The Utility of Novel Kidney Injury Biomarkers in Early Detection of CSA-AKI
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- Qualification for an elective cardiac surgery procedure with the use of CPB;
- Written consent for study enrolment.
- End-stage renal disease (eGFR < 15 mL/min/1.73 m2);
- Kidney artery stenosis in medical history;
- Active inflammatory disease;
- Active neoplasm;
- Mayor intraoperative complications causing hypotension;
- Mayor postoperative complications causing hypotension;
- Need for continuous renal replacement therapy (CRRT) within 6 h after the procedure.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Karim, H.; Yunus, M.; Saikia, M.; Kalita, J.; Mandal, M. Incidence and Progression of Cardiac Surgery-Associated Acute Kidney Injury and Its Relationship with Bypass and Cross Clamp Time. Ann. Card. Anaesth. 2017, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Schurle, A.; Koyner, J.L. CSA-AKI: Incidence, Epidemiology, Clinical Outcomes, and Economic Impact. J. Clin. Med. 2021, 10, 5746. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.W.; Endre, Z.H.; John, A.; Pickering, W. The Definition and Detection of Acute Kidney Injury. J. Ren. Inj. Prev. 2014, 3, 21–2521. [Google Scholar] [CrossRef]
- Walther, C.P.; Podoll, A.S.; Finkel, K.W. Summary of Clinical Practice Guidelines for Acute Kidney Injury. Hosp. Pract. 2014, 42, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef] [PubMed]
- Gavrić, A.; Kališnik, J.M. Novel Biomarkers for Early Diagnosis of Acute Kidney Injury after Cardiac Surgery in Adults. Kardiochir. Torakochir. Pol. 2016, 13, 31–38. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Z.; Hu, Y.; Sheng, Y.; Li, Y.; Song, J. Novel Potential Biomarker of Adult Cardiac Surgery-Associated Acute Kidney Injury. Front. Physiol. 2020, 11, 587204. [Google Scholar] [CrossRef]
- Udzik, J.; Waszczyk, A.; Safranow, K.; Biskupski, A.; Majer, K.; Kwiatkowski, S.; Kwiatkowska, E. Assessment and Prognosis in Csa-Aki Using Novel Kidney Injury Biomarkers: A Prospective Observational Study. Biology 2021, 10, 823. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Kalantari, H.; Rezaei, A.; Maracy, M.R.; Salehi, M.; Bahador, A.; Hassannejad, N.; Narimani, M.; Sanei, M.H.; Bayat, B.; et al. Clinical Study Immune-Mediated Cochleovestibular Disease. Bratisl. Lek. List. 2015, 116, 296–301. [Google Scholar] [CrossRef]
- Kalisnik, J.M.; Steblovnik, K.; Hrovat, E.; Jerin, A.; Skitek, M. Enhanced Detection of Cardiac Surgery-Associated Acute Kidney Injury by a Composite Biomarker Panel in Patients with Normal Preoperative Kidney Function. J. Cardiovasc. Dev. Dis. 2022, 9, 210. [Google Scholar] [CrossRef]
- Elmedany, S.M.; Naga, S.S.; Elsharkawy, R.; Mahrous, R.S.; Elnaggar, A.I. Novel Urinary Biomarkers and the Early Detection of Acute Kidney Injury after Open Cardiac Surgeries. J. Crit. Care 2017, 40, 171–177. [Google Scholar] [CrossRef]
- Parikh, C.R.; Thiessen-Philbrook, H.; Garg, A.X.; Kadiyala, D.; Shlipak, M.G.; Koyner, J.L.; Edelstein, C.L.; Devarajan, P.; Patel, U.D.; Zappitelli, M.; et al. Performance of Kidney Injury Molecule-1 and Liver Fatty Acid-Binding Protein and Combined Biomarkers of Aki after Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2013, 8, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Mishra, J.; Thiessen-Philbrook, H.; Dursun, B.; Ma, Q.; Kelly, C.; Dent, C.; Devarajan, P.; Edelstein, C.L. Urinary IL-18 Is an Early Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery. Kidney Int. 2006, 70, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Krawczeski, C.D.; Goldstein, S.L.; Woo, J.G.; Wang, Y.; Piyaphanee, N.; Ma, Q.; Bennett, M.; Devarajan, P. Temporal Relationship and Predictive Value of Urinary Acute Kidney Injury Biomarkers after Pediatric Cardiopulmonary Bypass. J. Am. Coll. Cardiol. 2011, 58, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- De Fontnouvelle, C.A.; Greenberg, J.H.; Thiessen-Philbrook, H.R.; Zappitelli, M.; Roth, J.; Kerr, K.F.; Devarajan, P.; Shlipak, M.; Coca, S.; Parikh, C.R.; et al. Interleukin-8 and Tumor Necrosis Factor Predict Acute Kidney Injury After Pediatric Cardiac Surgery. Ann. Thorac. Surg. 2017, 104, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.D.; Altmann, C.; Smits, G.; Krawczeski, C.D.; Edelstein, C.L.; Devarajan, P.; Faubel, S. Serum Interleukin-6 and Interleukin-8 Are Early Biomarkers of Acute Kidney Injury and Predict Prolonged Mechanical Ventilation in Children Undergoing Cardiac Surgery: A Case-Control Study. Crit. Care 2009, 13, R104. [Google Scholar] [CrossRef]
- Koyner, J.L.; Garg, A.X.; Coca, S.G.; Sint, K.; Thiessen-Philbrook, H.; Patel, U.D.; Shlipak, M.G.; Parikh, C.R. Biomarkers Predict Progression of Acute Kidney Injury after Cardiac Surgery. J. Am. Soc. Nephrol. 2012, 23, 905–914. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Signalling in Health and Disease. F1000Research 2020, 9, 1013. [Google Scholar] [CrossRef]
- Rose-John, S. Il-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Proinflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, J.; Yin, S.; Li, X.; Zhao, S.; Mou, T.; Luo, S. The Association between Interleukin-8 Gene-251 A/T Polymorphism and Sepsis A Protocol for Systematic Review and Meta Analysis. Medicine 2021, 100, e25483. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.K.; Lan, H.Y. Chemokines in Renal Injury. J. Am. Soc. Nephrol. 2011, 22, 802–809. [Google Scholar] [CrossRef]
- Wang, M.; Weng, X.; Chen, H.; Chen, Z.; Liu, X. Resveratrol Inhibits Tnf-α-Induced Inflammation to Protect against Renal Ischemia/Reperfusion Injury in Diabetic Rats1. Acta Cir. Bras. 2020, 35, e202000506. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari Godarzi, S.; Valizade Gorji, A.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant Effect of P-Coumaric Acid on Interleukin 1-β and Tumor Necrosis Factor-α in Rats with Renal Ischemic Reperfusion. Nefrología 2020, 40, 311–319. [Google Scholar] [CrossRef]
- Szumilas, D.; Wojnar, J.; Chudek, J. Lipokalina Zwiazana z Zelatynaza Neutrofilów Jako Marker Ostrego Uszkodzenia Nerek u Chorych Onkologicznych Leczonych Cisplatyna. Nowotwory 2016, 66, 160–166. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, K.; Nie, Z.; Li, Q.; Jin, F. Kidney Injury Molecule-1 (KIM-1): A Novel Kidney-Specific Injury Molecule Playing Potential Double-Edged Functions in Kidney Injury. Transplant. Rev. 2010, 24, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.A.; Engström, G.; Nilsson, J.; Almgren, P.; Petkovic, M.; Christensson, A.; Nilsson, P.M.; Melander, O.; Orho-Melander, M. Plasma Kidney Injury Molecule-1 (p-KIM-1) Levels and Deterioration of Kidney Function over 16 Years. Nephrol. Dial. Transplant. 2020, 35, 265–273. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Zhao, X.; Dong, G.; Li, C. Diagnostic and Prognostic Value of Neutrophil Gelatinase-Associated Lipocalin, Matrix Metalloproteinase-9, and Tissue Inhibitor of Matrix Metalloproteinases-1 for Sepsis in the Emergency Department: An Observational Study. Crit. Care 2014, 18, 634. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, C.L.; Orozco-Ortega, R.A. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Chronic Kidney Disease and Acute Kidney Injury: A Systematic Review of the Literature. Hippokratia 2018, 22, 99–104. [Google Scholar] [PubMed]
- Zhang, Z.; Humphreys, B.D.; Bonventre, J.V. Shedding of the Urinary Biomarker Kidney Injury Molecule-1 (KIM-1) Is Regulated by MAP Kinases and Juxtamembrane Region. J. Am. Soc. Nephrol. 2007, 18, 2704–2714. [Google Scholar] [CrossRef]
- Franke, E.I.; Vanderbrink, B.A.; Hile, K.L.; Zhang, H.; Cain, A.; Matsui, F.; Meldrum, K.K. Renal IL-18 Production Is Macrophage Independent during Obstructive Injury. PLoS ONE 2012, 7, e47417. [Google Scholar] [CrossRef]
- Vanderbrink, B.A.; Asanuma, H.; Hile, K.; Zhang, H.; Rink, R.C.; Meldrum, K.K. Interleukin-18 Stimulates a Positive Feedback Loop during Renal Obstruction via Interleukin-18 Receptor. J. Urol. 2011, 186, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Colston, J.T.; De La Rosa, S.D.; Rao, P.P.; Freeman, G.L. TNF-α and H2O2 Induce IL-18 and IL-18Rβ Expression in Cardiomyocytes via NF-ΚB Activation. Biochem. Biophys. Res. Commun. 2003, 303, 1152–1158. [Google Scholar] [CrossRef]
- Parikh, C.R.; Jani, A.; Melnikov, V.Y.; Faubel, S.; Edelstein, C.L. Urinary Interleukin-18 Is a Marker of Human Acute Tubular Necrosis. Am. J. Kidney Dis. 2004, 43, 405–414. [Google Scholar] [CrossRef]
- Parikh, C.R.; Jani, A.; Mishra, J.; Ma, Q.; Kelly, C.; Barasch, J.; Edelstein, C.L.; Devarajan, P. Urine NGAL and IL-18 Are Predictive Biomarkers for Delayed Graft Function Following Kidney Transplantation. Am. J. Transplant. 2006, 6, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Topete, L.M.; Rosner, M.H.; Ronco, C. Acute Kidney Injury Risk Assessment and the Nephrology Rapid Response Team. Blood Purif. 2017, 43, 82–88. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.T.; Kurth, M.J.; McLean, G.; Domanska, A.; Lamont, J.V.; Maguire, D.; Watt, J.; Fitzgerald, P.; Young, I.; Joseph, J.; et al. Stratifying Risk of Acute Kidney Injury in Pre and Post Cardiac Surgery Patients Using a Novel Biomarker-Based Algorithm and Clinical Risk Score. Sci. Rep. 2019, 9, 16963. [Google Scholar] [CrossRef]
- Mariscalco, G.; Lorusso, R.; Dominici, C.; Renzulli, A.; Sala, A. Acute Kidney Injury: A Relevant Complication after Cardiac Surgery. Ann. Thorac. Surg. 2011, 92, 1539–1547. [Google Scholar] [CrossRef]
- Mao, H.; Katz, N.; Ariyanon, W.; Blanca-Martos, L.; Adýbelli, Z.; Giuliani, A.; Danesi, T.H.; Kim, J.C.; Nayak, A.; Neri, M.; et al. Cardiac Surgery-Associated Acute Kidney Injury. CardioRenal Med. 2013, 3, 178–199. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Fonseca, J.A.; Outerelo, C.; Lopes, J.A. Acute Kidney Injury: From Diagnosis to Prevention and Treatment Strategies. J. Clin. Med. 2020, 9, 1704. [Google Scholar] [CrossRef]
- Spence, J.; LeManach, Y.; Chan, M.T.V.; Wang, C.Y.; Sigamani, A.; Xavier, D.; Pearse, R.; Alonso-Coello, P.; Garutti, I.; Srinathan, S.K.; et al. Association between Complications and Death within 30 Days after Noncardiac Surgery. Cmaj 2019, 191, E830–E837. [Google Scholar] [CrossRef]
- Chan, P.G.; Seese, L.; Aranda-Michel, E.; Sultan, I.; Gleason, T.G.; Wang, Y.; Thoma, F.; Kilic, A. Operative Mortality in Adult Cardiac Surgery: Is the Currently Utilized Definition Justified? J. Thorac. Dis. 2021, 13, 5582–5591. [Google Scholar] [CrossRef]
- Lin, X.; Yuan, J.; Zhao, Y.; Zha, Y. Urine Interleukin-18 in Prediction of Acute Kidney Injury: A Systemic Review and Meta-Analysis. J. Nephrol. 2015, 28, 7–16. [Google Scholar] [CrossRef]
- Arthur, J.M.; Hill, E.G.; Alge, J.L.; Lewis, E.C.; Neely, B.A.; Janech, M.G.; Tumlin, J.A.; Chawla, L.S.; Shaw, A.D. Evaluation of 32 Urine Biomarkers to Predict the Progression of Acute Kidney Injury after Cardiac Surgery. Kidney Int. 2014, 85, 431–438. [Google Scholar] [CrossRef]
- Shao, X.; Tian, L.; Xu, W.; Zhang, Z.; Wang, C.; Qi, C.; Ni, Z.; Mou, S. Diagnostic Value of Urinary Kidney Injury Molecule 1 for Acute Kidney Injury: A Meta-Analysis. PLoS ONE 2014, 9, e84131. [Google Scholar] [CrossRef] [PubMed]
- Haase-Fielitz, A.; Haase, M.; Devarajan, P. Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Acute Kidney Injury: A Critical Evaluation of Current Status. Ann. Clin. Biochem. 2014, 51, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Wagener, G.; Jan, M.; Kim, M.; Mori, K.; Barasch, J.M.; Sladen, R.N.; Lee, H.T. Association between increases in urinary neutrophil gelatinase–associated lipocalin and acute renal dysfunction after adult cardiac surgery. J. Am. Soc. Anesthesiol. 2006, 105, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Paper, O. Clinical Usefulness of Novel Biomarkers for the Detection of Acute Kidney Injury. Nephron Clin. Pract. 2010, 115, c66–c72. [Google Scholar] [CrossRef]
- Heise, D.; Rentsch, K.; Braeuer, A.; Friedrich, M.; Quintel, M. Comparison of Urinary Neutrophil Glucosaminidase-Associated Lipocalin, Cystatin C, and a 1-Microglobulin for Early Detection of Acute Renal Injury after Cardiac Surgery. Eur. J. Cardio-Thorac. Surg. 2011, 39, 38–43. [Google Scholar] [CrossRef]
- Zhou, F.; Luo, Q.; Wang, L.; Han, L. Diagnostic Value of Neutrophil Gelatinase-Associated Lipocalin for Early Diagnosis of Cardiac Surgery-Associated Acute Kidney Injury: A Meta-Analysis. Eur. J. Cardio-Thorac. Surg. 2016, 49, 746–755. [Google Scholar] [CrossRef]
- Goldman, J.; Becker, M.L.; Jones, B.; Clements, M.; Leeder, J.S. Development of Biomarkers to Optimize Pediatric Patient Management: What Makes Children Different? Biomark. Med. 2011, 5, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Yang, S.Y.; Ting, T.; Chiou, Y.; Shiao, C.C.; Wu, C.H. Comparative Accuracy of Biomarkers for the Prediction of Hospital-Acquired Acute Kidney Injury: A Systematic Review and Meta-Analysis. Crit. Care 2022, 26, 349. [Google Scholar] [CrossRef] [PubMed]
- Cody, E.M.; Bennett, M.R.; Gulati, G.; Ma, Q.; Altaye, M.; Devarajan, P.; Brunner, H.I. Successful Urine Multiplex Bead Assay to Measure Lupus Nephritis Activity. Kidney Int. Rep. 2021, 6, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
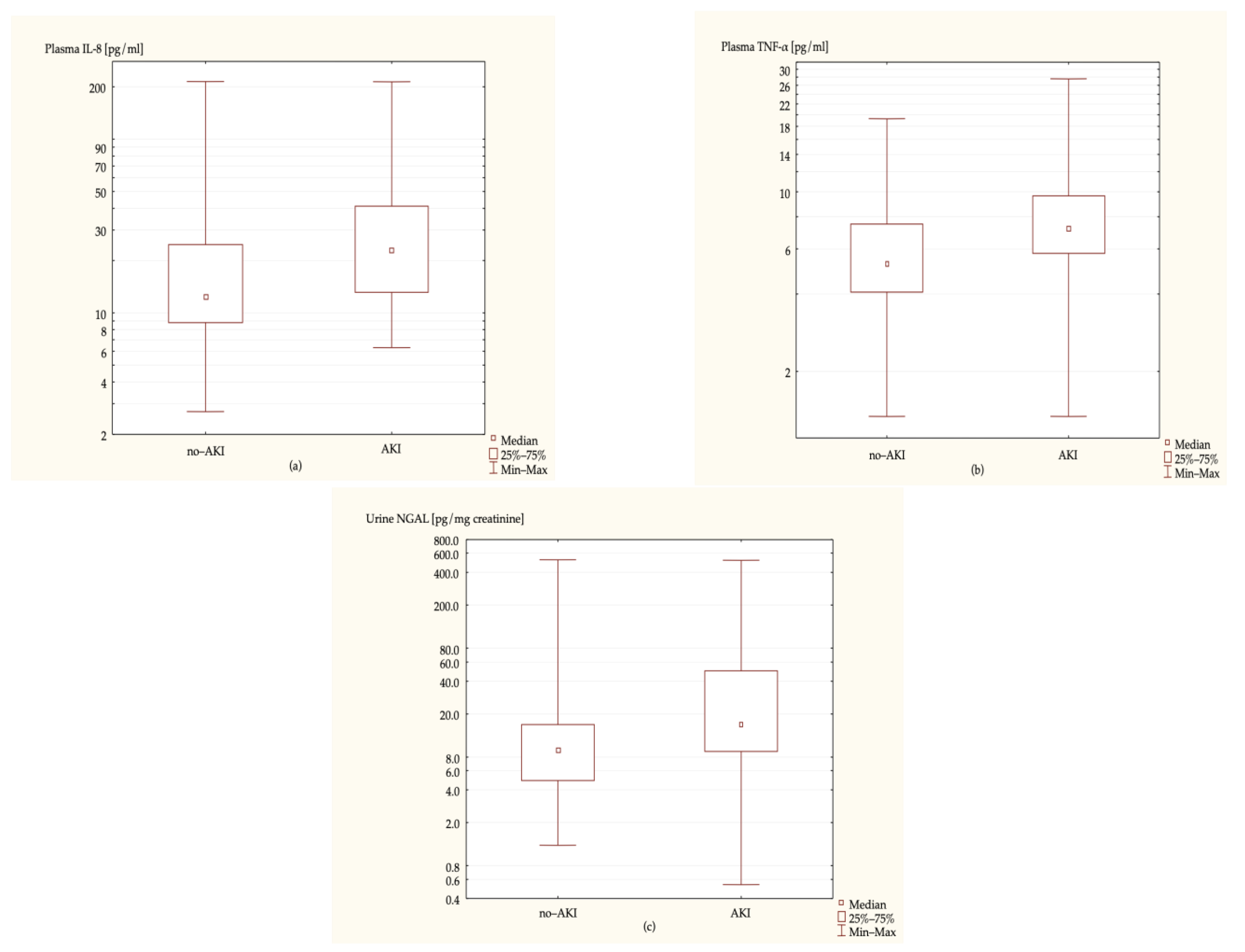
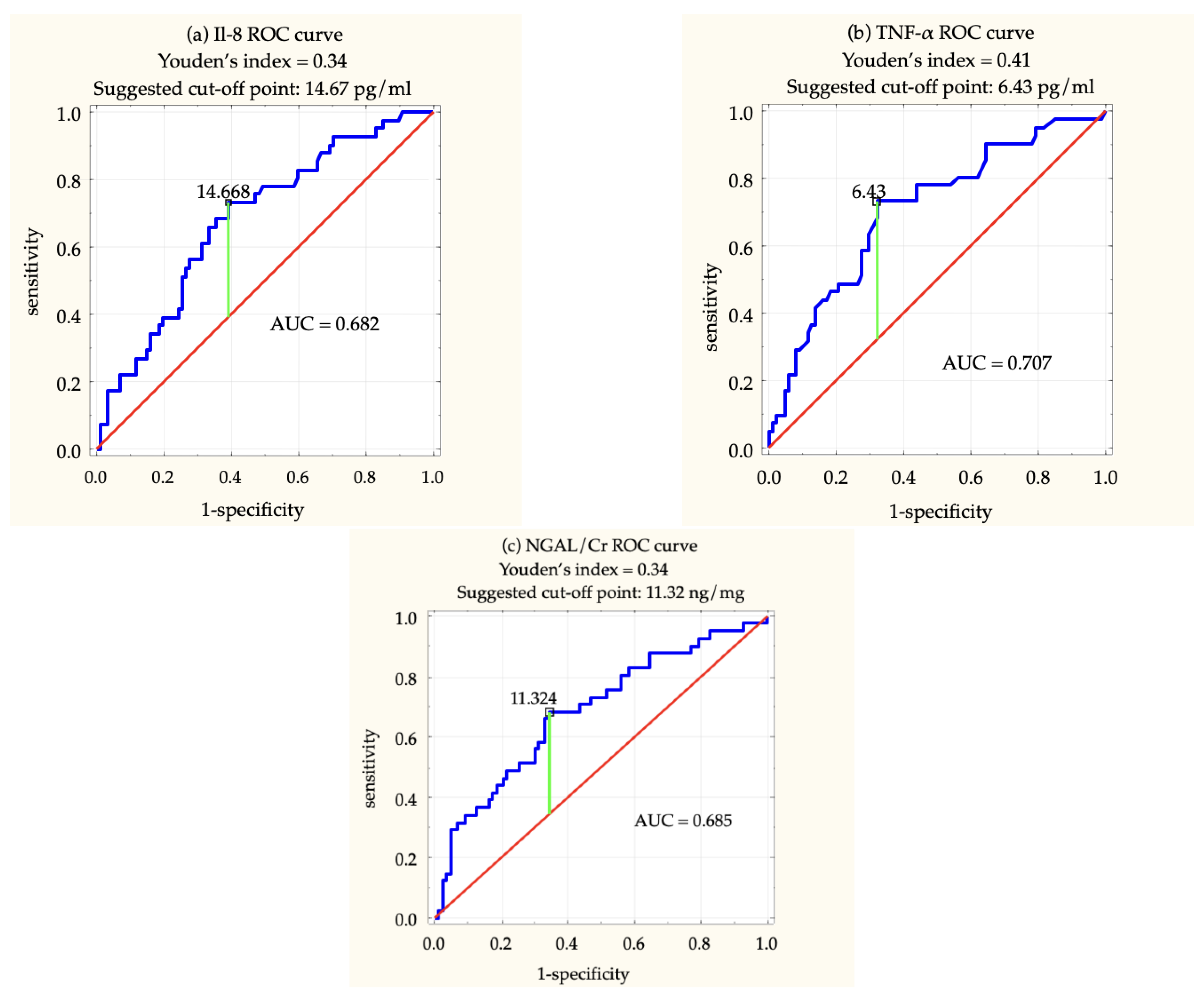
| AKI (n = 41) | no-AKI (n = 87) | p-Value | |
|---|---|---|---|
| Age M (Q1–Q3) (years) | 70 (67–75) | 67 (61–72) | 0.013 |
| Male n (%) | 25 (60.98) | 65 (74.71) | 0.147 |
| Female n (%) | 16 (39.02) | 22 (25.29) | |
| BMI M (Q1–Q3) | 27.85 (25.91–30.70) | 28.09 (25.53–31.10) | 0.815 |
| ES II M (Q1–Q3) | 3.81 (2.10–5.42) | 2.92 (1.70–4.77) | 0.267 |
| Hypertension n (%) | 35 (85.37) | 67 (77.01) | 0.349 |
| Diabetes n (%) | 15 (36.59) | 27 (31.03) | 0.550 |
| Dyslipidemia n (%) | 19 (46.34) | 38 (43.68) | 0.850 |
| CKD n (%) | 15 (36.59) | 7 (8.05) | <0.001 |
| Preoperative hematocrit (Q1–Q3) (%) | 39.50 (37.35–42.80) | 41.40 (39.30–43.20) | 0.045 |
| HbA1C M (Q1–Q3) [%] | 5.90 (5.70–6.70) | 5.90 (5.50–6.30) | 0.344 |
| Preoperative creatinine (Q1–Q3) (mg/dl) | 1.02 (0.89–1.26) | 0.89 (0.76–1.01) | 0.002 |
| Preoperative eGFR Q1–Q3) (mL/min/1.73 m2) | 65 (55–81) | 82 (71–93) | <0.001 |
| R | p-Value | |
|---|---|---|
| Age and TNF-α | 0.234 | 0.008 |
| Age and NGAL/Cr | 0.238 | 0.007 |
| Preoperative eGFR and IL-8 | −0.190 | 0.031 |
| Preoperative eGFR and TNF-α | −0.373 | <0.001 |
| Preoperative eGFR and NGAL/Cr | −0.226 | 0.010 |
| Mean HtCPB and IL-8 | −0.251 | 0.004 |
| (a) Logistic Regression Model Involving Age, Sex, Preoperative CKD, Plasma TNF-ɑ and Urine NGAL/Cr | |||
|---|---|---|---|
| OR | CI | p-Value | |
| Age | 1.03 | 0.97–1.09 | 0.377 |
| Sex | 0.94 | 0.34–2.59 | 0.910 |
| Preoperative CKD | 3.64 | 1.19–11.16 | 0.022 |
| Log TNF-ɑ | 3.19 | 1.20–8.49 | 0.019 |
| Log NGAL/Cr | 1.68 | 1.15–2.47 | 0.007 |
| (b) Logistic Regression Model Involving Age, Sex, Preoperative CKD, Plasma IL-8 and Urine NGAL/Cr | |||
| OR | CI | p-Value | |
| Age | 1.04 | 0.98–1.10 | 0.230 |
| Sex | 1.16 | 0.42–3.23 | 0.774 |
| Preoperative CKD | 5.03 | 1.71–14.78 | 0.003 |
| Log IL-8 | 1.88 | 1.07–3.30 | 0.026 |
| Log NGAL/Cr | 1.57 | 1.08–2.29 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udzik, J.; Waszczyk, A.; Wojciechowska-Koszko, I.; Kwiatkowski, P.; Roszkowska, P.; Rogulska, K.; Safranow, K.; Biskupski, A.; Kwiatkowski, S.; Kwiatkowska, E. The Utility of Novel Kidney Injury Biomarkers in Early Detection of CSA-AKI. Int. J. Mol. Sci. 2022, 23, 15864. https://doi.org/10.3390/ijms232415864
Udzik J, Waszczyk A, Wojciechowska-Koszko I, Kwiatkowski P, Roszkowska P, Rogulska K, Safranow K, Biskupski A, Kwiatkowski S, Kwiatkowska E. The Utility of Novel Kidney Injury Biomarkers in Early Detection of CSA-AKI. International Journal of Molecular Sciences. 2022; 23(24):15864. https://doi.org/10.3390/ijms232415864
Chicago/Turabian StyleUdzik, Jakub, Aleksandra Waszczyk, Iwona Wojciechowska-Koszko, Paweł Kwiatkowski, Paulina Roszkowska, Karolina Rogulska, Krzysztof Safranow, Andrzej Biskupski, Sebastian Kwiatkowski, and Ewa Kwiatkowska. 2022. "The Utility of Novel Kidney Injury Biomarkers in Early Detection of CSA-AKI" International Journal of Molecular Sciences 23, no. 24: 15864. https://doi.org/10.3390/ijms232415864
APA StyleUdzik, J., Waszczyk, A., Wojciechowska-Koszko, I., Kwiatkowski, P., Roszkowska, P., Rogulska, K., Safranow, K., Biskupski, A., Kwiatkowski, S., & Kwiatkowska, E. (2022). The Utility of Novel Kidney Injury Biomarkers in Early Detection of CSA-AKI. International Journal of Molecular Sciences, 23(24), 15864. https://doi.org/10.3390/ijms232415864






