652. [Google Scholar] [CrossRef]
- Kufel, A.; Kuciel, S. Hybrid Composites Based on Polypropylene with Basalt/Hazelnut Shell Fillers: The Influence of Temperature, Thermal Aging, and Water Absorption on Mechanical Properties. Polymers 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Merijs-Meri, R.; Zicans, J.; Ivanova, T.; Bochkov, I.; Varkale, M.; Franciszczak, P.; Bledzki, A.K.; Danilovas, P.P.; Gravitis, J.; Rubenis, K.; et al. Development and Characterization of Grain Husks Derived Lignocellulose Filler Containing Polypropylene Composites. Polym. Eng. Sci. 2019, 59, 2467–2473. [Google Scholar] [CrossRef]
- Ratnakumar, A.; Rathnayake, W.S.M.; Karunanayake, L.; Samarasekara, A.M.P.B.; Amarasinghe, D.A.S. Microcrystalline Cellulose as Reinforcing Agents for Polypropylene Composites. Trop. Agric. Res. 2020, 31, 106. [Google Scholar] [CrossRef]
- Sergi, C.; Sbardella, F.; Lilli, M.; Tirillò, J.; Calzolari, A.; Sarasini, F. Hybrid Cellulose–Basalt Polypropylene Composites with Enhanced Compatibility: The Role of Coupling Agent. Molecules 2020, 25, 4384. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannya, J.; Entezami, A.A.; Asl, A.K. Novel nanocomposites based on fatty acid modified cellulose nanofibers/poly(lactic acid): Morphological and physical properties. Food Packag. Shelf Life 2015, 5, 21–31. [Google Scholar] [CrossRef]
- Filho, A.S.; Parveen, S.; Rana, S.; Vanderlei, R.; Fangueiro, R. Micro-structure and mechanical properties of microcrystalline cellulose-sisal fiber reinforced cementitious composites developed using cetyltrimethylammonium bromide as the dispersing agent. Cellulose 2021, 28, 1663–1686. [Google Scholar] [CrossRef]
- Larsson, P.A.; Berglund, L.A.; Wågberg, L. Ductile All-Cellulose Nanocomposite Films Fabricated from Core–Shell Structured Cellulose Nanofibrils. Biomacromolecules 2014, 15, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, A.P.M.; Lona, L.M.F. Comparison between cellulose nanocrystal and microfibrillated cellulose as reinforcement of poly(vinyl acetate) composites obtained by either in situ emulsion polymerization or a simple mixing technique. Cellulose 2021, 28, 2273–2286. [Google Scholar] [CrossRef]
- Singha, A.S.; Thakur, V.K. Synthesis, Characterisation and Analysis of Hibiscus Sabdariffa Fibre Reinforced Polymer Matrix Based Composites. Polym. Polym. Compos. 2009, 17, 189–194. [Google Scholar] [CrossRef]
- Ashori, A.; Babaee, M.; Jonoobi, M.; Hamzeh, Y. Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohydr. Polym. 2014, 102, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Guo, D.; Yan, Q.; Yu, H.; Lyu, Q.; Han, L.; Zhou, C.; Xiao, W. Synthesis and Characterization of Corn Stover-Based Cellulose Triacetate Catalyzed by Ionic Liquid Phosphotungstate. Int. J. Mol. Sci. 2022, 23, 6783. [Google Scholar] [CrossRef]
- Mantanis, G.I. Chemical Modification of Wood by Acetylation or Furfurylation: A Review of the Present Scaled-up Technologies. BioResources 2017, 12, 4478–4489. [Google Scholar] [CrossRef]
- Potthast, A.; Ahn, K.; Becker, M.; Eichinger, T.; Kostic, M.; Böhmdorfer, S.; Jeong, M.J.; Rosenau, T. Acetylation of cellulose–Another pathway of natural cellulose aging during library storage of books and papers. Carbohydr. Polym. 2022, 287, 119323. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and characterization of modified cellulose nanofibers reinforced polylactic acid nanocomposite. Polym. Test. 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Lease, J.; Kawano, T.; Andou, Y. Esterification of Cellulose with Long Fatty Acid Chain through Mechanochemical Method. Polymers 2021, 13, 4397. [Google Scholar] [CrossRef]
- Li, D.; Henschen, J.; Ek, M. Esterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitable for preparation of surface-functionalised cellulose nanocrystals with high yield. Green Chem. 2017, 19, 5564–5567. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef]
- Ungerer, B.; Müller, U.; Potthast, A.; Herrero Acero, E.; Veigel, S. Chemical and physical interactions of regenerated cellulose yarns and isocyanate-based matrix systems. Sci. Rep. 2021, 11, 11647. [Google Scholar] [CrossRef]
- Williamson, S.L.; McCormick, C.L. Cellulose Derivatives Synthesized via Isocyanate and Activated Ester Pathways in Homogeneous Solutions of Lithium Chloride/N,N-Dimethylacetamide. J. Macromol. Sci. A 1998, 35, 1915–1927. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Dong, Y.; Lai, Y.; Wang, X.; Gao, M.; Xue, F.; Chen, X.; Ma, Y.; Wei, Y. Design and synthesis of amine-functionalized cellulose with multiple binding sites and their application in C C bond forming reactions. Int. J. Biol. Macromol. 2019, 130, 778–785. [Google Scholar] [CrossRef]
- Nypelö, T.; Berke, B.; Spirk, S.; Sirviö, J. Review: Periodate oxidation of wood polysaccharides—Modulation of hierarchies. Carbohydr. Polym. 2021, 252, 117105. [Google Scholar] [CrossRef]
- Brochier Salon, M.-C.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Gandini, A. Silane adsorption onto cellulose fibers: Hydrolysis and condensation reactions. J. Colloid Interface Sci. 2005, 289, 249–261. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Dhawale, P.V.; Sorate, C.S. Surface Modification of Cellulose with Silanes for Adhesive Application: Review. Open J. Polym. Chem. 2021, 11, 11–30. [Google Scholar] [CrossRef]
- Siuda, J.; Perdoch, W.; Mazela, B.; Zborowska, M. Catalyzed Reaction of Cellulose and Lignin with Methyltrimethoxysilane—FT-IR, 13C NMR and 29Si NMR Studies. Materials 2019, 12, 2006. [Google Scholar] [CrossRef] [PubMed]
- Aridoss, G.; Laali, K.K. Ionic Liquids as Novel Media and Catalysts for Electrophilic/Onium Ion Chemistry and Metal-Mediated Reactions. Green Chem. 2018, 555–608. [Google Scholar] [CrossRef]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies-a review. iForest 2017, 10, 895–908. [Google Scholar] [CrossRef]
- Ochiai, B.; Watanabe, T.; Hanzawa, C.; Akiyama, K.; Matsumura, Y.; Shimura, R.; Koda, T.; Nishioka, A. Milling in Seconds Accelerates Acetylation of Cellulose in Hours. ACS Omega 2019, 4, 17542–17546. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Morais, E.S.; da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Tenhunen, T.-M.; Hakalahti, M.; Kouko, J.; Salminen, A.; Härkäsalmi, T.; Pere, J.; Harlin, A.; Hänninen, T. Method for Forming Pulp Fibre Yarns Developed by a Design-driven Process. BioResources 2016, 11, 2492–2503. [Google Scholar] [CrossRef][Green Version]
- Moyer, P.; Smith, M.D.; Abdoulmoumine, N.; Chmely, S.C.; Smith, J.C.; Petridis, L.; Labbé, N. Relationship between lignocellulosic biomass dissolution and physicochemical properties of ionic liquids composed of 3-methylimidazolium cations and carboxylate anions. Phys. Chem. Chem. Phys. 2018, 20, 2508–2516. [Google Scholar] [CrossRef]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic Liquids—Promising but Challenging Solvents for Homogeneous Derivatization of Cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef]
- Taokaew, S.; Kriangkrai, W. Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides 2022, 3, 671–691. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Louis, H.; Temitope, H.A.; Philip, M.; Amos, P.I.; Magu, T.O.; Ozioma, A.U.; Amusan, O.O. Ionic liquids (ILs): Advances in biorefinery for the efficient conversion of lignocellulosic biomass. Asain J. Green Chem. 2019, 3, 288–417. [Google Scholar]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Troxell, S.; Quitevis, E.L.; Abidi, N. Substituent effects on cellulose dissolution in imidazolium-based ionic liquids. Cellulose 2018, 25, 6887–6900. [Google Scholar] [CrossRef]
- Chandrabhan, V.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [PubMed]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F. The effect of subcritical carbon dioxide on the dissolution of cellulose in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Cellulose 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Gravel, O.; Larachi, F. Torrefaction of ionic liquid impregnated lignocellulosic biomass and its comparison to dry torrefaction. Fuel 2013, 103, 814–826. [Google Scholar] [CrossRef]
- Pernak, J.; Zabielska-Matejuk, J.; Kropacz, A.; Foksowicz-Flaczyk, J. Ionic liquids in wood preservation. Holzforschung 2004, 58, 286–291. [Google Scholar] [CrossRef]
- Söyler, Z.; Meier, M.A.R. Sustainable functionalization of cellulose and starch with diallyl carbonate in ionic liquids. Green Chem. 2017, 19, 3899–3907. [Google Scholar] [CrossRef]
- Ma, K.; Jin, X.; Zheng, M.; Gao, H. Dissolution and functionalization of celluloses using 1,2,3-triazolium ionic liquid. Carbohydr. Polym. Technol. Appl. 2021, 2, 100109. [Google Scholar] [CrossRef]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic Liquids as Reaction Medium in Cellulose Functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef]
- Odalanowska, M.; Skrzypczak, A.; Borysiak, S. Innovative ionic liquids as functional agent for wood-polymer composites. Cellulose 2021, 28, 10589–10608. [Google Scholar] [CrossRef]
- Aghmih, K.; Wakrim, H.; Boukhriss, A.; El Bouchti, M.; Majid, S.; Gmouh, S. Rheological study of microcrystalline cellulose/pyridinium-based ionic liquids solutions. Polym. Bull. 2022, 79, 8987–8999. [Google Scholar] [CrossRef]
- Kerche, E.F.; Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Schrekker, H.S. The influence of ionic liquid concentration on microcrystalline cellulose modification. Carbohydr. Polym. Technol. Appl. 2022, 3, 100211. [Google Scholar] [CrossRef]
- Croitoru, C.; Varodi, A.M.; Timar, M.C.; Roata, I.C.; Stanciu, E.M.; Pascu, A. Wood-plastic composites based on HDPE and ionic liquid additives. J. Mater. Sci. 2018, 53, 4132–4143. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Kuroda, K.; Miyamura, K.; Satria, H.; Takada, K.; Ninomiya, K.; Takahashi, K. Hydrolysis of Cellulose Using an Acidic and Hydrophobic Ionic Liquid and Subsequent Separation of Glucose Aqueous Solution from the Ionic Liquid and 5-(Hydroxymethyl)furfural. ACS Sustain. Chem. Eng. 2016, 4, 3352–3356. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Liu, J.; Yin, D.; Su, S.; Ding, H. Hydrolysis of cellulose into reducing sugars in ionic liquids. Fuel 2016, 164, 46–50. [Google Scholar] [CrossRef]
- Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. High glucose yields from the hydrolysis of cellulose dissolved in ionic liquids. J. Chem. Eng. 2012, 181–182, 538–541. [Google Scholar] [CrossRef]
- Grząbka-Zasadzińska, A.; Skrzypczak, A.; Borysiak, S. The influence of the cation type of ionic liquid on the production of nanocrystalline cellulose and mechanical properties of chitosan-based biocomposites. Cellulose 2019, 26, 4827–4840. [Google Scholar] [CrossRef]
- Sultana, T.; Sultana, S.; Nur, H.P.; Khan, M.W. Studies on Mechanical, Thermal and Morphological Properties of Betel Nut Husk Nano Cellulose Reinforced Biodegradable Polymer Composites. J. Compos. Sci. 2020, 4, 83. [Google Scholar] [CrossRef]
- Abiaziem, C.V.; Williams, A.B.; Inegbenebor, A.I.; Onwordi, C.T.; Ehi-Eromosele, C.O.; Petrik, L.F. Preparation and Characterisation of Cellulose Nanocrystal from Sugarcane Peels by XRD, SEM and CP/MAS 13 C NMR. J. Phys. Conf. Ser. 2019, 1299, 012123. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Szentner, K.; Bartkowiak, M.; Peplińska, B.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Nanocellulose Production Using Ionic Liquids with Enzymatic Pretreatment. Materials 2021, 14, 3264. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Muhammad, N.; Sarwono, A.; Bustam, M.A.; Vignesh Kumar, M.; Rafiq, S. Preparation of Cellulose Nanocrystals Using an Ionic Liquid. J. Polym. Environ. 2011, 19, 726–731. [Google Scholar] [CrossRef]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass Bioenerg. 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Metzger, C.; Auber, D.; Dähnhardt-Pfeiffer, S.; Briesen, H. Agglomeration of cellulose nanocrystals: The effect of secondary sulfates and their use in product separation. Cellulose 2020, 27, 9839–9851. [Google Scholar] [CrossRef]
- Rojas, J.; Bedoya, M.; Ciro, Y. Current Trends in the Production of Cellulose Nanoparticles and Nanocomposites for Biomedical Applications. In Cellulose-Fundamental Aspects and Current Trends; Poletto, M., Ornaghi, H.L., Eds.; IntechOpen: London, UK, 2015. [Google Scholar]
- Doan, T.K.Q.; Chiang, K.Y. Characteristics and kinetics study of spherical cellulose nanocrystal extracted from cotton cloth waste by acid hydrolysis. Sustain. Environ. Res. 2022, 32, 26. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Md Jamil, S.N.A.; Abdan, K. Nanocellulose Extraction Using Ionic Liquids: Syntheses, Processes, and Properties. Front. Mater. 2022, 9, 919918. [Google Scholar] [CrossRef]
- Zielińska, D.; Siwińska-Ciesielczyk, K.; Bula, K.; Jesionowski, T.; Borysiak, S. TiO2/nanocellulose hybrids as functional additives for advanced polypropylene nanocomposites. Ind. Crops Prod. 2022, 176, 114314. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernández, B.; Ramos, J.A.; Mondragon, I. Thermal and crystallization studies of short flax fibre reinforced polypropylene matrix composites: Effect of treatments. Thermochim. Acta 2006, 440, 111–121. [Google Scholar] [CrossRef]
- Lenes, M.; Gregersen, Ø.W. Effect of surface chemistry and topography of sulphite fibres on the transcrystallinity of polypropylene. Cellulose 2006, 13, 345–355. [Google Scholar] [CrossRef]
- Raka, L.; Bogoeva-Gaceva, G. Crystallization of polypropylene: Application of differential scanning calorimetry part i. Isothermal and non-isothermal crystallization. Contributions 2017, 29. [Google Scholar] [CrossRef]
- Borysiak, S. Influence of cellulose polymorphs on the polypropylene crystallization. J Therm Anal Calorim 2013, 113, 281–289. [Google Scholar] [CrossRef][Green Version]
- Bartczak, Z.; Martuscelli, E.; Galeski, A. Primary spherulite nucleation in polypropylene-based blends and copolymers. In Polypropylene Structure, Blends and Composites; Karger-Kocsis, J., Ed.; Springer: Dordrecht, The Netherlands, 1995; Volume 2, pp. 25–49. [Google Scholar]
- Quan, H.; Li, Z.-M.; Yang, M.-B.; Huang, R. On transcrystallinity in semi-crystalline polymer composites. Compos. Sci. Technol. 2005, 65, 999–1021. [Google Scholar] [CrossRef]
- Chen, H.B.; Karger-Kocsis, J.; Wu, J.S.; Varga, J. Fracture toughness of α- and β-phase polypropylene homopolymers and random- and block-copolymers. Polymer 2002, 43, 6505–6514. [Google Scholar] [CrossRef]
- Varga, J. Β-modification of isotactic polypropylene: Preparation, structure, processing, properties, and application. J. Macromol. Sci. Phys. 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Yahya, R.O. Magnetic Graphene Oxide/Carboxymethyl-Imidazolium-Grafted Chitosan Schiff Base Nanocomposite: A New PdNPs Support for Efficient Catalytic Reduction of Hazardous Nitroarenes. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3813–3825. [Google Scholar] [CrossRef]
- Dragaun, H.; Hubeny, H.; Muschik, H. Shear-induced β-form crystallization in isotactic polypropylene. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 1779–1789. [Google Scholar] [CrossRef]
- Kaczmarek, D.K.; Czerniak, K.; Klejdysz, T. Dicationic ionic liquids as new feeding deterrents. Chem. Pap. 2018, 72, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Skrzypczak, A.; Lota, G.; Frackowiak, E. Synthesis and Properties of Trigeminal Tricationic Ionic Liquids. Eur. J. Chem. 2007, 13, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]

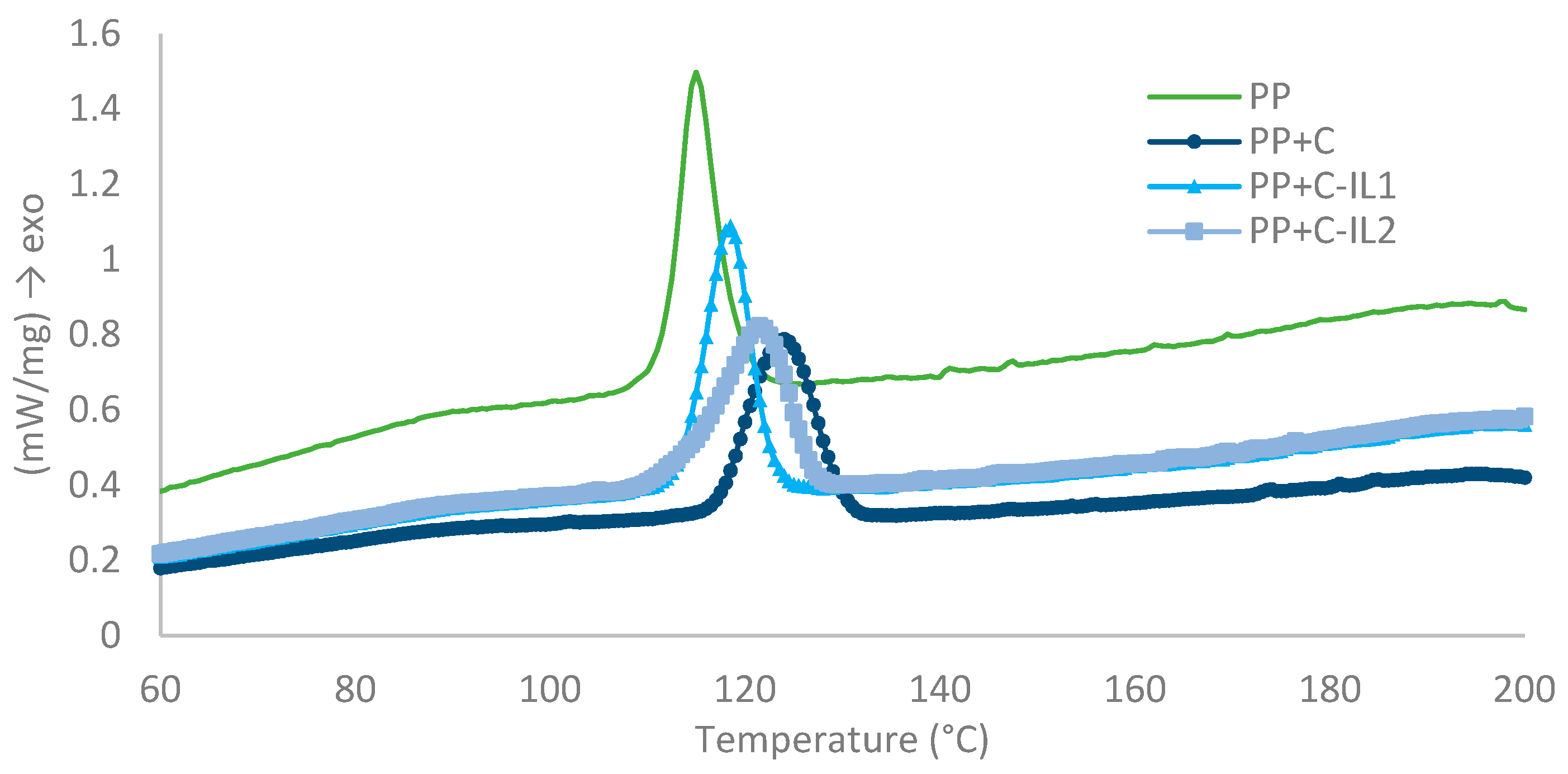

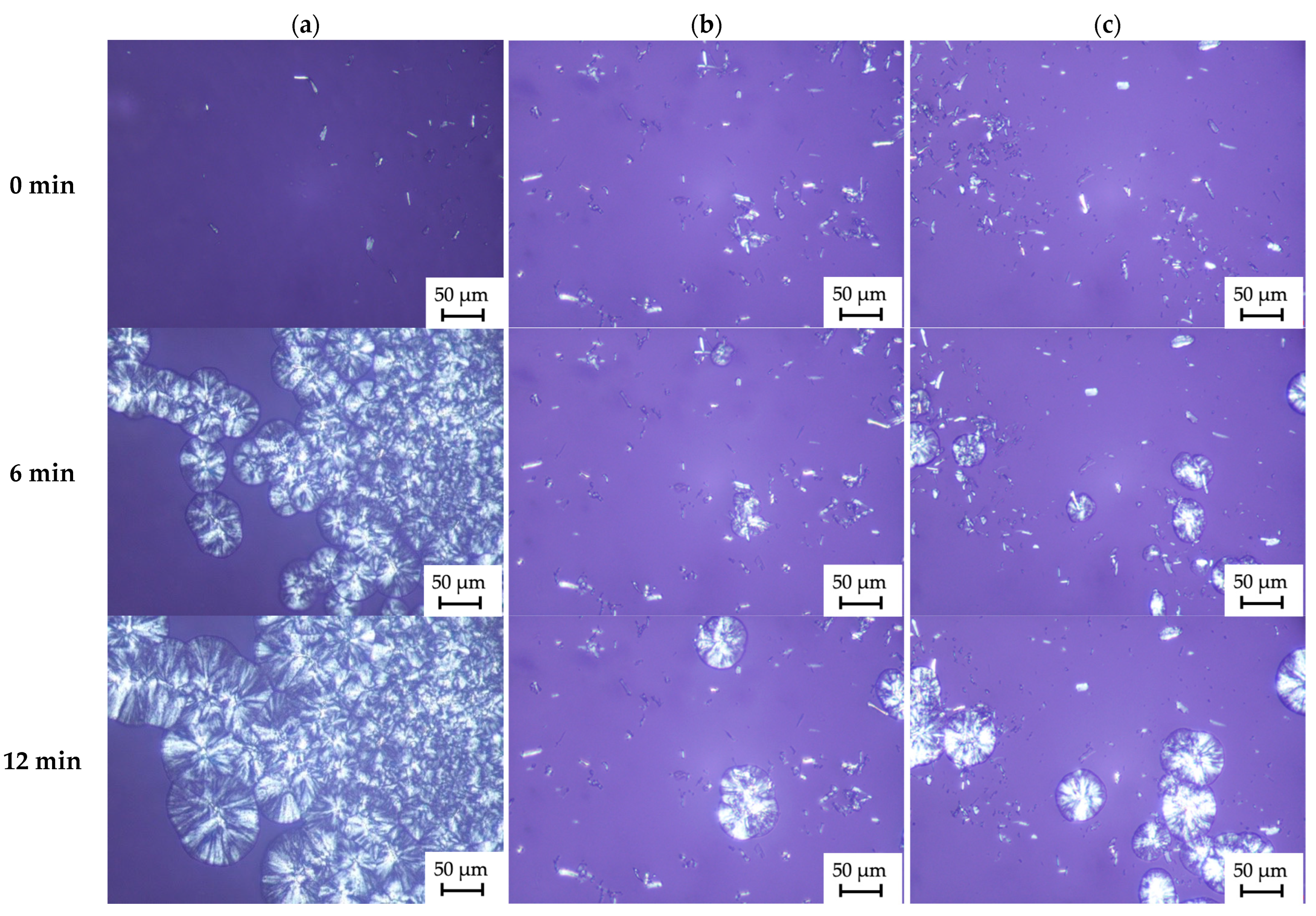
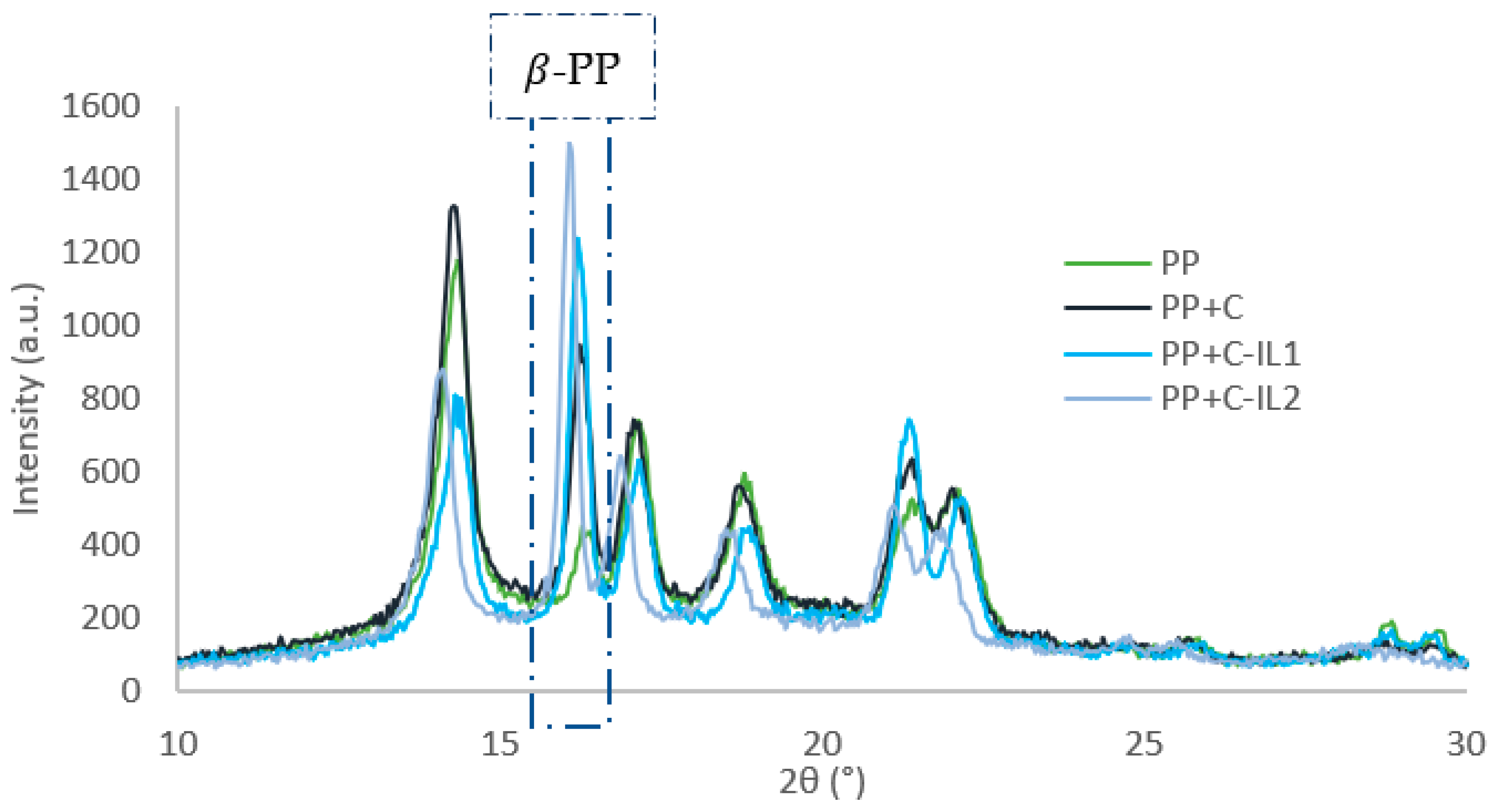
| Material | Temperature of Crystallization (°C) | Temperature of Melting (°C) | Half-Time of Crystallization (min) | Induction Time (min) | The Growth Rate of TCL (μm/min) |
|---|---|---|---|---|---|
| PP | 114.6 | 165.1 | 2.6 | 5 | 1.0 |
| PP + C | 123.8 | 165.2 | 1.4 | 1 | 2.7 |
| PP + C-IL1 | 118.3 | 164.6 | 1.6 | 2.5 | 1.7 |
| PP + C-IL2 | 121.3 | 165.1 | 1.8 | 4 | 1.3 |
| Material | Content of the -PP Form (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation at Break (%) | Impact Strength (kJ/m2) |
|---|---|---|---|---|---|
| PP | 9 | 29.0 (±0.12) | 1.07 (±0.06) | 303.3 (±27.3) | 48.3 (±1.2) |
| PP + C | 27 | 33.5 (±0.25) | 1.39 (±0.11) | 286.4 (±24.0) | 29.3 (±2.5) |
| PP + C-IL1 | 44 | 37.3 (±0.31) | 1.50 (±0.13) | 377.6 (±19.4) | 34.4 (±2.2) |
| PP + C-IL2 | 49 | 38.5 (±0.28) | 1.66 (±0.10) | 483.7 (±16.5) | 37.8 (±1.8) |
| Ionic Liquid | Chemical Name | Chemical Formula |
|---|---|---|
IL1 | 3,3′-[1,4-butane]-bis (1-carboxymethylimidazolium) di(bis(trifluoromethylsulfonyl)imide) | 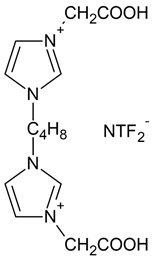 |
IL2 | 3,3′-[1,8-octane]-bis (1-carboxymethylimidazolium) di(bis(trifluoromethylsulfonyl)imide) | 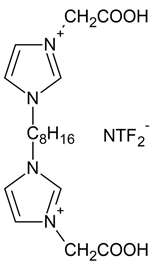 |
| Sample | Material |
|---|---|
| C | Nanocellulose |
| C-IL1 | Nanocellulose modified with IL1 |
| C-IL2 | Nanocellulose modified with IL2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Skrzypczak, A.; Peplińska, B.; Borysiak, S. Nanocellulose-Based Polymer Composites Functionalized with New Gemini Ionic Liquids. Int. J. Mol. Sci. 2022, 23, 15807. https://doi.org/10.3390/ijms232415807
Zielińska D, Skrzypczak A, Peplińska B, Borysiak S. Nanocellulose-Based Polymer Composites Functionalized with New Gemini Ionic Liquids. International Journal of Molecular Sciences. 2022; 23(24):15807. https://doi.org/10.3390/ijms232415807
Chicago/Turabian StyleZielińska, Daria, Andrzej Skrzypczak, Barbara Peplińska, and Sławomir Borysiak. 2022. "Nanocellulose-Based Polymer Composites Functionalized with New Gemini Ionic Liquids" International Journal of Molecular Sciences 23, no. 24: 15807. https://doi.org/10.3390/ijms232415807
APA StyleZielińska, D., Skrzypczak, A., Peplińska, B., & Borysiak, S. (2022). Nanocellulose-Based Polymer Composites Functionalized with New Gemini Ionic Liquids. International Journal of Molecular Sciences, 23(24), 15807. https://doi.org/10.3390/ijms232415807









