The Combination of Panobinostat and Melphalan for the Treatment of Patients with Multiple Myeloma
Abstract
1. Introduction
2. Results
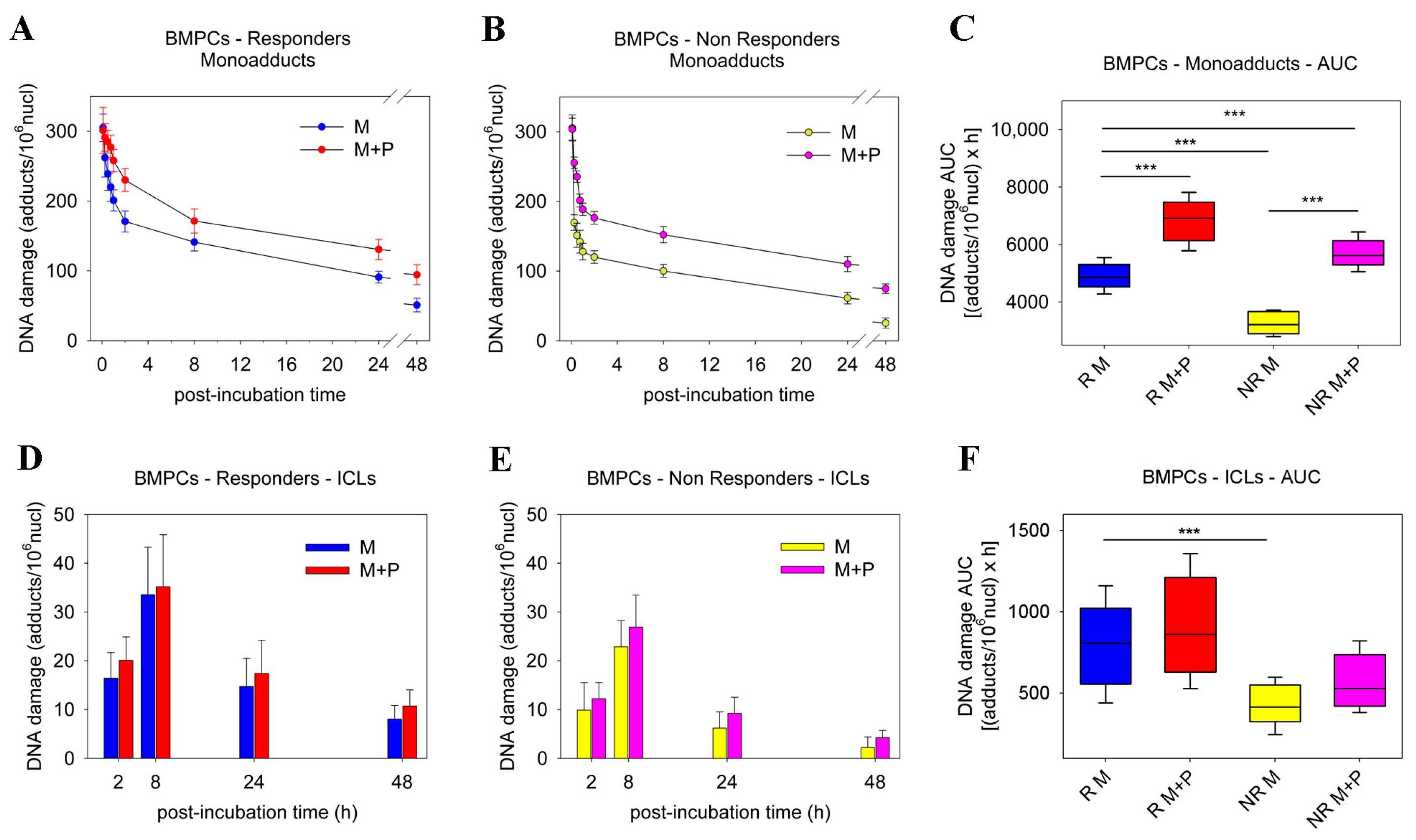
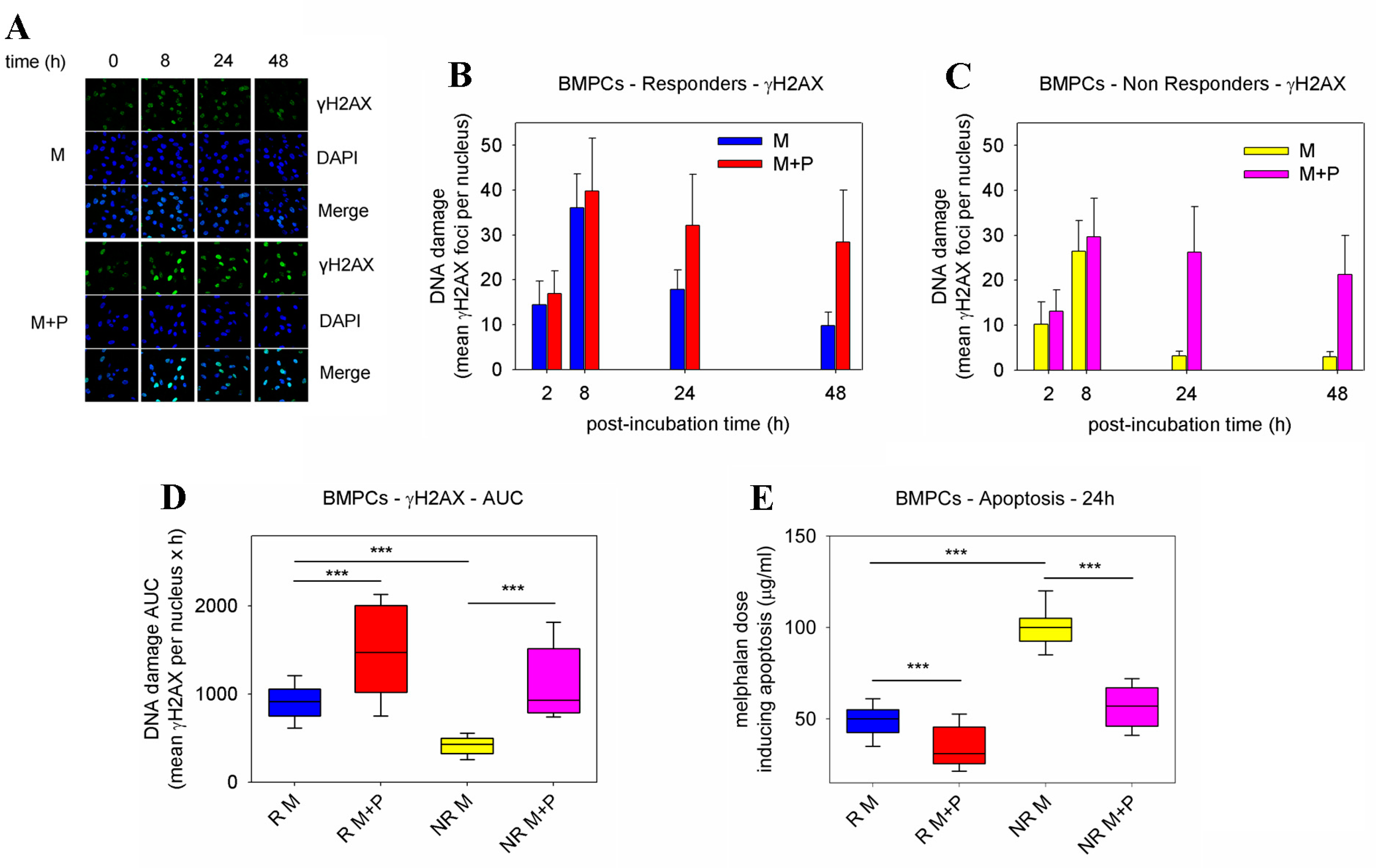
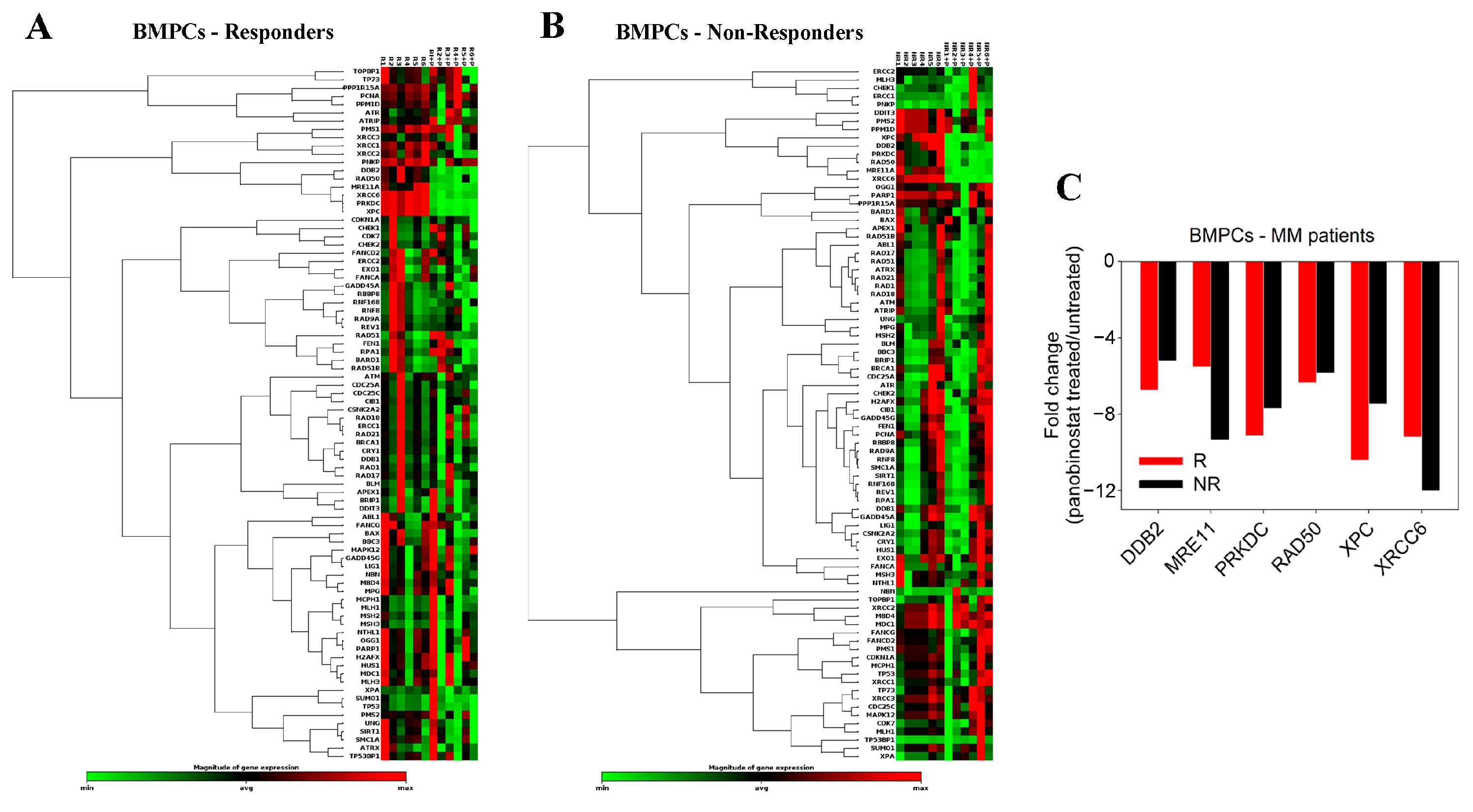
2.1. Pre-Treatment with Panobinostat Significantly Increased Melphalan Sensitivity of BMPCs
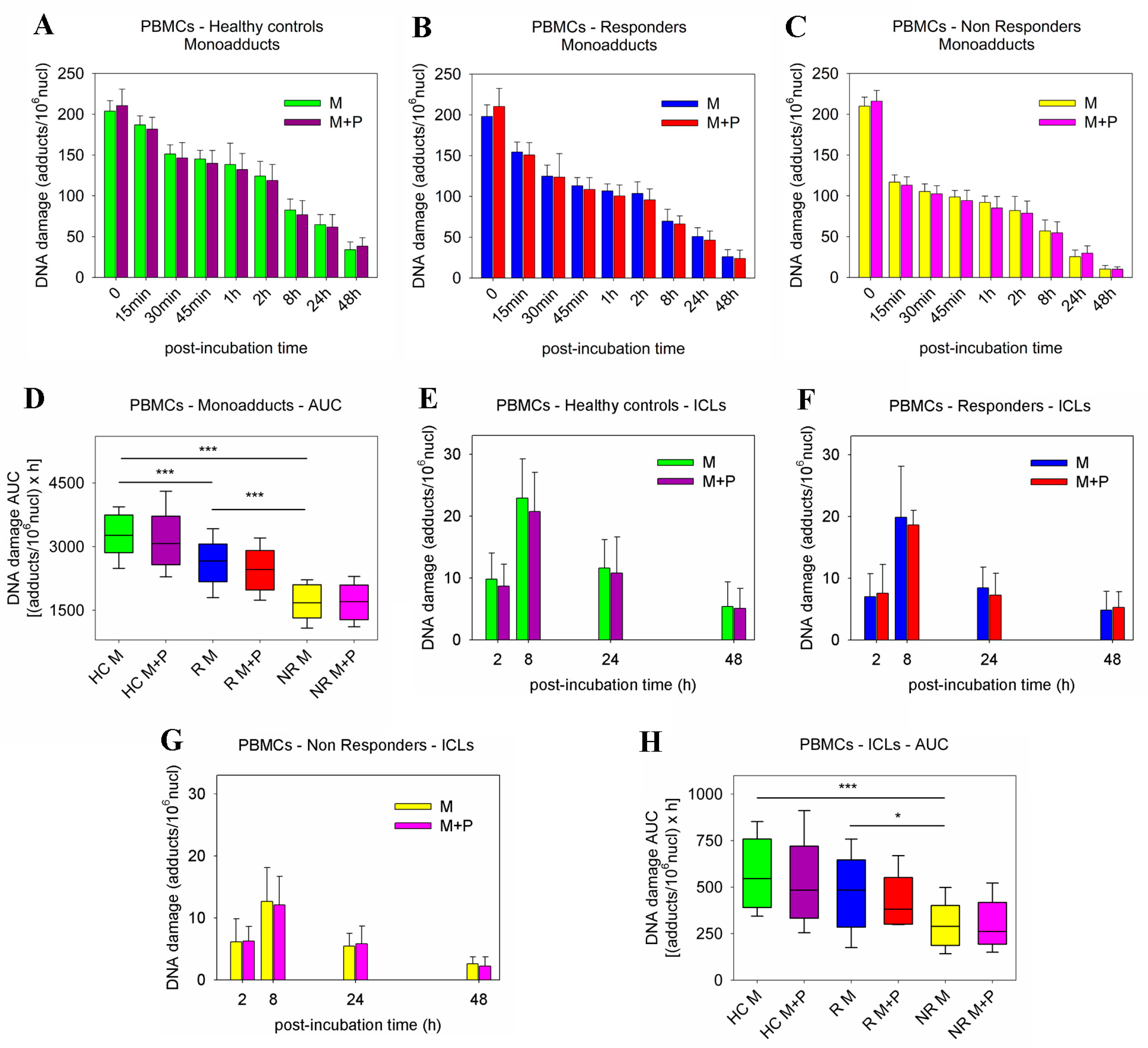
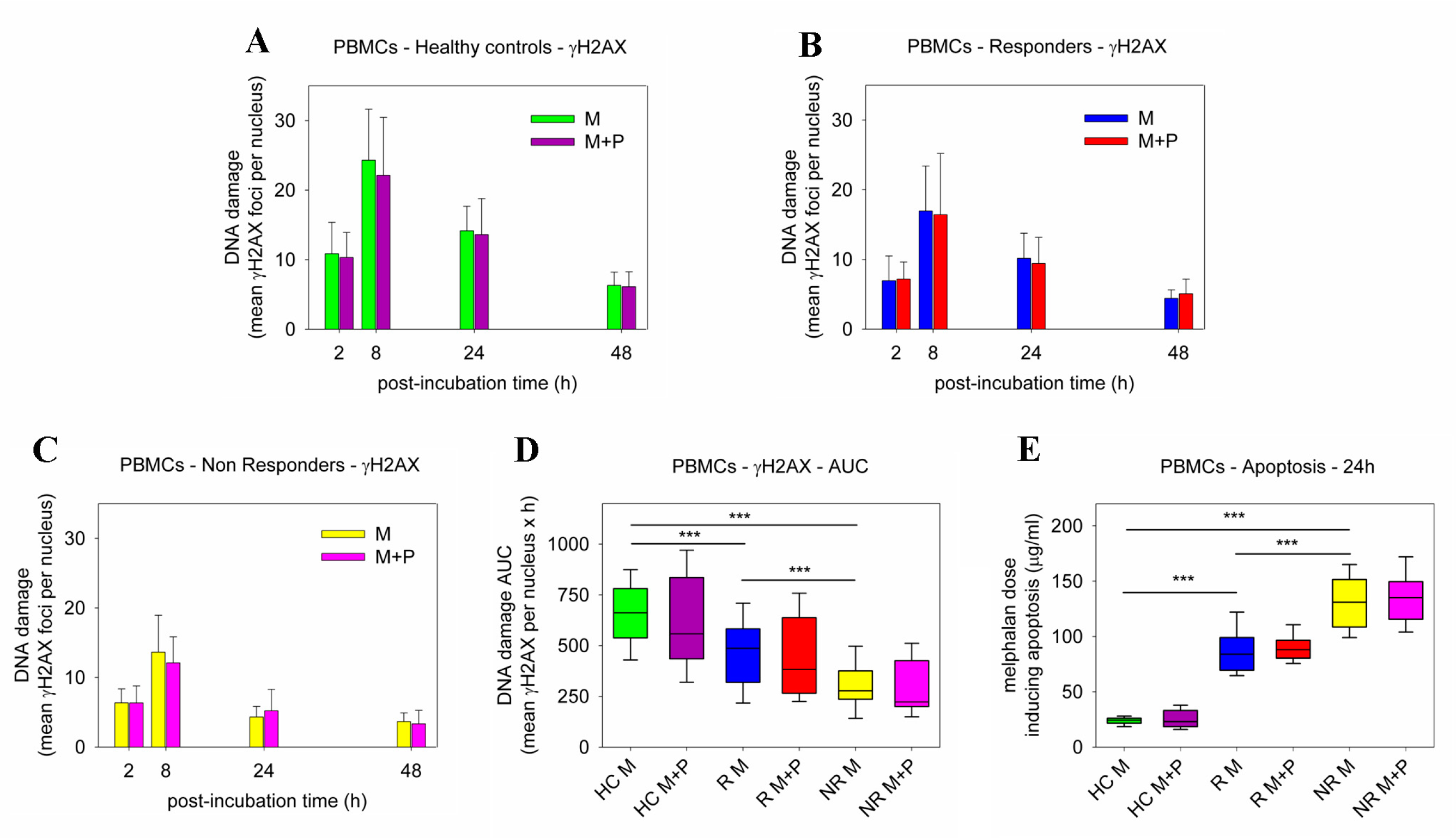
2.2. DDR Signals following Combined Treatment of PBMCs with Panobinostat and Melphalan
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Cell Treatment
4.3. Measurement of Gene-Specific Damage Repair
4.4. Measurement of γH2AX Foci
4.5. Apoptosis and Cell Viability Assay
4.6. Expression of DDR-Associated Genes
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; San-Miguel, J. Treatment of newly diagnosed myeloma in patients not eligible for transplantation. Curr. Hematol. Malig. Rep. 2011, 6, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Giralt, S. 200 mg/m2 melphalan-the gold standard for multiple myeloma. Nat. Rev. Clin. Oncol. 2010, 7, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Rajkumar, S.V.; Palumbo, A.; Moreau, P.; Orlowski, R.; Bladé, J.; Sezer, O.; Ludwig, H.; Dimopoulos, M.A.; Attal, M.; et al. International Myeloma Working Group. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood 2011, 117, 6063–6073. [Google Scholar] [CrossRef]
- Genadieva-Stavric, S.; Cavallo, F.; Palumbo, A. New approaches to management of multiple myeloma. Curr. Treat. Options Oncol. 2014, 15, 157–170. [Google Scholar] [CrossRef]
- Episkopou, H.; Kyrtopoulos, S.A.; Sfikakis, P.P.; Fousteri, M.; Dimopoulos, M.A.; Mullenders, L.H.; Souliotis, V.L. Association between transcriptional activity, local chromatin structure, and the efficiencies of both subpathways of nucleotide excision repair of melphalan adducts. Cancer Res. 2009, 69, 4424–4433. [Google Scholar] [CrossRef]
- Thompson, L.H.; Hinz, J.M. Cellular and molecular consequences of defective Fanconi anemia proteins in replication-coupled DNA repair: Mechanistic insights. Mutat. Res. 2009, 668, 54–72. [Google Scholar] [CrossRef]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef]
- Hanlon Newell, A.E.; Hemphill, A.; Akkari, Y.M.; Hejna, J.; Moses, R.E.; Olson, S.B. Loss of homologous recombination or non-homologous end-joining leads to radial formation following DNA interstrand crosslink damage. Cytogenet. Genome Res. 2008, 121, 174–180. [Google Scholar] [CrossRef]
- Helleday, T. Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis 2010, 31, 955–960. [Google Scholar] [CrossRef]
- Chakraborty, A.; Tapryal, N.; Venkova, T.; Horikoshi, N.; Pandita, R.K.; Sarker, A.H.; Sarkar, P.S.; Pandita, T.K.; Hazra, T.K. Classical non-homologous end-joining pathway utilizes nascent RNA for error-free double-strand break repair of transcribed genes. Nat. Commun. 2016, 7, 13049. [Google Scholar] [CrossRef] [PubMed]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Laubach, J.P.; Anderson, K.C.; Richardson, P.G. Investigational agents in immunotherapy: A new horizon for the treatment of multiple myeloma. Br. J. Haematol. 2018, 181, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Holstein, S.A.; Schlossman, R.L.; Anderson, K.C.; Attal, M.; McCarthy, P.L. Lenalidomide in combination or alone as maintenance therapy following autologous stem cell transplant in patients with multiple myeloma: A review of options for and against. Expert Opin. Pharmacother. 2017, 18, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [PubMed]
- Lakshmaiah, K.C.; Jacob, L.A.; Aparna, S.; Lokanatha, D.; Saldanha, S.C. Epigenetic therapy of cancer with histone d;acetylase inhibitors. J. Cancer Res. Ther. 2014, 10, 469–478. [Google Scholar]
- Raedler, L.A. Farydak (Panobinostat): First HDAC Inhibitor Approved for Patients with Relapsed Multiple Myeloma. Am. Health Drug Benefits 2016, 9, 84–87. [Google Scholar]
- Atadja, P. Development of the pan-DAC inhibitor panobinostat (LBH589): Successes and challenges. Cancer Lett. 2009, 280, 233–241. [Google Scholar] [CrossRef]
- Catley, L.; Weisberg, E.; Kiziltepe, T.; Tai, Y.T.; Hideshima, T.; Neri, P.; Tassone, P.; Atadja, P.; Chauhan, D.; Munshi, N.C.; et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood 2006, 108, 3441–3449. [Google Scholar] [CrossRef]
- Maiso, P.; Carvajal-Vergara, X.; Ocio, E.M.; López-Pérez, R.; Mateo, G.; Gutiérrez, N.; Atadja, P.; Pandiella, A.; San Miguel, J.F. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006, 66, 5781–5789. [Google Scholar] [CrossRef]
- Bruzzese, F.; Pucci, B.; Milone, M.R.; Ciardiello, C.; Franco, R.; Chianese, M.I.; Rocco, M.; Di Gennaro, E.; Leone, A.; Luciano, A.; et al. Panobinostat synergizes with zoledronic acid in prostate cancer and multiple myeloma models by increasing ROS and modulating mevalonate and p38-MAPK pathways. Cell Death Dis. 2013, 4, e878. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Shen, J.; Steinberg, J.; Li, M.; Wang, C.; Bonavida, B.; Chen, H.; Li, Z.W.; Berenson, J.R. The histone deacetylase inhibitor LBH589 enhances the anti-myeloma effects of chemotherapy in vitro and in vivo. Leuk. Res. 2011, 35, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Offidani, M.; Polloni, C.; Cavallo, F.; Liberati, A.M.; Ballanti, S.; Pulini, S.; Catarini, M.; Alesiani, F.; Corvatta, L.; Gentili, S.; et al. Phase II study of melphalan, thalidomide and prednisone combined with oral panobinostat in patients with relapsed/refractory multiple myeloma. Leuk. Lymphoma 2012, 53, 1722–1727. [Google Scholar] [CrossRef]
- Berenson, J.R.; Hilger, J.D.; Yellin, O.; Boccia, R.V.; Matous, J.; Dressler, K.; Ghazal, H.H.; Jamshed, S.; Kingsley, E.C.; Harb, W.A.; et al. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann. Hematol. 2014, 93, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Vieyra, C.V.; Berenson, J.R. The potential of panobinostat as a treatment option in patients with relapsed and refractory multiple myeloma. Ther. Adv. Hematol. 2014, 5, 197–210. [Google Scholar] [CrossRef]
- Teo, E.C.; Valdez, B.C.; Ji, J.; Li, Y.; Liu, Y.; Brammer, J.E.; Hosing, C.; Nieto, Y.; Champlin, R.E.; Andersson, B.S. Synergistic cytotoxicity of busulfan, melphalan, gemcitabine, panobinostat, and bortezomib in lymphoma cells. Leuk. Lymphoma 2016, 57, 2644–2652. [Google Scholar] [CrossRef]
- Offidani, M.; Corvatta, L.; Liberati, A.M.; Pulini, S.; Ballanti, S.; Bringhen, S. Updated results of a phase 2 study of panobinostat combined with melphalan, thalidomide and prednisone (MPT) in relapsed/refractory multiple myeloma. Leuk. Lymphoma 2018, 59, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Souliotis, V.L.; Dimopoulos, M.A.; Episkopou, H.G.; Kyrtopoulos, S.A.; Sfikakis, P.P. Preferential in vivo DNA repair of melphalan-induced damage in human genes is greatly affected by the local chromatin structure. DNA Repair 2006, 5, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Gkotzamanidou, M.; Terpos, E.; Bamia, C.; Munshi, N.C.; Dimopoulos, M.A.; Souliotis, V.L. DNA repair of myeloma plasma cells correlates with clinical outcome: The effect of the nonhomologous end-joining inhibitor SCR7. Blood 2016, 128, 1214–1225. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Souliotis, V.L.; Anagnostopoulos, A.; Papadimitriou, C.; Sfikakis, P.P. Extent of damage and repair in the p53 tumor-suppressor gene after treatment of myeloma patients with high-dose melphalan and autologous blood stem-cell transplantation is individualized and may predict clinical outcome. J. Clin. Oncol. 2005, 23, 4381–4389. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Souliotis, V.L.; Anagnostopoulos, A.; Bamia, C.; Pouli, A.; Baltadakis, I.; Terpos, E.; Kyrtopoulos, S.A.; Sfikakis, P.P. Melphalan-induced DNA damage in vitro as a predictor for clinical outcome in multiple myeloma. Haematologica 2007, 92, 1505–1512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoyanova, T.; Roy, N.; Kopanja, D.; Raychaudhuri, P.; Bagchi, S. DDB2 (damaged-DNA binding protein 2) in nucleotide excision repair and DNA damage response. Cell Cycle 2009, 8, 4067–4071. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Hsiao, Y.P.; Lu, C.T.; Huang, C.H.; Chao, W.R.; Lin, Y.T.; Su, H.A.; Chang, S.L.; Chung, J.G. Xeroderma pigmentosum complementation group C protein (XPC) expression in basal cell carcinoma. In Vivo 2015, 29, 35–38. [Google Scholar] [PubMed]
- Stracker, T.H.; Petrini, J.H. The MRE11 complex: Starting from the ends. Nat. Rev. Mol. Cell Biol. 2011, 12, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Matsumoto, Y.; Okumoto, M.; Suzuki, N.; Yamate, J. Variations in Prkdc encoding the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) and susceptibility to radiation-induced apoptosis and lymphomagenesis. Oncogene 2001, 20, 3609–3619. [Google Scholar] [CrossRef]
- Heikkinen, K.; Rapakko, K.; Karppinen, S.M.; Erkko, H.; Knuutila, S.; Lundán, T.; Mannermaa, A.; Børresen-Dale, A.L.; Borg, A.; Barkardottir, R.B.; et al. RAD50 and NBS1 are breast cancer susceptibility genes associated with genomic instability. Carcinogenesis 2006, 27, 1593–1599. [Google Scholar] [CrossRef]
- Li, R.; Yang, Y.; An, Y.; Zhou, Y.; Liu, Y.; Yu, Q.; Lu, D.; Wang, H.; Jin, L.; Zhou, W.; et al. Genetic polymorphisms in DNA double-strand break repair genes XRCC5, XRCC6 and susceptibility to hepatocellular carcinoma. Carcinogenesis 2011, 32, 530–536. [Google Scholar] [CrossRef]
- Lee, J.H.; Choy, M.L.; Ngo, L.; Foster, S.S.; Marks, P.A. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc. Natl. Acad. Sci. USA 2010, 107, 14639–14644. [Google Scholar] [CrossRef]
- Munshi, A.; Tanaka, T.; Hobbs, M.L.; Tucker, S.L.; Richon, V.M.; Meyn, R.E. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of γH2AX foci. Mol. Cancer Ther. 2006, 5, 1967–1974. [Google Scholar] [CrossRef]
- Munshi, A.; Kurland, J.F.; Nishikawa, T.; Tanaka, T.; Hobbs, M.L.; Tucker, S.L.; Ismail, S.; Stevens, C.; Meyn, R.E. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin. Cancer Res. 2005, 11, 4912–4922. [Google Scholar] [CrossRef]
- Rosato, R.R.; Almenara, J.A.; Maggio, S.C.; Coe, S.; Atadja, P.; Dent, P.; Grant, S. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol. Cancer Ther. 2008, 7, 3285–3297. [Google Scholar] [CrossRef]
- Xiao, W.; Graham, P.H.; Hao, J.; Chang, L.; Ni, J.; Power, C.A.; Dong, Q.; Kearsley, J.H.; Li, Y. Combination therapy with the histone deacetylase inhibitor LBH589 and radiation is an effective regimen for prostate cancer cells. PLoS ONE 2013, 8, e74253. [Google Scholar] [CrossRef]
- Shen, J.; Huang, C.; Jiang, L.; Gao, F.; Wang, Z.; Zhang, Y.; Bai, J.; Zhou, H.; Chen, Q. Enhancement of cisplatin induced apoptosis by suberoylanilidehydroxamic acid in human oral squamous cell carcinoma cell lines. Biochem. Pharmacol. 2007, 73, 1901–1909. [Google Scholar] [CrossRef]
- Falck, J.; Coates, J.; Jackson, S.P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005, 434, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Fandy, T.E.; Herman, J.G.; Kerns, P.; Jiemjit, A.; Sugar, E.A.; Choi, S.H.; Yang, A.S.; Aucott, T.; Dauses, T.; Odchimar-Reissig, R.; et al. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood 2009, 114, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Fiskus, W.; Pranpat, M.; Balasis, M.; Bali, P.; Estrella, V.; Kumaraswamy, S.; Rao, R.; Rocha, K.; Herger, B.; Lee, F.; et al. Cotreatment with vorinostat (suberoylanilidehydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinibmesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells. Clin. Cancer Res. 2006, 12, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Dai, Y.; Grant, S. Histone deacetylase inhibitor (HDACI) mechanisms of action: Emerging insights. Pharmacol. Ther. 2014, 143, 323–336. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Gkotzamanidou, M.; Terpos, E.; Bamia, C.; Kyrtopoulos, S.A.; Sfikakis, P.P.; Dimopoulos, M.A.; Souliotis, V.L. Progressive changes in chromatin structure and DNA damage response signals in bone marrow and peripheral blood during myelomagenesis. Leukemia 2014, 28, 1113–1121. [Google Scholar] [CrossRef]
- Souliotis, V.L.; Vougas, K.; Gorgoulis, V.G.; Sfikakis, P.P. Defective DNA repair and chromatin organization in patients with quiescent systemic lupus erythematosus. Arthritis Res. Ther. 2016, 18, 182. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Patient | |||
|---|---|---|---|---|
| No. | Age (years) | % of Total | ||
| Sex | Female | 12 | 46.2 | |
| Male | 14 | 53.8 | ||
| Age | Median | 60 | ||
| Range | 42–66 | |||
| Ig subtype | IgG | 12 | 46.2 | |
| IgA | 9 | 34.8 | ||
| IgM | 0 | 0 | ||
| IgE | 0 | 0 | ||
| FLCs | 5 | 19.2 | ||
| Non-secretory | 0 | 0 | ||
| ISS stage | I | 5 | 19.2 | |
| II | 8 | 30.8 | ||
| III | 13 | 50.0 | ||
| Response to HDM | Responders | 17 | 65.4 | |
| Non-Responders | 9 | 34.6 | ||
| High-risk cytogenetics * | Responders | 6 | 35.3 | |
| Non-Responders | 4 | 44.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkotzamanidou, M.; Terpos, E.; Dimopoulos, M.A.; Souliotis, V.L. The Combination of Panobinostat and Melphalan for the Treatment of Patients with Multiple Myeloma. Int. J. Mol. Sci. 2022, 23, 15671. https://doi.org/10.3390/ijms232415671
Gkotzamanidou M, Terpos E, Dimopoulos MA, Souliotis VL. The Combination of Panobinostat and Melphalan for the Treatment of Patients with Multiple Myeloma. International Journal of Molecular Sciences. 2022; 23(24):15671. https://doi.org/10.3390/ijms232415671
Chicago/Turabian StyleGkotzamanidou, Maria, Evangelos Terpos, Meletios A. Dimopoulos, and Vassilis L. Souliotis. 2022. "The Combination of Panobinostat and Melphalan for the Treatment of Patients with Multiple Myeloma" International Journal of Molecular Sciences 23, no. 24: 15671. https://doi.org/10.3390/ijms232415671
APA StyleGkotzamanidou, M., Terpos, E., Dimopoulos, M. A., & Souliotis, V. L. (2022). The Combination of Panobinostat and Melphalan for the Treatment of Patients with Multiple Myeloma. International Journal of Molecular Sciences, 23(24), 15671. https://doi.org/10.3390/ijms232415671








