Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing’s Syndrome
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Human Subjects
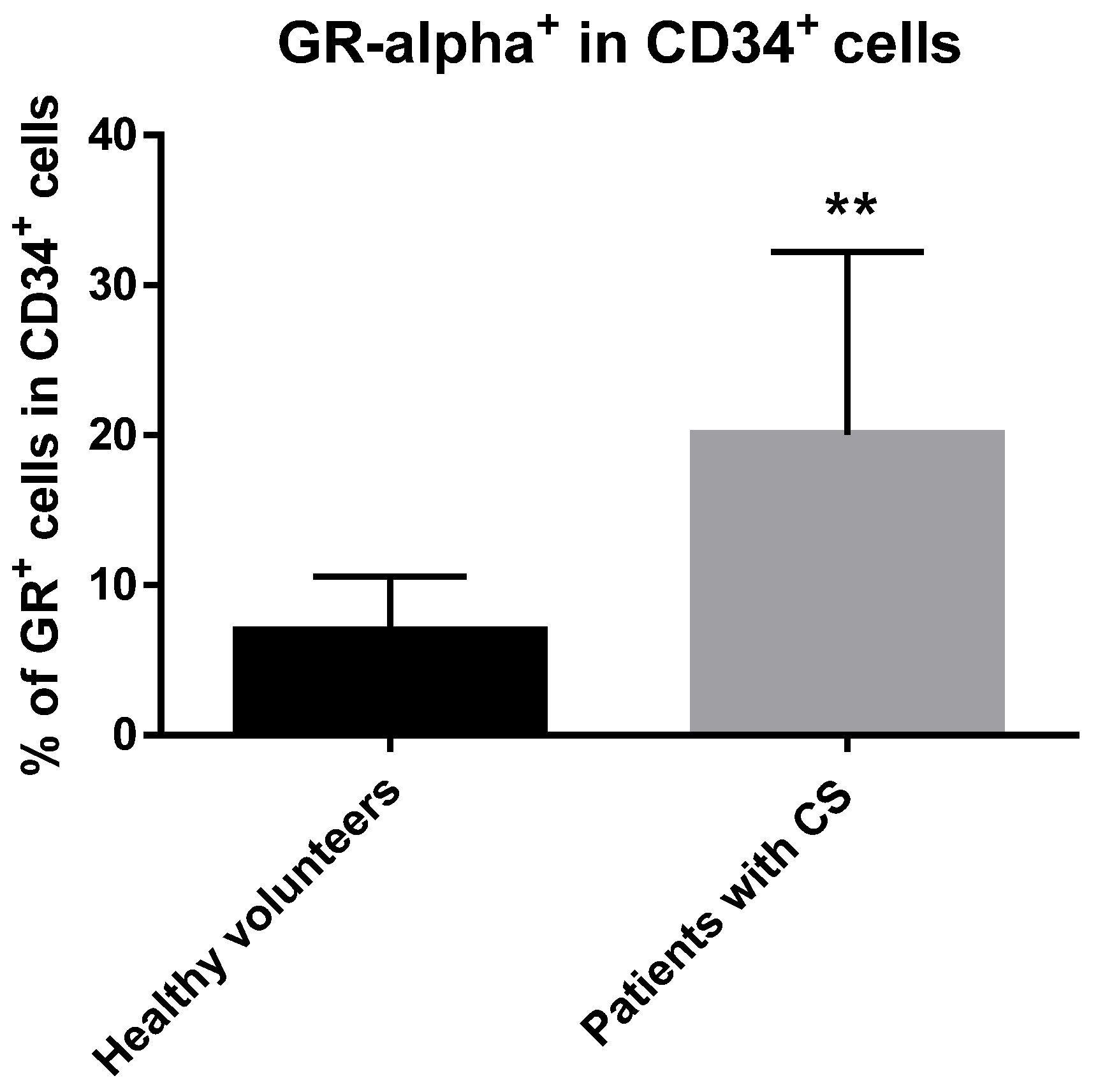
2.2. GRα Is Expressed at the Protein Level in CD34+ Hematopoietic Cells from CS Patients
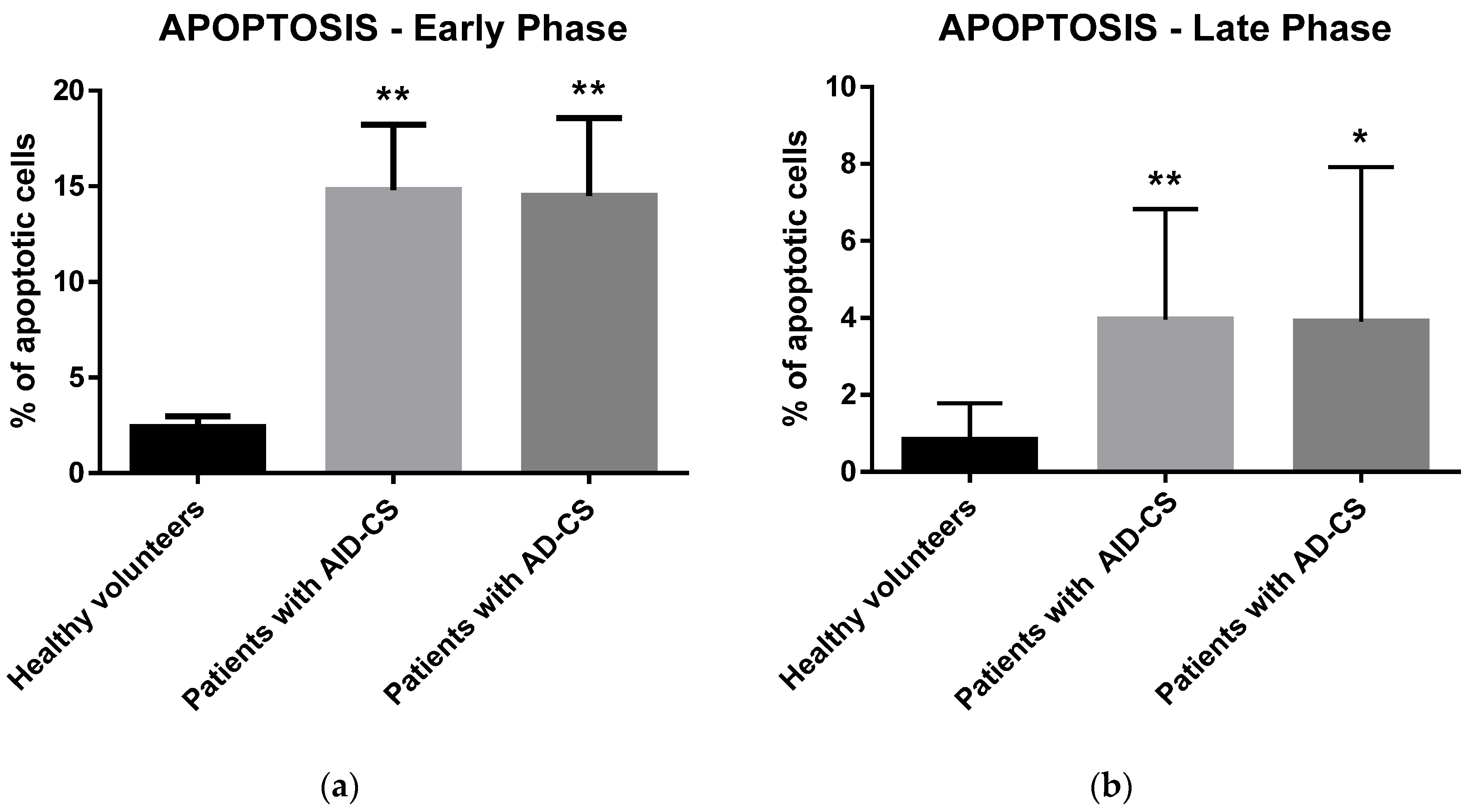
2.3. Glucocorticoids Induce Apoptosis in CD34+ Hematopoietic Cells from CS Patients
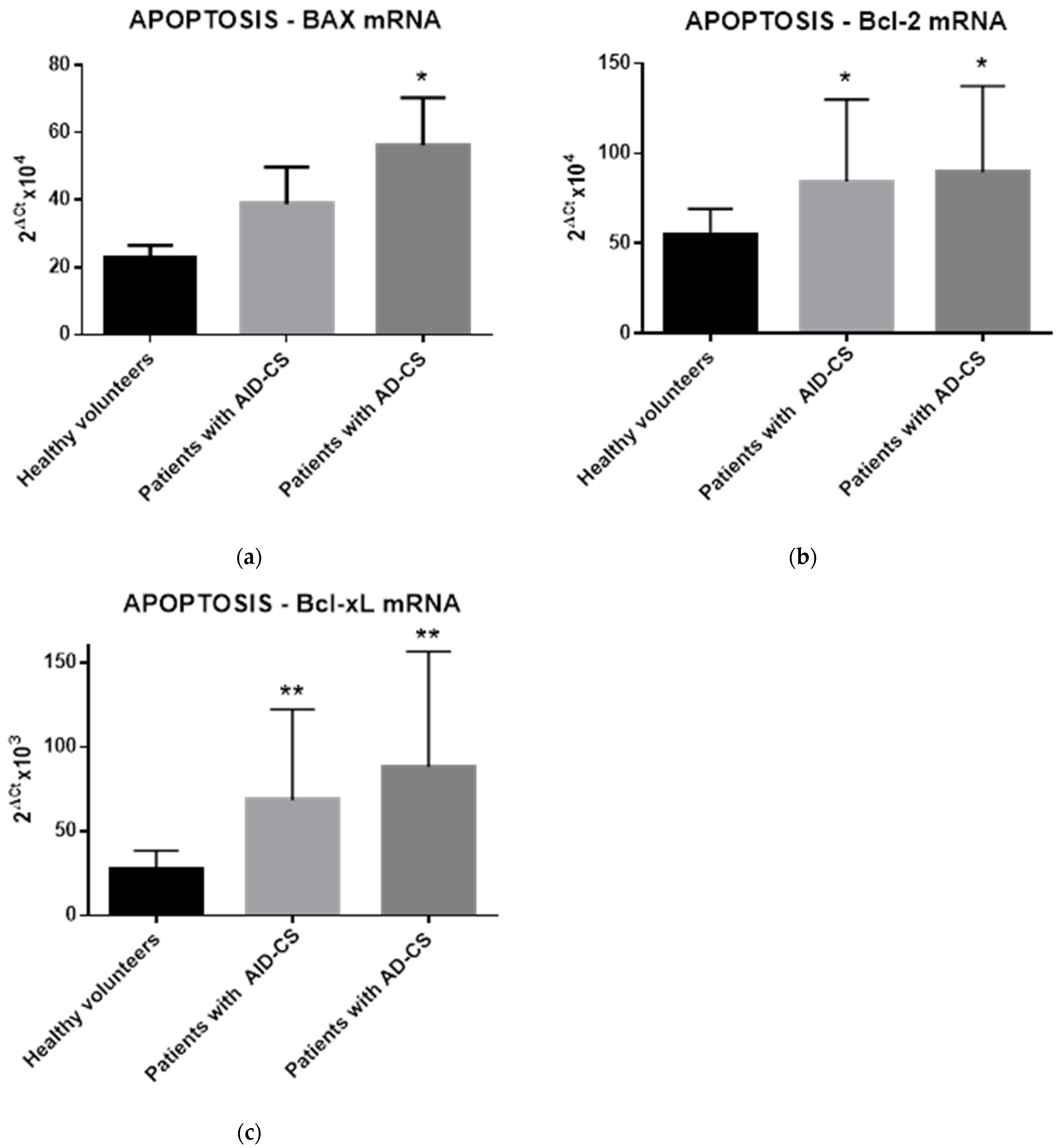
2.4. Glucocorticoids Modulate the Gene Expression of Selected Apoptosis-Regulating Proteins in CD34+ Hematopoietic Cells from CS Patients
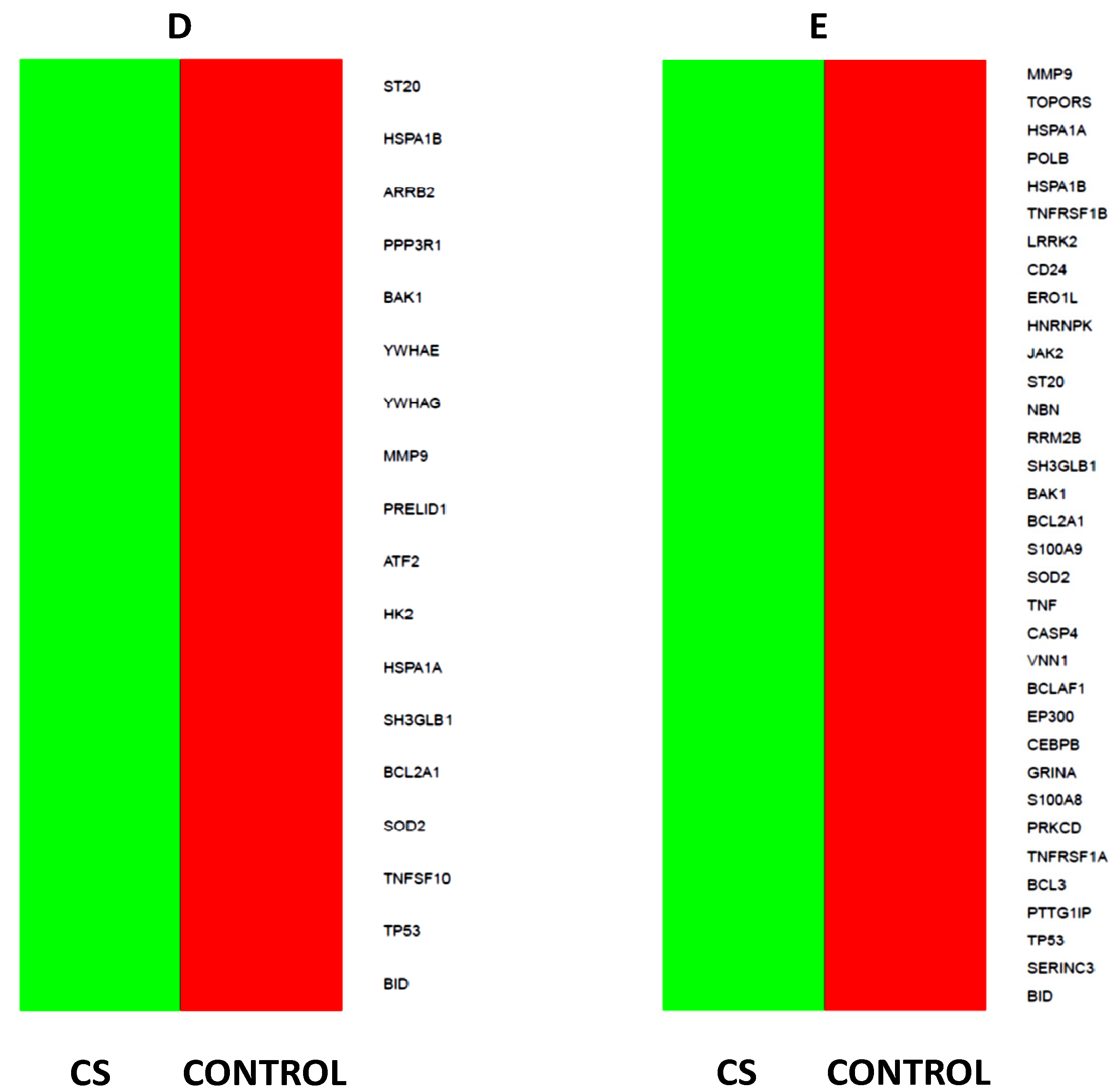
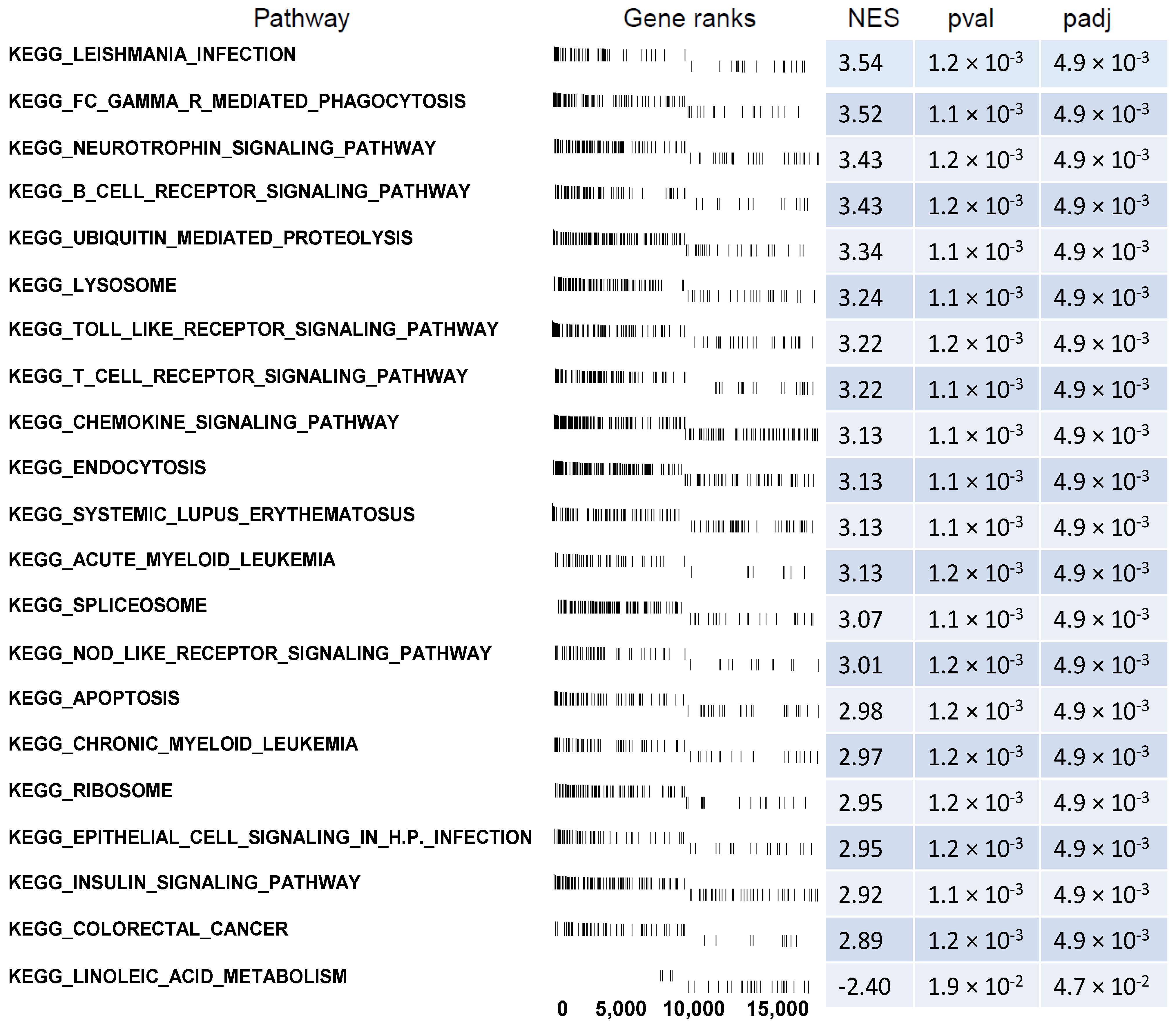
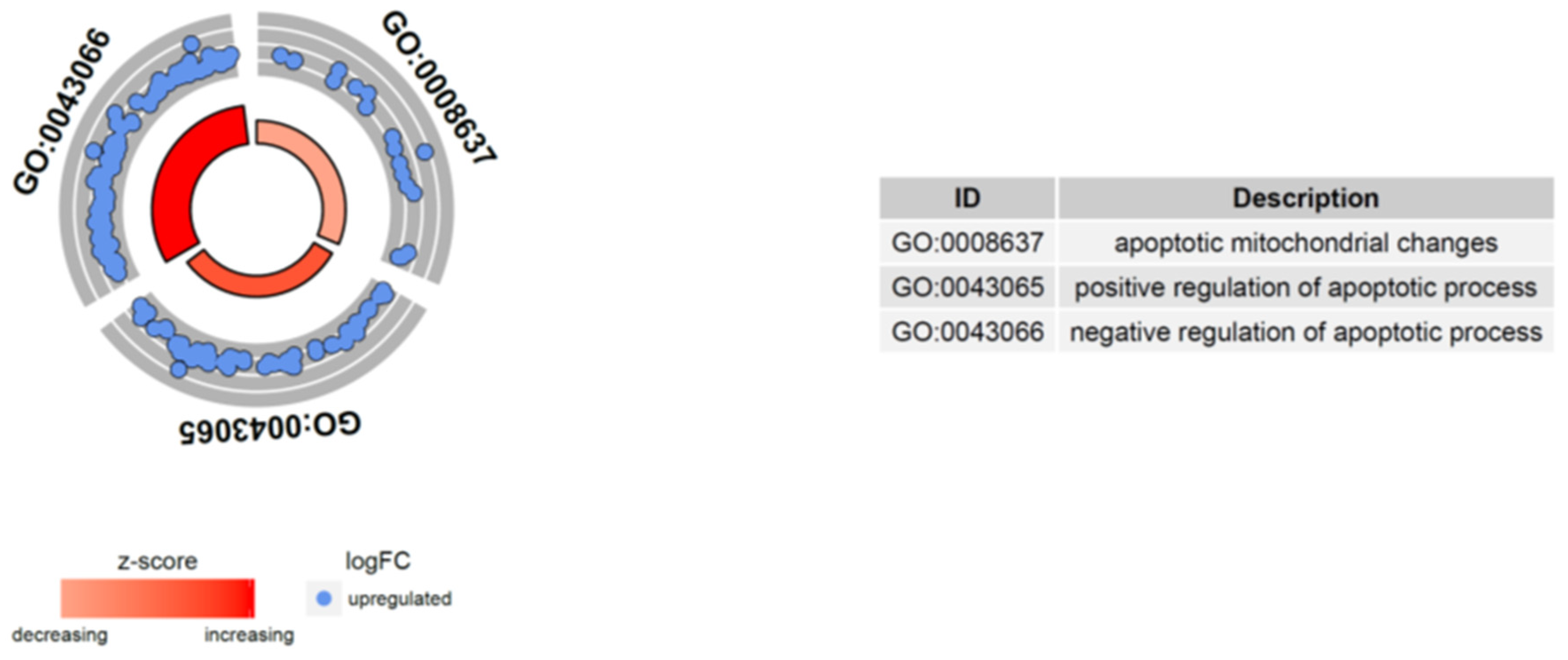
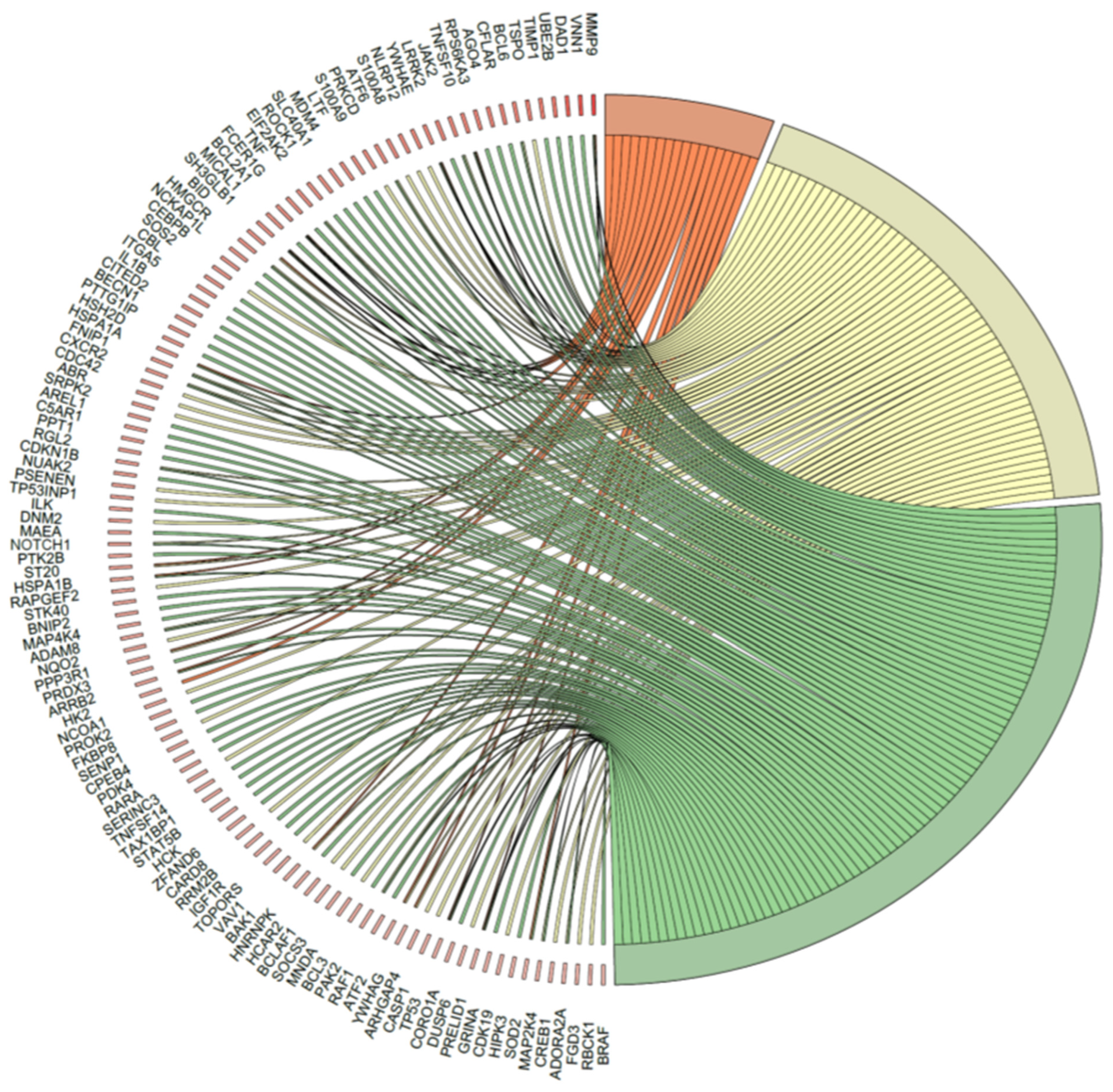
2.5. Microarray Analysis of Gene Expression Changes in CD34+ Hematopoietic Progenitor Cells from CS Patients
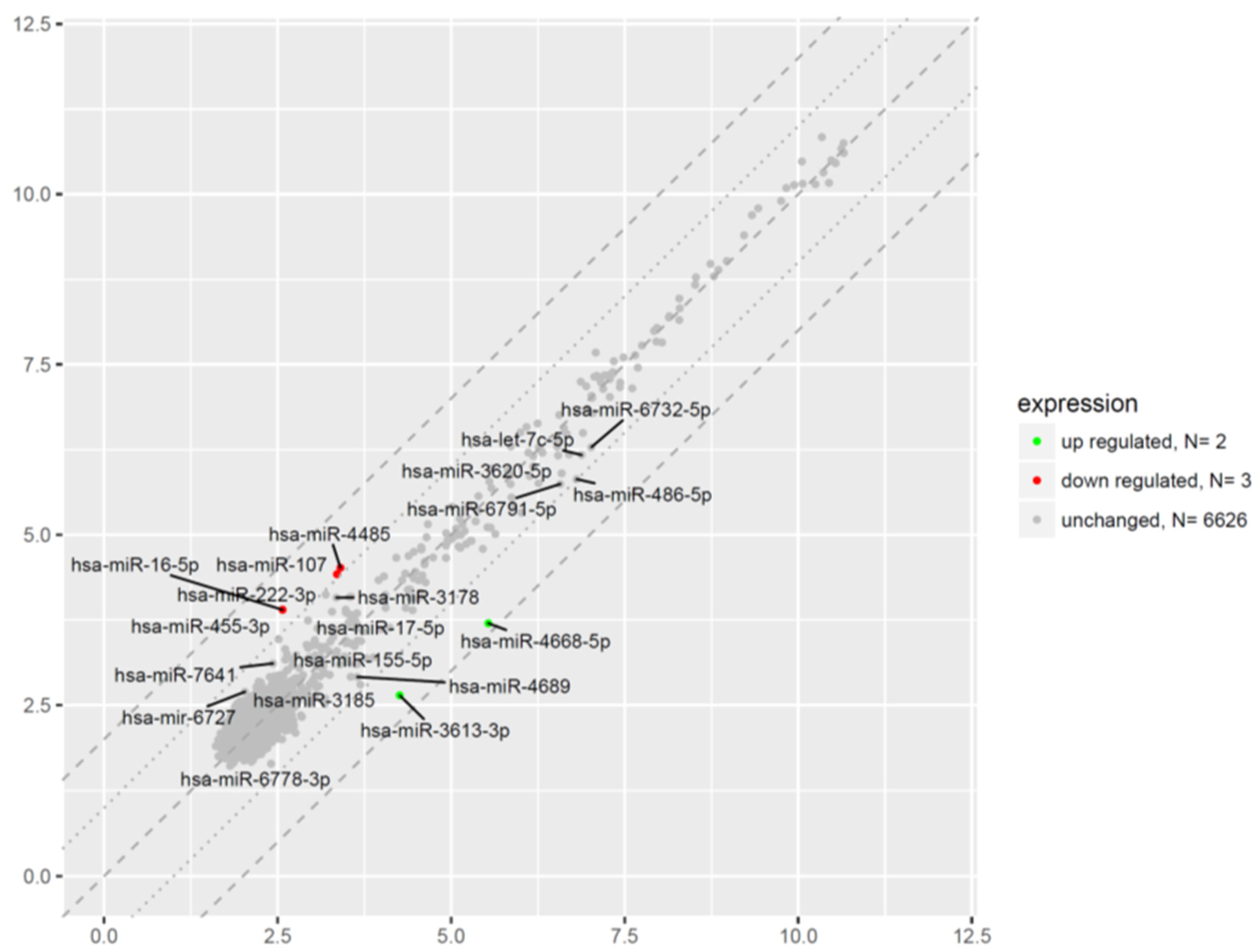
2.6. Microarray Analysis of miRNA Expression Changes in CD34+ Hematopoietic Progenitor Cells from CS Patients
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Blood Sample Collection
4.3. Cell Isolation
4.4. Flow Cytometry
4.5. Apoptosis Detection
4.6. RNA Isolation and Gene Expression Analysis
4.7. RNA and MiRNA Isolation for Microarrays
4.8. Affymetrix GeneChip Whole Transcriptome Microarray
4.9. Affymetrix GeneChip miRNA Microarray
4.10. Microarrays Data Analysis
4.11. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masri-Iraqi, H.; Robenshtok, E.; Tzvetov, G.; Manistersky, Y.; Shimon, I. Elevated white blood cell counts in Cushing’s disease: Association with hypercortisolism. Pituitary 2014, 17, 436–440. [Google Scholar] [CrossRef]
- Nakagawa, M.; Terashima, T.; D’yachkova, Y.; Bondy, G.; Hogg, J.; van Eeden, S. Glucocorticoid-induced granulocytosis: Contribution of marrow release and demargination of intravascular granulocytes. Circulation 1998, 98, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Liles, W.; Dale, D.; Klebanoff, S. Glucocorticoids inhibit apoptosis of human neutrophils. Blood 1995, 86, 3181–3188. [Google Scholar] [CrossRef] [PubMed]
- Zen, M.; Canova, M.; Campana, C.; Bettio, S.; Nalotto, L.; Rampudda, M.; Ramonda, R.; Iaccarino, L.; Doria, A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun. Rev. 2011, 10, 305–310. [Google Scholar] [CrossRef]
- Pitzalis, C.; Pipitone, N.; Bajocchi, G.; Hall, M.; Goulding, N.; Lee, A.; Kingsley, G.; Lanchbury, J.; Panayi, G. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: An additional mechanism for their anti-inflammatory and immunosuppressive effect. J. Immunol. 1997, 158, 5007–5016. [Google Scholar]
- Kawa, M.P.; Grymula, K.; Paczkowska, E.; Baskiewicz-Masiuk, M.; Dabkowska, E.; Koziolek, M.; Tarnowski, M.; Kłos, P.; Dziedziejko, V.; Kucia, M.; et al. Clinical relevance of thyroid dysfunction in human haematopoiesis: Biochemical and molecular studies. Eur. J. Endocrinol. 2010, 162, 295–305. [Google Scholar] [CrossRef]
- Kawa, M.P.; Stecewicz, I.; Piecyk, K.; Paczkowska, E.; Rogińska, D.; Sobuś, A.; Łuczkowska, K.; Pius-Sadowska, E.; Gawrych, E.; Petriczko, E.; et al. The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency. Int. J. Mol. Sci. 2017, 18, 111. [Google Scholar] [CrossRef]
- Kawa, M.P.; Stecewicz, I.; Piecyk, K.; Pius-Sadowska, E.; Paczkowska, E.; Rogińska, D.; Sobuś, A.; Łuczkowska, K.; Gawrych, E.; Petriczko, E.; et al. Effects of growth hormone therapeutic supplementation on hematopoietic stem/progenitor cells in children with growth hormone deficiency: Focus on proliferation and differentiation capabilities. Endocrine 2015, 50, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Newell-Price, J.; Bertagna, X.; Grossman, A.B.; Nieman, L.K. Cushing’s syndrome. Lancet 2006, 367, 1605–1617. [Google Scholar] [CrossRef]
- Nishioka, H.; Yamada, S. Cushing’s Disease. J. Clin. Med. 2019, 8, 1951. [Google Scholar] [CrossRef]
- Stratakis, C.A. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin-independent Cushing syndrome). Endocr. Dev. 2008, 13, 117–132. [Google Scholar] [PubMed]
- MacLeod, C.; Hadoke, P.; Nixon, M. Glucocorticoids: Fuelling the Fire of Atherosclerosis or Therapeutic Extinguishers? Int. J. Mol. Sci. 2021, 22, 7622. [Google Scholar] [CrossRef] [PubMed]
- Loerz, C.; Maser, E. The cortisol-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 in skeletal muscle in the pathogenesis of the metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2017, 174, 65–71. [Google Scholar] [CrossRef]
- Morgan, S.; Hassan-Smith, Z.; Lavery, G. Mechanisms in Endocrinlogy: Tissue-specific activation of cortisol in Cushing’s syndrome. Eur. J. Endocrinol. 2016, 175, R83–R89. [Google Scholar] [CrossRef]
- Motavalli, R.; Majidi, T.; Pourlak, T.; Abediazar, S.; Shoja, M.M.; Vahed, S.Z.; Etemadi, J. The clinical significance of the glucocorticoid receptors: Genetics and epigenetics. J. Steroid Biochem. Mol. Biol. 2021, 213, 105952. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Berezikov, E. Evolution of microRNA diversity and regulation in animals. Nat. Rev. Genet. 2011, 12, 846–860. [Google Scholar] [CrossRef]
- Romero, A.; Eckel, J. Organ Crosstalk and the Modulation of Insulin Signaling. Cells 2021, 10, 2082. [Google Scholar] [CrossRef]
- Pérez-García, A.; Torrecilla-Parra, M.; Fernández-de Frutos, M.; Martín-Martín, Y.; Pardo-Marqués, V.; Ramírez, C.M. Posttranscriptional Regulation of Insulin Resistance: Implications for Metabolic Diseases. Biomolecules 2022, 12, 208. [Google Scholar] [CrossRef]
- Kaur, P.; Kotru, S.; Singh, S.; Munshi, A. Role of miRNAs in diabetic neuropathy: Mechanisms and possible interventions. Mol. Neurobiol. 2022, 59, 1836–1849. [Google Scholar] [CrossRef]
- Pierouli, K.; Papageorgiou, L.; Mitsis, T.; Papakonstantinou, E.; Diakou, I.; Leptidis, S.; Sigala, M.; Dragoumani, K.; Spandidos, D.A.; Bacopoulou, F.; et al. Role of microRNAs and long non-coding RNAs in glucocorticoid signaling. Int. J. Mol. Med. 2022, 50, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gou, X.; Jiang, T.; Ouyang, J. The effects of microRNAs on glucocorticoid responsiveness. J. Cancer Res. Clin. Oncol. 2017, 143, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Kawa, M.P.; Sobuś, A.; Litwińska, Z.; Osowicz-Korolonek, L.; Cymbaluk-Płoska, A.; Stecewicz, I.; Zagrodnik, E.; Romanowska, H.; Walczak, M.; Syrenicz, A.; et al. Expression of selected angiogenesis-related small microRNAs in patients with abnormally increased secretion of glucocorticoids. Endokrynol. Pol. 2019, 70, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Butz, H.; Mészáros, K.; Likó, I.; Patocs, A. Wnt-Signaling Regulated by Glucocorticoid-Induced miRNAs. Int. J. Mol. Sci. 2021, 22, 11778. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.M.; Gadkari, M.; Howe, K.N.; Sun, J.; Kardava, L.; Kumar, P.; Kumari, S.; Hu, Z.; Fraser, I.D.C.; Moir, S.; et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. J. Exp. Med. 2019, 216, 384–406. [Google Scholar] [CrossRef]
- Vidović, A.; Gotovac, K.; Vilibić, M.; Sabioncello, A.; Jovanović, T.; Rabatić, S.; Folnegović-Šmalć, V.; Dekaris, D. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation 2011, 18, 199–211. [Google Scholar] [CrossRef]
- Heideveld, E.; Hampton-O’Neil, L.A.; Cross, S.J.; van Alphen, F.P.J.; van den Biggelaar, M.; Toye, A.M.; van den Akker, E. Glucocorticoids induce differentiation of monocytes towards macrophages that share functional and phenotypical aspects with erythroblastic island macrophages. Haematologica 2018, 103, 395–405. [Google Scholar] [CrossRef]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell. Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef]
- Peffer, M.E.; Chandran, U.R.; Luthra, S.; Volonte, D.; Galbiati, F.; Garabedian, M.J.; Monaghan, A.P.; DeFranco, D.B. Caveolin-1 regulates genomic action of the glucocorticoid receptor in neural stem cells. Mol. Cell Biol. 2014, 34, 2611–2623. [Google Scholar] [CrossRef]
- Cabral-Teixeira, J.; Martinez-Fernandez, A.; Cai, W.; Terzic, A.; Mercola, M.; Willems, E. Cholesterol-derived glucocorticoids control early fate specification in embryonic stem cells. Stem Cell. Res. 2015, 15, 88–95. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, E.K.; Kwack, M.H.; Sung, Y.K. Effects of dexamethasone, a synthetic glucocorticoid, on human periodontal ligament stem cells. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Kwan, W.; Cortes, M.; Frost, I.; Esain, V.; Theodore, L.N.; Liu, S.Y.; Budrow, N.; Goessling, W.; North, T.E. The Central Nervous System Regulates Embryonic HSPC Production via Stress-Responsive Glucocorticoid Receptor Signaling. Cell Stem Cell. 2016, 19, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Huang, X.; Cooper, S.; Broxmeyer, H.E. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat. Med. 2017, 23, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Vidović, A.; Vilibić, M.; Sabioncello, A.; Gotovac, K.; Rabatić, S.; Folnegović-Smalc, V.; Dekaris, D. Circulating lymphocyte subsets, natural killer cell cytotoxicity, and components of hypothalamic-pituitary-adrenal axis in Croatian war veterans with posttraumatic stress disorder: Cross-sectional study. Croat. Med. J. 2007, 48, 198–206. [Google Scholar] [PubMed]
- Prenek, L.; Boldizsár, F.; Kugyelka, R.; Ugor, E.; Berta, G.; Németh, P.; Berki, T. The regulation of the mitochondrial apoptotic pathway by glucocorticoid receptor in collaboration with Bcl-2 family proteins in developing T cells. Apoptosis 2017, 22, 239–253. [Google Scholar] [CrossRef]
- Costa, K.M.D.A.; Valente, R.C.; Silva, J.M.C.D.A.; Paiva, L.S.D.E.; Rumjanek, V.M. Glucocorticoid susceptibility and in vivo ABCB1 activity differ in murine B cell subsets. An. Acad. Bras. Cienc. 2018, 90, 3081–3097. [Google Scholar] [CrossRef]
- Gruver-Yates, A.L.; Quinn, M.A.; Cidlowski, J.A. Analysis of glucocorticoid receptors and their apoptotic response to dexamethasone in male murine B cells during development. Endocrinology 2014, 155, 463–474. [Google Scholar] [CrossRef]
- Schweingruber, N.; Fischer, H.J.; Fischer, L.; van den Brandt, J.; Karabinskaya, A.; Labi, V.; Villunger, A.; Kretzschmar, B.; Huppke, P.; Simons, M.; et al. Chemokine-mediated redirection of T cells constitutes a critical mechanism of glucocorticoid therapy in autoimmune CNS responses. Acta Neuropathol. 2014, 127, 713–729. [Google Scholar] [CrossRef]
- Bier, J.; Steiger, S.M.; Reichardt, H.M.; Lühder, F. Protection of Antigen-Primed Effector T Cells From Glucocorticoid-Induced Apoptosis in Cell Culture and in a Mouse Model of Multiple Sclerosis. Front. Immunol. 2021, 12, 671258. [Google Scholar] [CrossRef]
- Rasmussen, M.L.; Gama, V. A connection in life and death: The BCL-2 family coordinates mitochondrial network dynamics and stem cell fate. Int. Rev. Cell Mol. Biol. 2020, 353, 255–284. [Google Scholar]
- Herold, M.; McPherson, K.; Reichardt, H. Glucocorticoids in T cell apoptosis and function. Cell Mol. Life Sci. 2006, 63, 60–72. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, C.J.; Zhang, X.; Zhai, Z.S.; Wang, Y.Z.; Hu, M.X.; Xu, X.L.; Zhang, H.W.; Qin, T. Glucocorticoid receptor regulates expression of microRNA-22 and downstream signaling pathway in apoptosis of pancreatic acinar cells. World J. Gastroenterol. 2018, 24, 5120–5130. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.N.; Tang, Y.L.; Ke, Z.Y.; Chen, Y.Q.; Luo, X.Q.; Zhang, H.; Huang, L.B. MiR-124 contributes to glucocorticoid resistance in acute lymphoblastic leukemia by promoting proliferation, inhibiting apoptosis and targeting the glucocorticoid receptor. J. Steroid Biochem. Mol. Biol. 2017, 172, 62–68. [Google Scholar] [CrossRef]
- Erlacher, M.; Michalak, E.M.; Kelly, P.N.; Labi, V.; Niederegger, H.; Coultas, L.; Adams, J.M.; Strasser, A.; Villunger, A. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005, 106, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Müller, N.; McPherson, K.G.; Reichardt, H.M. Glucocorticoids engage different signal transduction pathways to induce apoptosis in thymocytes and mature T cells. J. Immunol. 2006, 176, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Machaliński, B.; Paczkowski, M.; Kawa, M.; Paczkowska, E.; Ostrowski, M. An optimization of isolation of early hematopoietic cells from heparinized cadaveric organ donors. Transpl. Proc. 2003, 35, 3096–3100. [Google Scholar] [CrossRef]
- Gródecka-Szwajkiewicz, D.; Ulańczyk, Z.; Zagrodnik, E.; Łuczkowska, K.; Rogińska, D.; Kawa, M.P.; Stecewicz, I.; Safranow, K.; Machaliński, B. Differential Secretion of Angiopoietic Factors and Expression of MicroRNA in Umbilical Cord Blood from Healthy Appropriate-For-Gestational-Age Preterm and Term Newborns-in Search of Biomarkers of Angiogenesis-Related Processes in Preterm Birth. Int. J. Mol. Sci. 2020, 21, 1305. [Google Scholar] [CrossRef]
- Gródecka-Szwajkiewicz, D.; Ulańczyk, Z.; Zagrodnik, E.; Łuczkowska, K.; Rogińska, D.; Kawa, M.P.; Stecewicz, I.; Safranow, K.; Ustianowski, P.; Szymański, S.; et al. Comparative Analysis of Global Gene Expression and Complement Components Levels in Umbilical Cord Blood from Preterm and Term Neonates: Implications for Significant Downregulation of Immune Response Pathways related to Prematurity. Int. J. Med. Sci. 2020, 17, 1840–1853. [Google Scholar] [CrossRef]






| Gene Symbol | Gene Name | Gene Function According to GO Biological Processes Database | miRNA |
|---|---|---|---|
| CARD8 | Caspase Recruitment Domain Family Member 8 | programmed cell death (GO:0012501), regulation of apoptotic process (GO:0042981), regulation of I-kappaB kinase/NF-kappaB signaling (GO:0043122), negative regulation of I-kappaB kinase/NF-kappaB signaling (GO:0043124), negative regulation of NF-kappaB transcription factor activity (GO:0032088), activation of cysteine-type endopeptidase activity involved in apoptotic process (GO:0043280), positive regulation of interleukin-1 beta production (GO:0032731), inflammatory response (GO:0006954), negative regulation of NLRP3 inflammasome complex assembly (GO:1900226) | miRNA-16 |
| LITAF | Lipopolysaccharide Induced TNF Factor | regulation of cytokine production (GO:0001817), regulation of macrophage cytokine production (GO:0010935), response to lipopolysaccharide (GO:0032496), cellular response to lipopolysaccharide (GO:0071222), positive regulation of I-kappaB kinase/NF-kappaB signaling (GO:0043123), negative regulation of NIK/NF-kappaB signaling (GO:1901223) | miRNA-16 |
| TP53 | Tumor Suppressor Protein P53 | apoptotic process (GO:0006915), regulation of apoptotic process (GO:0042981), positive regulation of apoptotic process (GO:0043065), intrinsic apoptotic signaling pathway (GO:0097193), positive regulation of intrinsic apoptotic signaling pathway (GO:2001244), regulation of fibroblast apoptotic process (GO:2000269), cardiac muscle cell apoptotic process (GO:0010659), positive regulation of cardiac muscle cell apoptotic process (GO:0010666), programmed cell death (GO:0012501), intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator (GO:0042771), regulation of neuron apoptotic process (GO:0043523), positive regulation of neuron apoptotic process (GO:0043525), regulation of thymocyte apoptotic process (GO:0070242), positive regulation of thymocyte apoptotic process (GO:0070245) | miRNA-16 |
| HSPA1A | Heat Shock Protein Family A (Hsp70) Member 1A | regulation of cell death (GO:0010941), negative regulation of apoptotic process (GO:0043066), negative regulation of mitochondrial outer membrane permeabilization involved in apoptotic process (GO:1901029), negative regulation of extrinsic apoptotic signaling pathway in absence of ligand (GO:2001240), cellular response to oxidative stress (GO:0034605), negative regulation of endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathway (GO:1902236), negative regulation of cell growth (GO:0030308), regulation of protein ubiquitination (GO:0031396), negative regulation of protein ubiquitination (GO:0031397), positive regulation of tumor necrosis factor-mediated signaling pathway (GO:1903265) | miRNA-16 |
| HSPA1B | Heat Shock Protein Family A (Hsp70) Member 1B | negative regulation of apoptotic process (GO:0043066), negative regulation of extrinsic apoptotic signaling pathway in absence of ligand (GO:2001240), negative regulation of cell death (GO:0060548), cellular response to steroid hormone stimulus (GO:0071383), cellular response to oxidative stress (GO:0034599), positive regulation of NF-kappaB transcription factor activity (GO:0051092), positive regulation of tumor necrosis factor-mediated signaling pathway (GO:1903265), positive regulation of interleukin-8 production (GO:0032757) | miRNA-16 |
| GABARAP | GABA Type A Receptor-Associated Protein | apoptotic process (GO:0006915), extrinsic apoptotic signaling pathway via death domain receptors (GO:0008625), autophagy (GO:0006914), autophagy of mitochondrion (GO:0000422), autophagosome assembly (GO:0000045), macroautophagy (GO:0016236), positive regulation of proteasomal ubiquitin-dependent protein catabolic process (GO:0032436), regulation of Rac protein signal transduction (GO:0035020) | miRNA-16 |
| PPP2CA | Protein Phosphatase 2 Catalytic Subunit Alpha | apoptotic process (GO:0006915), response to organic substance (GO:0010033), negative regulation of cell growth (GO:0030308), negative regulation of epithelial to mesenchymal transition (GO:0010719) | miRNA-107 |
| RAD21 | Cohesin Complex Component | apoptotic process (GO:0006915), cellular response to DNA damage stimulus (GO:0006974), double-strand break repair (GO:0006302), replication-born double-strand break repair via sister chromatid exchange (GO:1990414) | miRNA-107 |
| UBE2B | Ubiquitin Conjugating Enzyme E2 B | apoptotic process (GO:0006915), negative regulation of apoptotic process (GO:0043066), response to xenobiotic stimulus (GO:0009410), cellular response to DNA damage stimulus (GO:0006974), DNA repair (GO:0006281), ubiquitin-dependent protein catabolic process (GO:0006511), protein polyubiquitination (GO:0000209), protein monoubiquitination (GO:0006513) | miRNA-107 |
| No. | Gene Name | Gene Symbol | Primer Direction | Primer Sequence |
|---|---|---|---|---|
| 1 | B-cell CLL/lymphoma 2 | BCL-2 | Sense Antisense | GCC GGT TCA GGT ACT CAG TCA T CAT GTG TGT GGA GAG CGT CAA |
| 2 | B-cell lymphoma-extra large | BCL-XL | Sense Antisense | CTC AGC GCT TGC TTT AC CGC ACA GCA GCA GTT TGG |
| 3 | BCL2-associated X protein | BAX | Sense Antisense | GTT GCG GTC AGA AAA CAT GTC GCC GCC GTG GAC ACA |
| 4 | Beta-2-microglobulin | BMG | Sense Antisense | AAT GCG GCA TCT TCA AAC CT TGA CTT TGT CAC AGC CCA AGA TA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawa, M.P.; Sobuś, A.; Pius-Sadowska, E.; Łuczkowska, K.; Rogińska, D.; Wnęk, S.; Paczkowska, E.; Walczak, M.; Syrenicz, A.; Machaliński, B. Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing’s Syndrome. Int. J. Mol. Sci. 2022, 23, 15794. https://doi.org/10.3390/ijms232415794
Kawa MP, Sobuś A, Pius-Sadowska E, Łuczkowska K, Rogińska D, Wnęk S, Paczkowska E, Walczak M, Syrenicz A, Machaliński B. Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing’s Syndrome. International Journal of Molecular Sciences. 2022; 23(24):15794. https://doi.org/10.3390/ijms232415794
Chicago/Turabian StyleKawa, Miłosz P., Anna Sobuś, Ewa Pius-Sadowska, Karolina Łuczkowska, Dorota Rogińska, Szymon Wnęk, Edyta Paczkowska, Mieczysław Walczak, Anhelli Syrenicz, and Bogusław Machaliński. 2022. "Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing’s Syndrome" International Journal of Molecular Sciences 23, no. 24: 15794. https://doi.org/10.3390/ijms232415794
APA StyleKawa, M. P., Sobuś, A., Pius-Sadowska, E., Łuczkowska, K., Rogińska, D., Wnęk, S., Paczkowska, E., Walczak, M., Syrenicz, A., & Machaliński, B. (2022). Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing’s Syndrome. International Journal of Molecular Sciences, 23(24), 15794. https://doi.org/10.3390/ijms232415794





