Immunopathological Inflammation in the Evolution of Mucositis and Peri-Implantitis
Abstract
1. Introduction
2. Results and Discussion
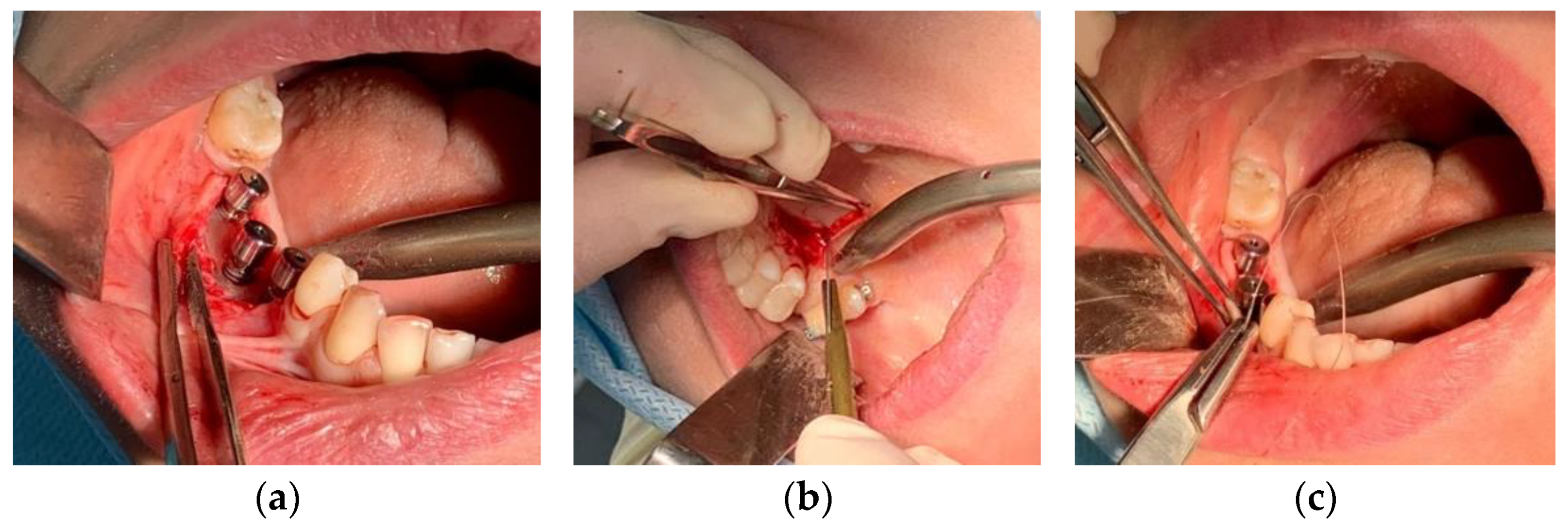
2.1. Results of Soft Tissue Biopsy Specimens
2.1.1. Results of Histological Studies
2.1.2. Results of X-ray Studies
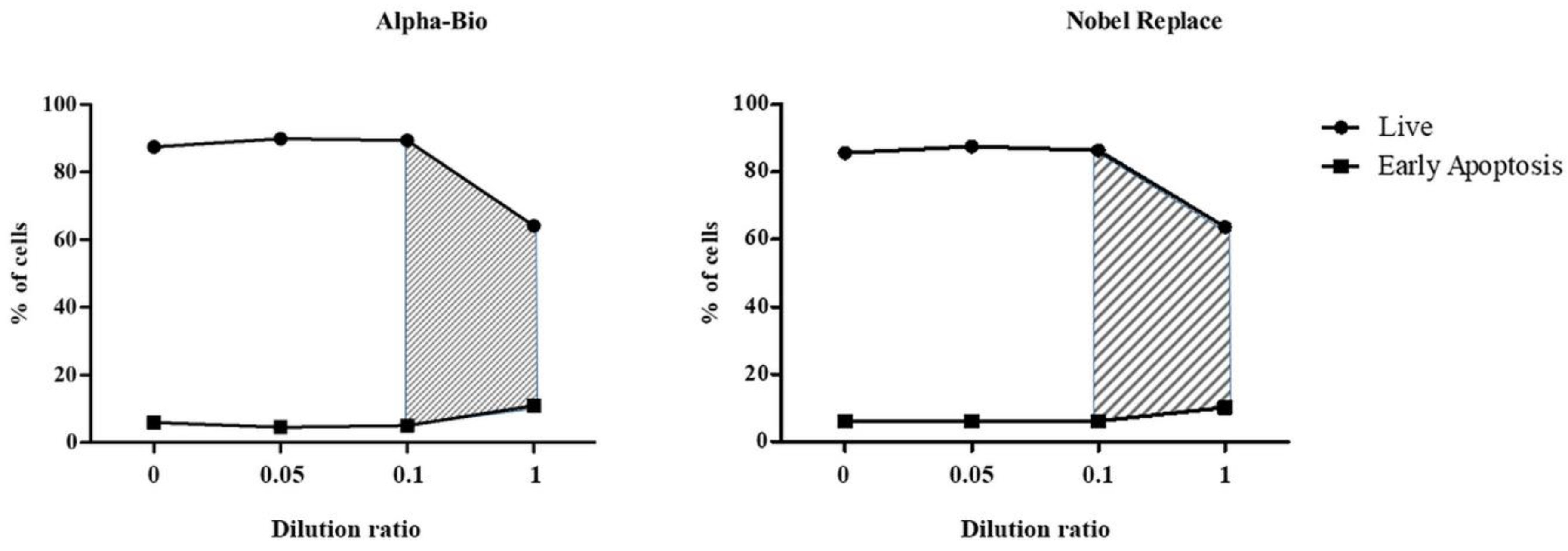
2.2. Results of the Supernatant Studies
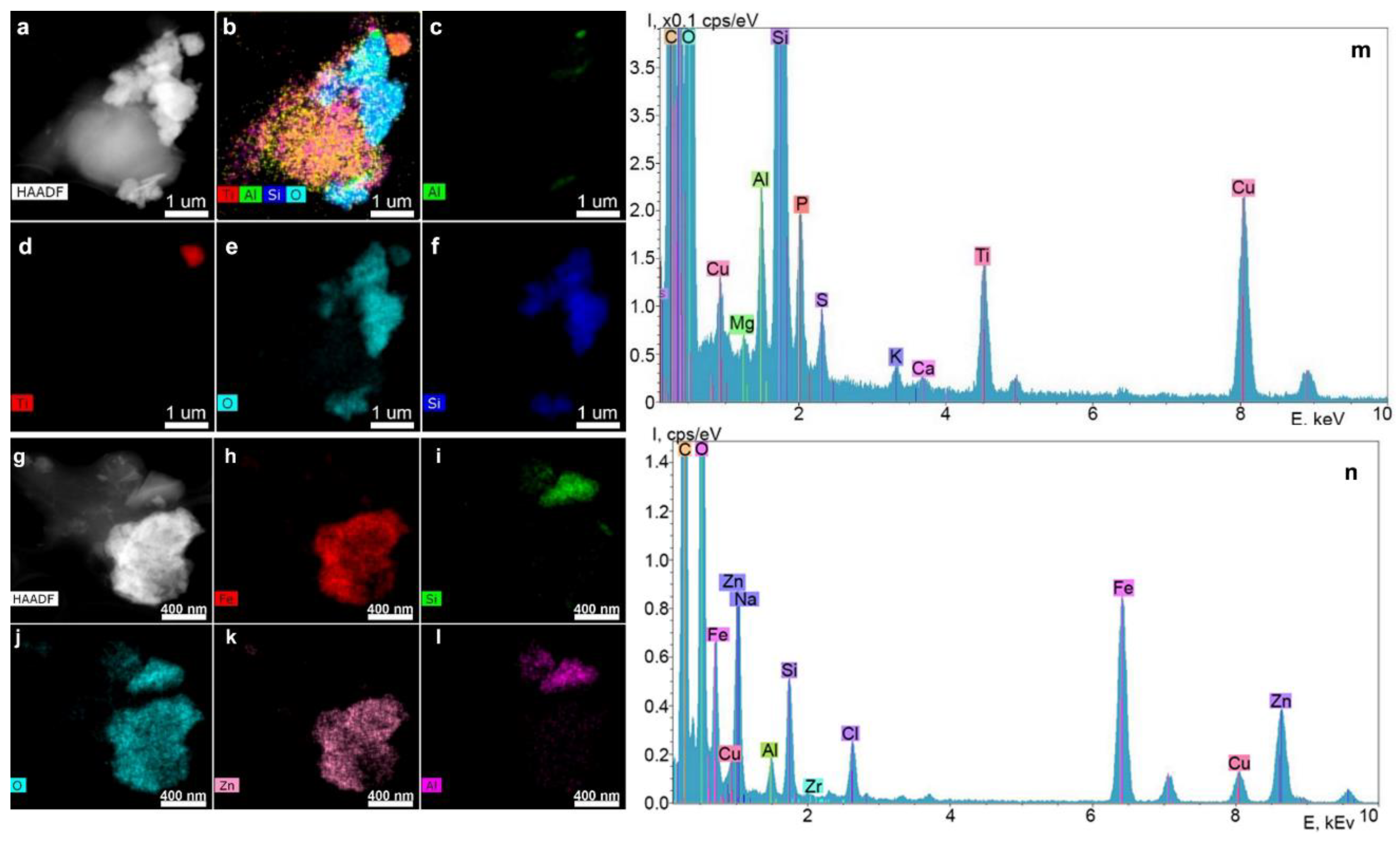
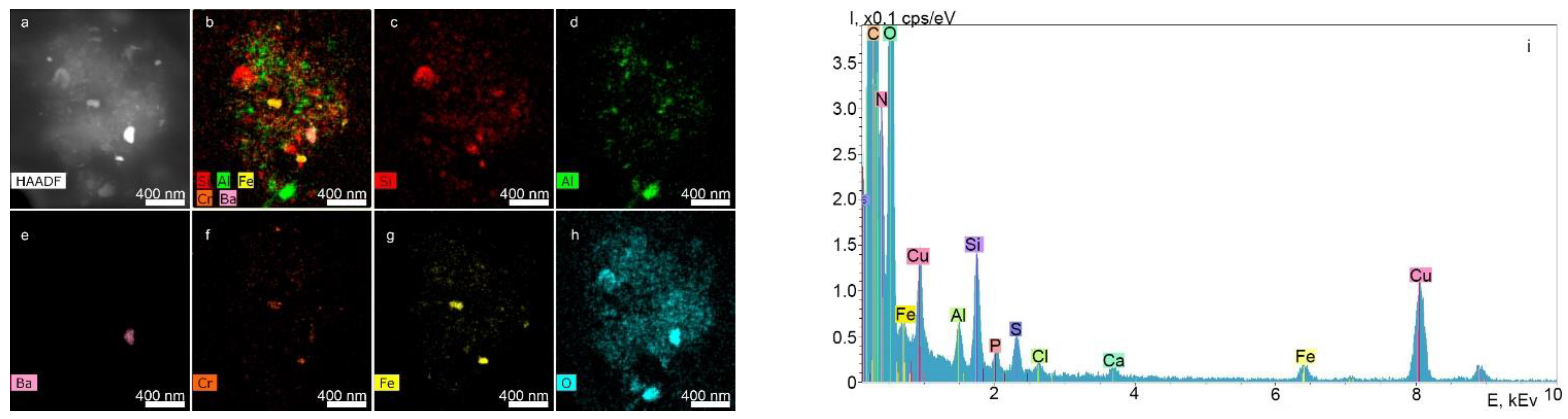
2.2.1. STEM and EDX Results of the Supernatants Obtained from the Surfaces of the Nobel Replace and Alpha-Bio Dental Implant Systems at a Dilution Ratio of 1.0 before Filtration
2.2.2. Results of DLS of Supernatants Obtained from the Surfaces of the Nobel Replace and Alpha-Bio Dental Implant Systems at a Dilution Ratio of 1.0 after Filtration
- Frequency of occurrence: Nobel Replace, 6.4 kcps; Alpha-Bio, 6.0 kcps;
- NSMP size: Nobel Replace, 103.31 nm; Alpha-Bio, 187.75 nm;
- Polydispersity: Nobel Replace, 0.744%; Alpha-Bio, 0.480%.
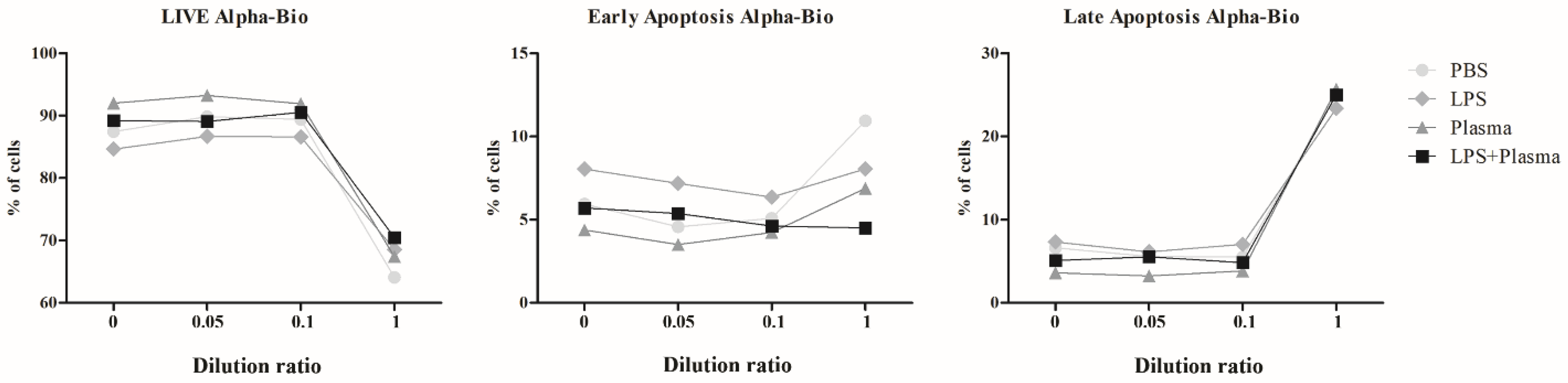
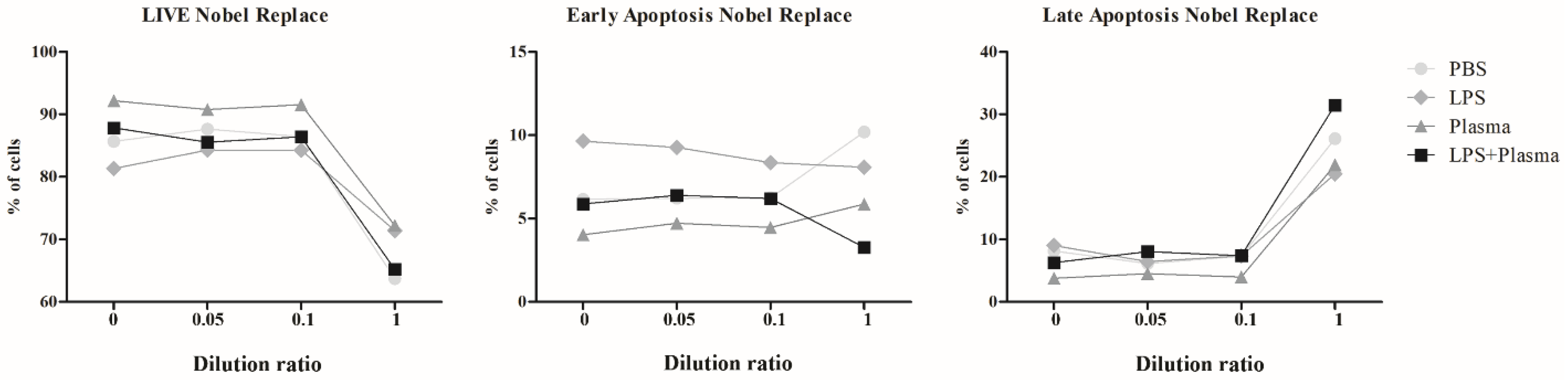
2.2.3. Results of Co-Culturing Supernatants Obtained from the Surfaces of Nobel Replace and Alpha-Bio Dental Implants with Lipopolysaccharide Induction and the Addition of Fresh Human Blood Plasma
3. Materials and Methods
3.1. Clinical Case
3.2. Histological Studies
3.3. X-ray Microtomography (XMCT)
3.4. X-ray Fluorescence Analysis (XRF)
3.5. Electron Microscopy
3.6. Experimental Laboratory Study
3.6.1. Preparation of a Suspension of Nanoscale Particles from the Surface of the Nobel Replace and Alpha-Bio Dental Implant Systems
3.6.2. Dynamic Light Scattering (DLS)
3.6.3. Cell Cultivation
3.6.4. Assessment of Cell Viability after Interactions with NSMP
3.6.5. Assessment of Cell Activation after Interactions with NSMP
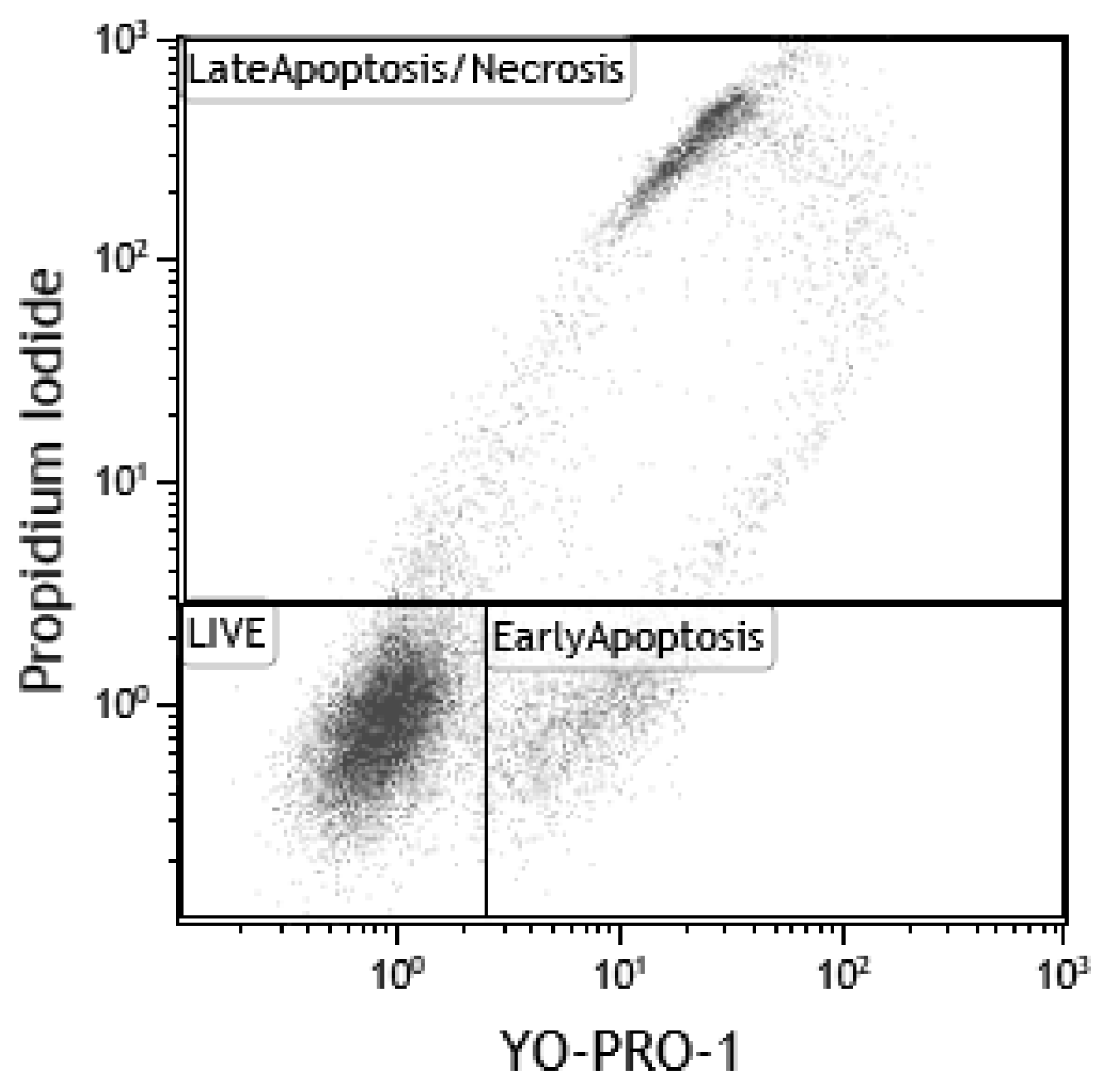
3.6.6. Flow Cytometry (FC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, A.; Alhamad, M.; Ampadi Ramachandran, R.; Shukla, A.; Barão, V.A.; Sukotjo, C.; Mathew, M.T. Peri-Implantitis in Relation to Titanium Corrosion: Current Status and Future Perspectives. J. Bio-Tribo-Corros. 2022, 8, 46. [Google Scholar] [CrossRef]
- Pettersson, M.; Pettersson, J.; Molin Thorén, M.; Johansson, A. Release of titanium after insertion of dental implants with different surface characteristics—An ex vivo animal study. Acta Biomater. Odontol. Scand. 2017, 3, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Ishikawa, T.; Sato, S.; Kihara, H.; Taira, M.; Sasaki, M.; Kondo, H. Uptake of Nanotitania by Gingival Epithelial Cells Promotes Inflammatory Response and Is Accelerated by Porphyromonas gingivalis Lipopolysaccharide: An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 8084. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Grandin, H.M.; Ofec, R. Retrospective cohort study of 4591 dental implants: Analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J. Periodontol. 2019, 90, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Thomsen, P.; Kunrath, M.F. The Role of Titanium Particles and Ions in the Pathogenesis of Peri-Implantitis. J. Bone Metab. 2022, 29, 145–154. [Google Scholar] [CrossRef]
- Ivanovski, S.; Bartold, P.M.; Huang, Y. The role of foreign body response in peri-implantitis: What is the evidence? Periodontol. 2000 2022, 90, 176–185. [Google Scholar] [CrossRef]
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased levels of dissolved titanium are associated with peri-implantitis—A cross-sectional study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef]
- Alhamad, M.; Barão, V.A.R.; Sukotjo, C.; Cooper, L.F.; Mathew, M.T. Ti-Ions and/or Particles in Saliva Potentially Aggravate Dental Implant Corrosion. Materials 2021, 14, 5733. [Google Scholar] [CrossRef]
- Golasik, M.; Herman, M.; Piekoszewski, W. Toxicological aspects of soluble titanium—A review of in vitro and in vivo studies. Metallomics 2016, 8, 1227–1242. [Google Scholar] [CrossRef]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Molin Thorén, M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J. Periodontal Res. 2017, 52, 21–32. [Google Scholar] [CrossRef]
- Silva-Bermudez, L.S.; Sevastyanova, T.N.; Schmuttermaier, C.; De La Torre, C.; Schumacher, L.; Klüter, H.; Kzhyshkowska, J. Titanium Nanoparticles Enhance Production and Suppress Stabilin-1-Mediated Clearance of GDF-15 in Human Primary Macrophages. Front. Immunol. 2021, 12, 5310. [Google Scholar] [CrossRef] [PubMed]
- Labis, V.V.; Bazikyan, E.A.; Volkov, A.V.; Sizova, S.V.; Khaydukov, S.V.; Asadchikov, V.E.; Buzmakov, A.V.; Kozlov, I.G. Role of Immune Mechanisms in Oral Microflora in the Pathogenesis of Periimplantitis; Bulletin of Orenburg Scientific Center, Ural Branch of Russian Academy of Sciences: Orenburg, Russia, 2019; Volume 3, pp. 1–15. [Google Scholar]
- Labis, V.V.; Bazikyan, E.A.; Kozlov, I.G.; Sizova, S.V.; Hajdukov, S.V. Nanosized Particles—Participants of Osseointrgration; Bulletin of Orenburg Scientific Center, Ural Branch of Russian Academy of Sciences: Orenburg, Russia, 2016; Volume 1, pp. 1–18. [Google Scholar]
- Labis, V.V.; Sizova, S.V.; Hajdukov, S.V.; Bazikyan, E.A.; Kozlov, I.G. The role of nanosized particles in the osseointegration mechanisms of dental implants. Russ. J. Immunol. 2015, 19, 48–53. [Google Scholar]
- Labis, V.V. New view on the bioinertness of dental implants. Med. Immunol. 2011, 13, 299–550. [Google Scholar]
- Labis, V.V.; Bazikyan, E.A.; Sizova, S.V.; Khaydukov, S.V.; Kozlov, I.G. Nanoscale particles and their role in the development of periimplantites. Russ. J. Immunol. 2016, 10, 451–453. [Google Scholar]
- Labis, V.; Bazikyan, E.; Zhigalina, O.; Sizova, S.; Oleinikov, V.; Khmelenin, D.; Dyachkova, I.; Zolotov, D.; Buzmakov, A.; Asadchikov, V.; et al. Assessment of dental implant surface stability at the nanoscale level. Dent. Mater. 2022, 38, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Zhigalina, O.M.; Khmelenin, D.N.; Labis, V.V.; Bazikyan, E.A.; Sizova, S.V.; Khaidukov, S.V.; Asadchikov, V.E.; Buzmakov, A.V.; Krivonosov, Y.S.; Zolotov, D.A.; et al. Electron Microscopy of the Surface of Dental Implants and Metal-Containing Nanoparticles Obtained in Supernatants. Crystallogr. Rep. 2019, 64, 798–805. [Google Scholar] [CrossRef]
- Clinical Documentation Booklet. Available online: https://alpha-bio.net/ (accessed on 2 April 2022).
- Product Catalog 2017/18, Complete Assortment. Available online: https://www.nobelbiocare.com/nobelreplace (accessed on 2 April 2022).
- van Aarle, W.; Palenstijn, W.J.; Cant, J.; Janssens, E.; Bleichrodt, F.; Dabravolski, A.; De Beenhouwer, J.; Joost Batenburg, K.; Sijbers, J. Fast and flexible X-ray tomography using the ASTRA toolbox. Opt. Express 2016, 24, 25129. [Google Scholar] [CrossRef]
- Bazikyan, E.A.; Labis, V.V.; Kozlov, I.G.; Khajdukov, S.V.; Labis, Y.V. Method of Personalised Selection of Dental Implant Based on Titanium Oxide Alloys. Patent 2611013 C1, 2015. Available online: https://yandex.ru/patents/doc/RU2611013C1_20170217 (accessed on 20 October 2022).
- Murphy, R.M. Static and dynamic light scattering of biological macromolecules: What can we learn? Curr. Opin. Biotechnol. 1997, 8, 25–30. [Google Scholar] [CrossRef]
- Idziorek, T.; Estaquier, J.; De Bels, F.; Ameisen, J.-C. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J. Immunol. Methods 1995, 185, 249–258. [Google Scholar] [CrossRef]
- Nurkhametova, D.; Kudryavtsev, I.; Guselnikova, V.; Serebryakova, M.; Giniatullina, R.R.; Wojciechowski, S.; Tore, F.; Rizvanov, A.; Koistinaho, J.; Malm, T.; et al. Activation of P2X7 Receptors in Peritoneal and Meningeal Mast Cells Detected by Uptake of Organic Dyes: Possible Purinergic Triggers of Neuroinflammation in Meninges. Front. Cell. Neurosci. 2019, 13, 45. [Google Scholar] [CrossRef]
- Mindukshev, I.; Kudryavtsev, I.; Serebriakova, M.; Trulioff, A.; Gambaryan, S.; Sudnitsyna, J.; Khmelevskoy, D.; Voitenko, N.; Avdonin, P.; Jenkins, R.; et al. Flow Cytometry and Light Scattering Technique in Evaluation of Nutraceuticals. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–332. [Google Scholar]
- Dubashynskaya, N.V.; Golovkin, A.S.; Kudryavtsev, I.V.; Prikhodko, S.S.; Trulioff, A.S.; Bokatyi, A.N.; Poshina, D.N.; Raik, S.V.; Skorik, Y.A. Mucoadhesive cholesterol-chitosan self-assembled particles for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2020, 158, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, C. Advanced Analysis of Variance; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99. [Google Scholar] [CrossRef] [PubMed]

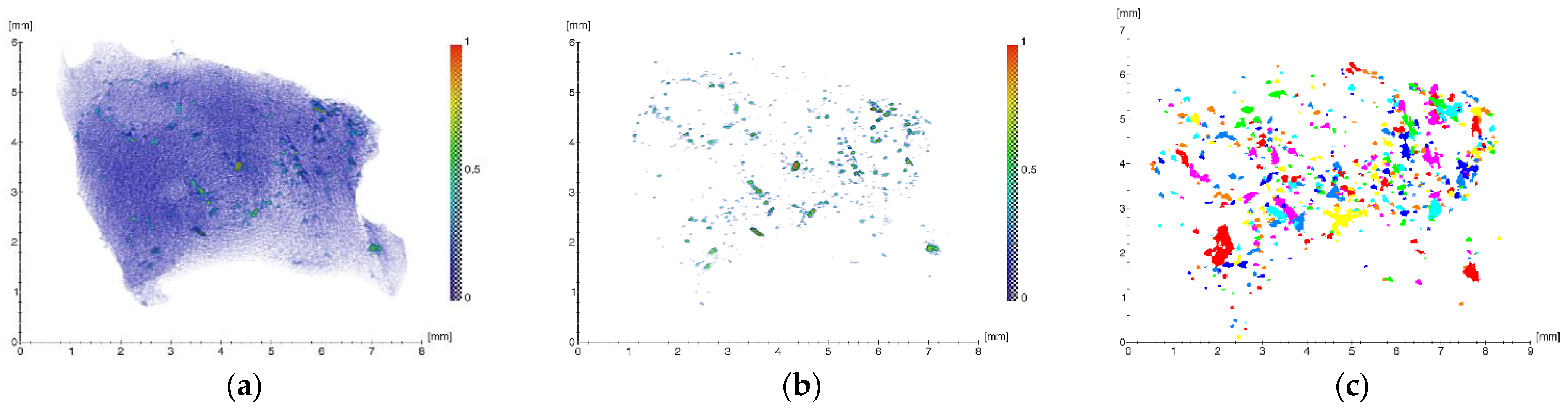



| Volume, µm3 | EqD, µm | Linear Absorption Coefficient µ, mm−1 | |
|---|---|---|---|
| Min | 729 | 11.2 | 0.22 |
| Max | 6,833,646 | 235.4 | 0.6 |
| Mean | 197,803 | 51.6 | 0.27 |
| Control | Alpha-Bio 1/20 | Alpha-Bio 1/10 | Alpha-Bio 1 | |
|---|---|---|---|---|
| Control | 1.37 ± 0.04 | 1.66 ± 0.08 | 1.85 ± 0.06 | 4.53 ± 0.30 |
| LPS | 9.80 ± 1.09 | 11.79 ± 0.81 | 13.06 ± 1.12 | 19.4 ± 0.42 |
| Plasma | 2.16 ± 0.57 | 1.87 ± 0.12 | 2.94 ± 0.94 | 14.1 ± 2.06 |
| LPS + plasma | 25.8 ± 0.04 | 26.8 ± 0.60 | 25.7 ± 0.54 | 22.8 ± 0.96 |
| Control | Nobel Replace 1/20 | Nobel Replace 1/10 | Nobel Replace 1 | |
|---|---|---|---|---|
| Control | 1.10 ± 0.08 | 1.32 ± 0.054 | 1.38 ± 0.024 | 2.15 ± 0.030 |
| LPS | 11.09 ± 0.43 | 14.41 ± 0.76 | 12.05 ± 0.36 | 19.20 ± 1.19 |
| Plasma | 1.00 ± 0.09 | 1.24 ± 0.11 | 1.21 ± 0.04 | 4.70 ± 1.47 |
| LPS + plasma | 25.9 ± 0.64 | 26.8 ± 1.69 | 26.9 ± 0.49 | 26.3 ± 2.60 |
| Control | Alpha-Bio 1/20 | Alpha-Bio 1/10 | Alpha-Bio 1 | ||
|---|---|---|---|---|---|
| Control | Live | 87.45 ± 0.48 | 89.86 ± 0.26 | 89.41 ± 0.72 | 64.10 ± 1.07 |
| Early apoptosis | 5.94 ± 0.73 | 4.56 ± 0.12 | 5.08 ± 0.49 | 10.94 ± 0.25 | |
| Late apoptosis/necrosis | 6.61 ± 1.00 | 5.58 ± 0.25 | 5.51 ± 0.49 | 24.96 ± 0.83 | |
| LPS | Live | 84.66 ± 0.62 | 86.68 ± 0.88 | 86.61 ± 1.09 | 68.6 ± 1.26 |
| Early apoptosis | 8.04 ± 0.34 | 7.18 ± 0.94 | 6.36 ± 0.70 | 8.05 ± 0.85 | |
| Late apoptosis/necrosis | 7.30 ± 0.59 | 6.13 ± 0.20 | 7.04 ± 0.82 | 23.4 ± 2.11 | |
| Plasma | Live | 92.0 ± 0.24 | 93.3 ± 0.21 | 91.9 ± 0.67 | 67.4 ± 2.34 |
| Early apoptosis | 4.39 ± 0.12 | 3.51 ± 0.39 | 4.23 ± 0.28 | 6.88 ± 0.60 | |
| Late apoptosis/necrosis | 3.60 ± 0.33 | 3.23 ± 0.21 | 3.84 ± 0.40 | 25.7 ± 1.75 | |
| LPS + plasma | Live | 89.2 ± 0.43 | 89.1 ± 0.56 | 90.5 ± 0.24 | 70.5 ± 4.64 |
| Early apoptosis | 5.70 ± 0.31 | 5.37 ± 0.29 | 4.62 ± 0.30 | 4.50 ± 0.12 | |
| Late apoptosis/necrosis | 5.09 ± 0.57 | 5.53 ± 0.30 | 4.84 ± 0.52 | 25.0 ± 4.67 |
| Control | Nobel Replace 1/20 | Nobel Replace 1/10 | Nobel Replace 1 | ||
|---|---|---|---|---|---|
| Control | Live | 85.69 ± 1.93 | 87.64 ± 1.33 | 86.44 ± 0.37 | 63.70 ± 1.35 |
| Early apoptosis | 6.15 ± 0.18 | 6.22 ± 1.31 | 6.24 ± 0.17 | 10.20 ± 0.87 | |
| Late apoptosis/necrosis | 8.16 ± 1.77 | 6.14 ± 0.94 | 7.31 ± 0.51 | 26.10 ± 2.21 | |
| LPS | Live | 81.34 ± 1.83 | 84.28 ± 0.60 | 84.29 ± 0.57 | 71.4 ± 0.30 |
| Early apoptosis | 9.64 ± 1.53 | 9.26 ± 0.32 | 8.35 ± 0.59 | 8.08 ± 0.41 | |
| Late apoptosis/necrosis | 9.02 ± 0.69 | 6.46 ± 0.31 | 7.36 ± 0.08 | 20.5 ± 0.17 | |
| Plasma | Live | 92.20 ± 0.14 | 90.8 ± 0.06 | 91.6 ± 0.76 | 72.23 ± 0.99 |
| Early apoptosis | 4.03 ± 0.23 | 4.72 ± 0.23 | 4.47 ± 0.64 | 5.86 ± 0.18 | |
| Late apoptosis/necrosis | 3.77 ± 0.35 | 4.50 ± 0.25 | 3.97 ± 0.21 | 21.9 ± 0.82 | |
| LPS + plasma | Live | 87.8 ± 0.40 | 85.6 ± 0.74 | 86.4 ± 0.39 | 65.2 ± 5.85 |
| Early apoptosis | 5.89 ± 0.89 | 6.39 ± 0.48 | 6.21 ± 0.39 | 3.27 ± 0.33 | |
| Late apoptosis/necrosis | 6.28 ± 0.72 | 8.04 ± 0.43 | 7.38 ± 0.19 | 31.5 ± 5.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labis, V.; Bazikyan, E.; Sizova, S.; Oleinikov, V.; Trulioff, A.; Serebriakova, M.; Kudryavtsev, I.; Zhigalina, O.; Khmelenin, D.; Dyachkova, I.; et al. Immunopathological Inflammation in the Evolution of Mucositis and Peri-Implantitis. Int. J. Mol. Sci. 2022, 23, 15797. https://doi.org/10.3390/ijms232415797
Labis V, Bazikyan E, Sizova S, Oleinikov V, Trulioff A, Serebriakova M, Kudryavtsev I, Zhigalina O, Khmelenin D, Dyachkova I, et al. Immunopathological Inflammation in the Evolution of Mucositis and Peri-Implantitis. International Journal of Molecular Sciences. 2022; 23(24):15797. https://doi.org/10.3390/ijms232415797
Chicago/Turabian StyleLabis, Varvara, Ernest Bazikyan, Svetlana Sizova, Vladimir Oleinikov, Andrey Trulioff, Maria Serebriakova, Igor Kudryavtsev, Olga Zhigalina, Dmitry Khmelenin, Irina Dyachkova, and et al. 2022. "Immunopathological Inflammation in the Evolution of Mucositis and Peri-Implantitis" International Journal of Molecular Sciences 23, no. 24: 15797. https://doi.org/10.3390/ijms232415797
APA StyleLabis, V., Bazikyan, E., Sizova, S., Oleinikov, V., Trulioff, A., Serebriakova, M., Kudryavtsev, I., Zhigalina, O., Khmelenin, D., Dyachkova, I., Zolotov, D., Asadchikov, V., Volkov, A., Khaidukov, S., & Kozlov, I. (2022). Immunopathological Inflammation in the Evolution of Mucositis and Peri-Implantitis. International Journal of Molecular Sciences, 23(24), 15797. https://doi.org/10.3390/ijms232415797







