Alterations in Kidney Structures Caused by Age Vary According to Sex and Dehydration Condition
Abstract
1. Introduction
2. Results
2.1. Kidney and Body Weight
2.2. Sodium Plasma Concentration
2.3. The Relative Expression of Slc12a3 mRNA in the Renal Cortex
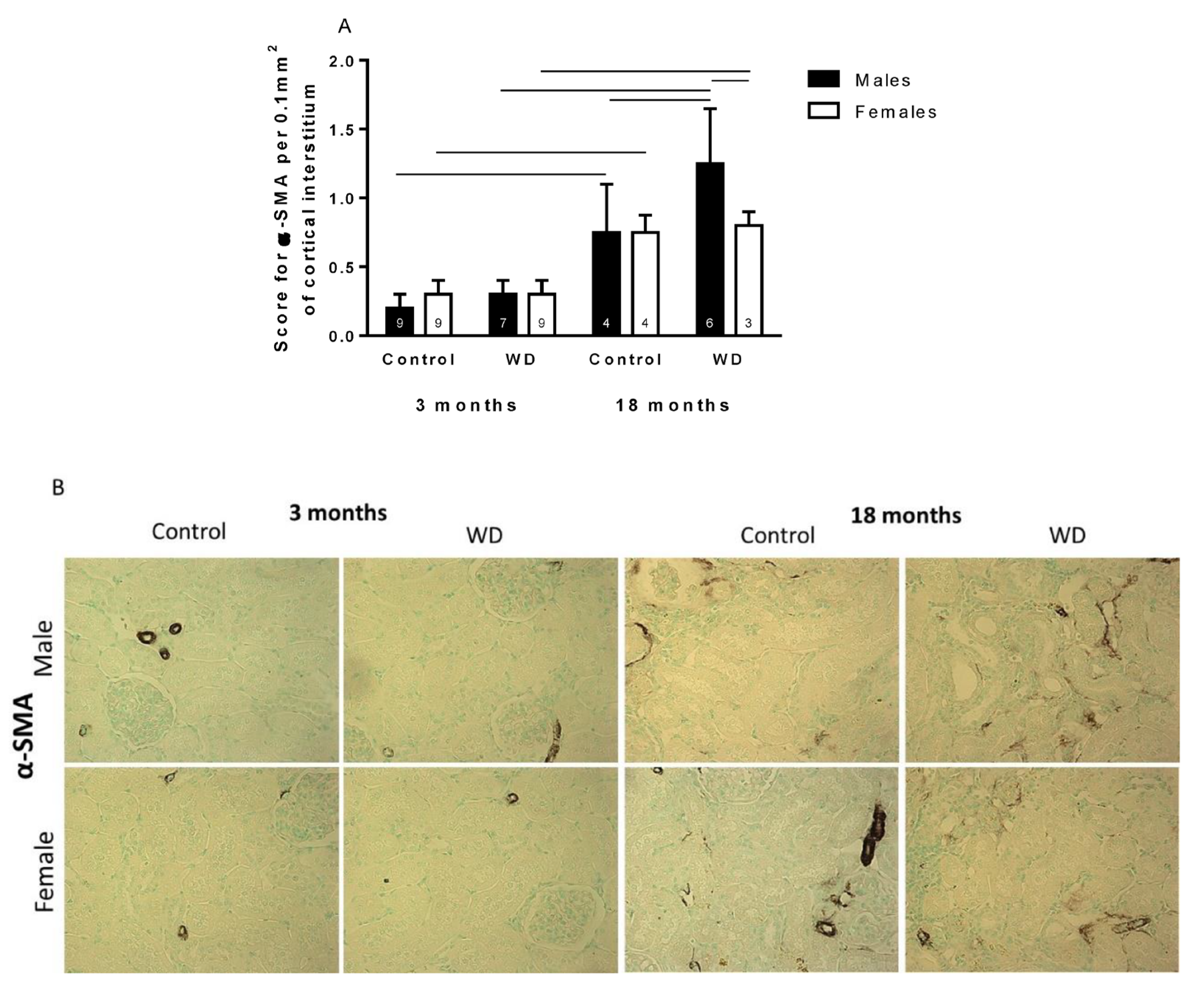
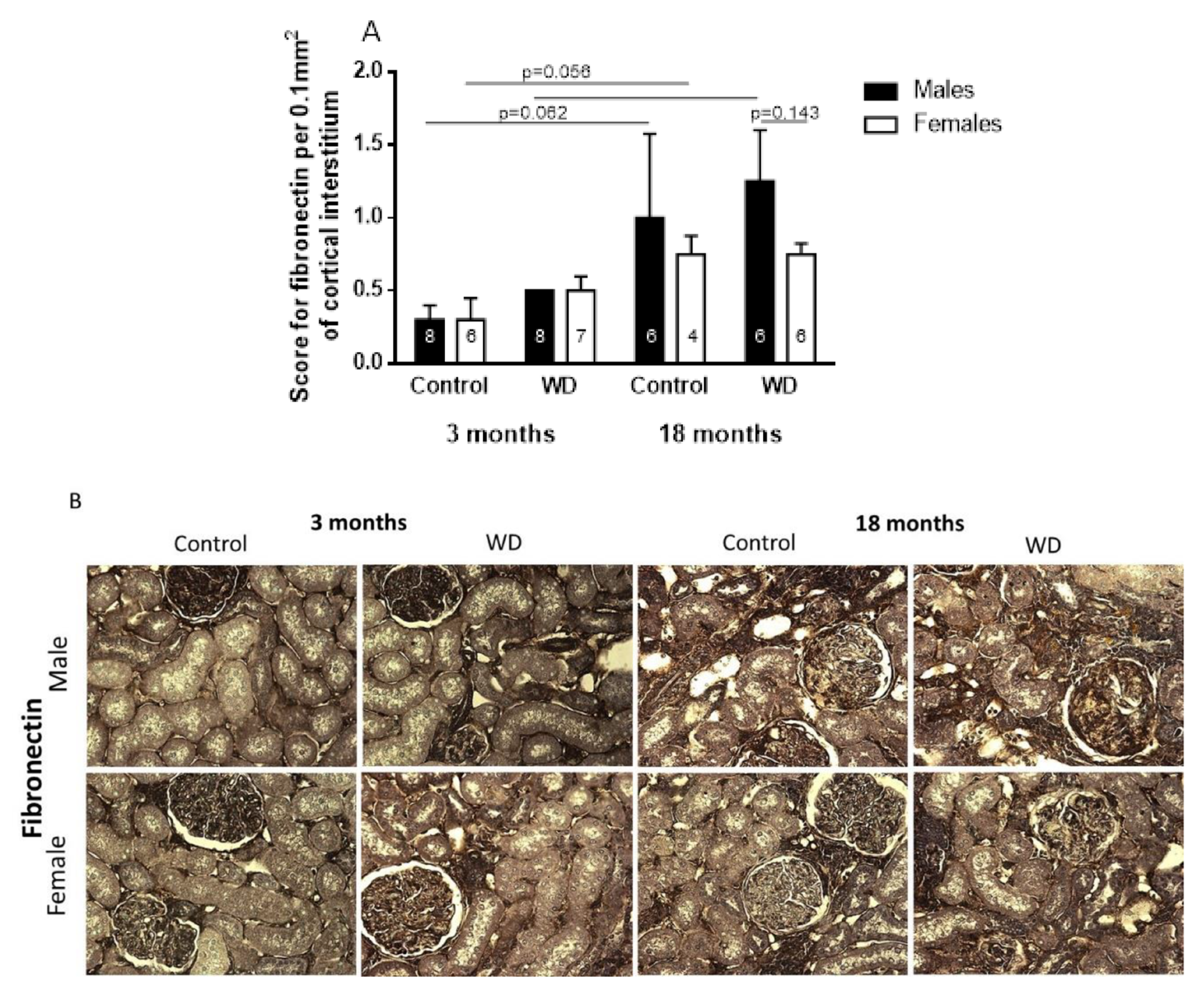
2.4. Immunohistochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Model and Experimental Design
4.2. Experimental Methods
4.3. Determination of Plasma Sodium
4.4. Tissue Collection
4.5. Microdissection, RNA Isolation, and Semiquantitative Real-Time PCR
4.6. Immunohistochemical Studies
4.7. Statistical Analyses
4.8. Study Limitations
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atherton, B.J.C. Renal Physiology. Br. J. Anaesth. 1972, 44, 236–245. [Google Scholar] [CrossRef]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes With the Aging Kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Anderson, S. The aging kidney-physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef]
- Rodrfguez-Puyol, D. The aging kidney. Kidney Int. 1998, 54, 2247–2265. [Google Scholar] [CrossRef]
- Sato, Y.; Yanagita, M. Resident fibroblasts in the kidney: A major driver of fibrosis and inflammation. Inflamm. Regen. 2017, 37, 17. [Google Scholar] [CrossRef]
- Gekle, M. Kidney and aging—A narrative review. Exp. Gerontol. 2017, 87, 153–155. [Google Scholar] [CrossRef]
- Sands, J.M. Urinary Concentration and Diluition in the Aging Kidney. Semin. Nephrol. 2009, 29, 579–586. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Riazi, S.; Khan, O.; Klein, J.; Sugimura, Y.; Verbalis, J.; Ecelbarger, C. Renal ENaC subunit, Na-K-2Cl and Na-Cl cotransporter abundances in aged, water-restricted F344 × Brown Norway rats. Kidney Int. 2006, 69, 304–312. [Google Scholar] [CrossRef][Green Version]
- Stachenfeld, N.S. Hormonal changes during menopause and the impact on fluid regulation. Reprod. Sci. 2014, 21, 555–561. [Google Scholar] [CrossRef]
- Begg, D.P. Disturbances of thirst and fluid balance associated with aging. Physiol. Behav. 2017, 178, 28–34. [Google Scholar] [CrossRef]
- Hooper, L. Why, oh why, are so many older adults not drinking enough fluid? J. Acad. Nutr. Diet. 2016, 116, 774–778. [Google Scholar] [CrossRef]
- Borra, S.I.; Beredo, R.; Kleinfeld, M. Hypernatremia in the aging: Causes, manifestations, and outcome. J. Natl. Med. Assoc. 1995, 87, 220–224. [Google Scholar]
- Kasiske, B.L.; Umen, A.J. The influence of age, sex, race, and body habitus on kidney weight in humans. J. Urol. 1986, 136, 194. [Google Scholar] [CrossRef]
- McLachlan, M.; Wasserman, P. Changes in sizes and distensibility of the aging kidney. Br. J. Radiol. 1981, 54, 488–491. [Google Scholar] [CrossRef]
- Bankir, L.; Fischer, C.; Fischer, S.; Jukkala, K.; Specht, H.-C.; Kriz, W.; Hähnel, B.; Hartmann, I.; Ertel, I.; Coutaud, C. Adaptation of the rat kidney to altered water intake and urine concentration. Pflüg. Arch. Eur. J. Physiol. 1988, 412, 42–53. [Google Scholar] [CrossRef]
- Heo, N.J.; Son, M.-J.; Lee, J.W.; Jung, J.Y.; Kim, S.; Oh, Y.K.; Na, K.Y.; Yoon, H.-J.; Joo, K.W.; Han, J.S. Effect of estradiol on the expression of renal sodium transporters in rats. Climacteric 2013, 16, 265–273. [Google Scholar] [CrossRef]
- Cognuck, S.Q.; Reis, W.L.; Silva, M.S.; Almeida-Pereira, G.; De Barba, L.K.; Zorro, S.V.; Mecawi, A.S.; Franci, C.R.; Elias, L.L.K.; Antunes-Rodrigues, J. Sex- and age-dependent differences in the hormone and drinking responses to water deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R567–R578. [Google Scholar] [CrossRef]
- Haymana, C.; Sonmez, A.; Aydogdu, A.; Tapan, S.; Basaran, Y.; Meric, C.; Baskoy, K.; Taslipinar, A.; Yilmaz, M.I.; Azal, O. Effect of testosterone replacement therapy on vitamin D and FGF-23 levels in congenital hypogonadism. Endokrynol. Pol. 2017, 68, 311–616. [Google Scholar] [CrossRef][Green Version]
- Andrukhova, O.; Slavic, S.; Smorodchenko, A.; Zeitz, U.; Shalhoub, V.; Lanske, B.; Pohl, E.E.; Erben, R.G. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 2014, 6, 744–759. [Google Scholar] [CrossRef]
- Mutig, K.; Saritas, T.; Uchida, S.; Kahl, T.; Borowski, T.; Paliege, A.; Böhlick, A.; Bleich, M.; Shan, Q.; Bachmann, S. Short-term stimulation of the thiazide-sensitive Na-Cl cotransporter by vasopressin involves phosphorylation and membrane translocation. Am. J. Physiol.-Ren. Physiol. 2010, 298, 502–509. [Google Scholar] [CrossRef]
- Karam, Z.; Tuazon, J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2013, 29, 555–564. [Google Scholar] [CrossRef] [PubMed]
- La Cruz, C.I.-D.; Ruiz-Torres, P.; del Moral, R.G.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D. Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am. J. Physiol.-Ren. Physiol. 2000, 278, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Almatamar, A.; Chaabane, I.; Salem, S.; Touati, H.; Remana, S.; Chevalier, C.; Moudilou, E.N.; Exbrayat, J.-M.; Barka-Dahane, Z. Kidney functional morphology variations between spring and winter in the Saharan male lizard Uromastyx acanthinura (Sauria, Agamidae), with special reference to body water economy. Tissue Cell 2020, 67, 101448. [Google Scholar] [CrossRef] [PubMed]
- Baiardi, G.; Macova, M.; Armando, I.; Ando, H.; Tyurmin, D.; Saavedra, J.M. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul. Pept. 2005, 124, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Pawluczyk, I.Z.A.; Harris, K.P.G. Effect of angiotensin type 2 receptor over-expression on the rat mesangial cell fibrotic phenotype: Effect of gender. JRAAS J. Renin-Angiotensin-Aldosterone Syst. 2012, 13, 221–231. [Google Scholar] [CrossRef]
- Hashimoto, N.; Maeshima, Y.; Satoh, M.; Odawara, M.; Sugiyama, H.; Kashihara, N.; Matsubara, H.; Yamasaki, Y.; Makino, H. Overexpression of angiotensin type 2 receptor ameliorates glomerular injury in a mouse remnant kidney model. Am. J. Physiol.-Ren. Physiol. 2004, 286, 2–5. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Shinozaki, M.; Hirakata, H.; Tamaki, K.; Hirano, T.; Tokumoto, M.; Goto, H.; Seiya, O.; Fujishima, M. Locally activated renin-angiotensin system associated with TGF-β1 as a major factor for renal injury induced by chronic inhibition of nitric oxide synthase in rats. J. Am. Soc. Nephrol. 2000, 11, 616–624. [Google Scholar] [CrossRef]
- de Almeida, L.F.; Francescato, H.D.C.; Antunes-Rodrigues, J.; de Paula, F.J.A.; da Silva, C.G.A.; Costa, R.S.; Coimbra, T.M. Imbalance of pro-and anti-angiogenic factors due to maternal vitamin D deficiency causes renal microvasculature alterations affecting the adult kidney function. Nutrients 2019, 11, 1929. [Google Scholar] [CrossRef]


| Parameters | Sex | Age (Months) | |||
|---|---|---|---|---|---|
| 3 | 18 | ||||
| Ctrl | WD | Ctrl | WD | ||
| Number of Animals | M | 10 | 10 | 8 | 8 |
| F | 12 | 12 | 7 | 6 | |
| Kidney weight (g/100g bw) | M | 0.35 (0.02) ac | 0.38 (0.03)bcg | 0.31 (0.02) ad | 0.35 (0.02)bdh |
| F | 0.33 (0.02) e | 0.35 (0.02) fg | 0.29 (0.03) e | 0.28 (0.02) fh | |
| Body weight (g) | M | 561.5 (39.5) iot | 476 (49.4) kom | 810.1 (147.9)iv | 733 (123) ky |
| F | 383.7 (19) pjt | 334.2 (20.3)qjm | 550 (98.6) pv | 466.8 (59.4) qy | |
| Na+ Plasma (mEq/L) | M | 131.3 (10.9) xz | 143.5 (4.7) x | 138.6 (11.8) | 146.6 (3.2) |
| F | 138.0 (3.1) rz | 147.6 (4.6) r | 141.6 (2.1) s | 145.7 (4.7) s | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cognuck, S.Q.; Almeida, L.F.d.; Reis, W.L.; Silva, M.S.; Almeida-Pereira, G.; Zorro, S.V.; Mecawi, A.S.; Coimbra, T.M.; Elias, L.L.K.; Antunes-Rodrigues, J. Alterations in Kidney Structures Caused by Age Vary According to Sex and Dehydration Condition. Int. J. Mol. Sci. 2022, 23, 15672. https://doi.org/10.3390/ijms232415672
Cognuck SQ, Almeida LFd, Reis WL, Silva MS, Almeida-Pereira G, Zorro SV, Mecawi AS, Coimbra TM, Elias LLK, Antunes-Rodrigues J. Alterations in Kidney Structures Caused by Age Vary According to Sex and Dehydration Condition. International Journal of Molecular Sciences. 2022; 23(24):15672. https://doi.org/10.3390/ijms232415672
Chicago/Turabian StyleCognuck, Susana Quirós, Lucas Ferreira de Almeida, Wagner L. Reis, Márcia S. Silva, Gislaine Almeida-Pereira, Sandra V. Zorro, André S. Mecawi, Terezila Machado Coimbra, Lucila L. K. Elias, and José Antunes-Rodrigues. 2022. "Alterations in Kidney Structures Caused by Age Vary According to Sex and Dehydration Condition" International Journal of Molecular Sciences 23, no. 24: 15672. https://doi.org/10.3390/ijms232415672
APA StyleCognuck, S. Q., Almeida, L. F. d., Reis, W. L., Silva, M. S., Almeida-Pereira, G., Zorro, S. V., Mecawi, A. S., Coimbra, T. M., Elias, L. L. K., & Antunes-Rodrigues, J. (2022). Alterations in Kidney Structures Caused by Age Vary According to Sex and Dehydration Condition. International Journal of Molecular Sciences, 23(24), 15672. https://doi.org/10.3390/ijms232415672






