Plasma and Peritoneal Fluid Fibronectin and Collagen IV Levels as Potential Biomarkers of Endometriosis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Populatoin
4.2. Reagents
4.3. Procedures
4.3.1. Chip Preparation
4.3.2. Antibody Immobilization
4.3.3. SPRi Measurement
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J. Med. Life 2014, 7, 349–357. [Google Scholar] [PubMed]
- Smolarz, B.; Szyllo, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, K.; Reckenbeil, L.; Brauer, D.; Sczesny, R.; Diebolder, H.; Runnebaum, I.B. Cycle-related Diarrhea and Dysmenorrhea are Independent Predictors of Peritoneal Endometriosis, Cycle-related Dyschezia is an Independent Predictor of Rectal Involvement. Geburtshilfe Frauenheilkd. 2020, 80, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Broi, M.G.D.; Ferriani, R.A.; Navarro, P.A. Ethiopathogenic mechanisms of endometriosis-related infertility. JBRA Assist. Reprod. 2019, 23, 273–280. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, V.L.; De Franciscis, P.; Barra, F.; Schiattarella, A.; Torok, P.; Shah, M.; Karaman, E.; Marques Cerentini, T.; Di Guardo, F.; Gullo, G.; et al. Quality of life in women with endometriosis: A narrative overview. Minerva Med. 2020, 111, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ghai, V.; Jan, H.; Shakir, F.; Haines, P.; Kent, A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J. Obstet. Gynaecol. 2020, 40, 83–89. [Google Scholar] [CrossRef]
- Wrobel, M.; Wielgos, M.; Laudanski, P. Diagnostic delay of endometriosis in adults and adolescence-current stage of knowledge. Adv. Med. Sci. 2022, 67, 148–153. [Google Scholar] [CrossRef]
- Golawski, K.; Soczewica, R.; Kacperczyk-Bartnik, J.; Manka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczynski, R.; Piekarski, P.; Banaszewska, B.; et al. The Role of Cadherin 12 (CDH12) in the Peritoneal Fluid among Patients with Endometriosis and Endometriosis-Related Infertility. Int. J. Environ. Res. Public Health 2022, 19, 11586. [Google Scholar] [CrossRef]
- Bartnik, P.; Kacperczyk-Bartnik, J.; Goławski, K.; Sierdziński, J.; Mańka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczyński, R.; Piekarski, P.; et al. Plasma and Peritoneal Fluid ZEB Levels in Patients with Endometriosis and Infertility. Biomedicines 2022, 10, 2460. [Google Scholar] [CrossRef]
- Kacperczyk-Bartnik, J.; Bartnik, P.; Goławski, K.; Sierdziński, J.; Mańka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczyński, R.; Piekarski, P.; et al. Plasma and Peritoneal Poly (ADP-Ribose) Polymerase Levels in Patients with Endometriosis. Biomedicines 2022, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Garzon, S.; Gotte, M.; Vigano, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Szamatowicz, J.; Laudanski, P.; Tomaszewska, I.; Szamatowicz, M. Chemokine growth-regulated-alpha: A possible role in the pathogenesis of endometriosis. Gynecol. Endocrinol. 2002, 16, 137–141. [Google Scholar] [CrossRef]
- Zalecka, J.; Pankiewicz, K.; Issat, T.; Laudanski, P. Molecular Mechanisms Underlying the Association between Endometriosis and Ectopic Pregnancy. Int. J. Mol. Sci. 2022, 23, 3490. [Google Scholar] [CrossRef] [PubMed]
- Sankiewicz, A.; Laudanski, P.; Romanowicz, L.; Hermanowicz, A.; Roszkowska-Jakimiec, W.; Debek, W.; Gorodkiewicz, E. Development of surface plasmon resonance imaging biosensors for detection of ubiquitin carboxyl-terminal hydrolase L1. Anal. Biochem. 2015, 469, 4–11. [Google Scholar] [CrossRef]
- Laudanski, P.; Gorodkiewicz, E.; Ramotowska, B.; Charkiewicz, R.; Kuzmicki, M.; Szamatowicz, J. Determination of cathepsins B, D and G concentration in eutopic proliferative endometrium of women with endometriosis by the surface plasmon resonance imaging (SPRI) technique. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 80–83. [Google Scholar] [CrossRef]
- Laudanski, P.; Szamatowicz, J.; Oniszczuk, M. Profiling of peritoneal fluid of women with endometriosis by chemokine protein array. Adv. Med. Sci. 2006, 51, 148–152. [Google Scholar]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, P.; Charkiewicz, R.; Kuzmicki, M.; Szamatowicz, J.; Swiatecka, J.; Mroczko, B.; Niklinski, J. Profiling of selected angiogenesis-related genes in proliferative eutopic endometrium of women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 172, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sankiewicz, A.; Romanowicz, L.; Laudanski, P.; Zelazowska-Rutkowska, B.; Puzan, B.; Cylwik, B.; Gorodkiewicz, E. SPR imaging biosensor for determination of laminin-5 as a potential cancer marker in biological material. Anal. Bioanal. Chem. 2016, 408, 5269–5276. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr. Top. Dev. Biol. 2018, 130, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Musiime, M.; Chang, J.; Hansen, U.; Kadler, K.E.; Zeltz, C.; Gullberg, D. Collagen Assembly at the Cell Surface: Dogmas Revisited. Cells 2021, 10, 662. [Google Scholar] [CrossRef]
- Weinberg, S.H.; Mair, D.B.; Lemmon, C.A. Mechanotransduction Dynamics at the Cell-Matrix Interface. Biophys. J. 2017, 112, 1962–1974. [Google Scholar] [CrossRef]
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Collagen Fibril Assembly and Function. Curr. Top. Dev. Biol. 2018, 130, 107–142. [Google Scholar] [CrossRef]
- Boudko, S.P.; Danylevych, N.; Hudson, B.G.; Pedchenko, V.K. Basement membrane collagen IV: Isolation of functional domains. Methods Cell Biol. 2018, 143, 171–185. [Google Scholar] [CrossRef]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.E.; Schwarzbauer, J.E. Collaboration of fibronectin matrix with other extracellular signals in morphogenesis and differentiation. Curr. Opin. Cell Biol. 2016, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.W.; Halter, M.; Tona, A.; Bhadriraju, K.; Plant, A.L. Using surface plasmon resonance imaging to probe dynamic interactions between cells and extracellular matrix. Cytom. A 2010, 77, 895–903. [Google Scholar] [CrossRef]
- Gorodkiewicz, E. The Surface Plasmon Resonance Imaging sensor for papain based on immobilized cystatin. Protein Pept. Lett. 2007, 14, 443–445. [Google Scholar] [CrossRef]
- Oldak, L.; Chludzinska-Kasperuk, S.; Milewska, P.; Grubczak, K.; Reszec, J.; Gorodkiewicz, E. Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy. Biomedicines 2022, 10, 2290. [Google Scholar] [CrossRef] [PubMed]
- Gorodkiewicz, E.; Lukaszewski, Z. Recent Progress in Surface Plasmon Resonance Biosensors (2016 to Mid-2018). Biosensors 2018, 8, 132. [Google Scholar] [CrossRef]
- Adachi, M.; Nasu, K.; Tsuno, A.; Yuge, A.; Kawano, Y.; Narahara, H. Attachment to extracellular matrices is enhanced in human endometriotic stromal cells: A possible mechanism underlying the pathogenesis of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 85–88. [Google Scholar] [CrossRef]
- Holzer, I.; Machado Weber, A.; Marshall, A.; Freis, A.; Jauckus, J.; Strowitzki, T.; Germeyer, A. GRN, NOTCH3, FN1, and PINK1 expression in eutopic endometrium-potential biomarkers in the detection of endometriosis—A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2723–2732. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Kubik, P.; Chrobak, A.; Pajak, J.; Chelmonska-Soyta, A.; Orczyk-Pawilowicz, M. Fibronectin Molecular Status in Plasma of Women with Endometriosis and Fertility Disorders. Int. J. Mol. Sci. 2021, 22, 11410. [Google Scholar] [CrossRef]
- Kauma, S.; Clark, M.R.; White, C.; Halme, J. Production of fibronectin by peritoneal macrophages and concentration of fibronectin in peritoneal fluid from patients with or without endometriosis. Obstet. Gynecol. 1988, 72, 13–18. [Google Scholar]
- Matalliotaki, C.; Matalliotakis, M.; Rahmioglu, N.; Mavromatidis, G.; Matalliotakis, I.; Koumantakis, G.; Zondervan, K.; Spandidos, D.A.; Goulielmos, G.N.; Zervou, M.I. Role of FN1 and GREB1 gene polymorphisms in endometriosis. Mol. Med. Rep. 2019, 20, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Nassif, J.; Abbasi, S.A.; Nassar, A.; Abu-Musa, A.; Eid, A.A. The role of NADPH-derived reactive oxygen species production in the pathogenesis of endometriosis: A novel mechanistic approach. J. Biol. Regul. Homeost. Agents 2016, 30, 31–40. [Google Scholar] [PubMed]
- Beliard, A.; Donnez, J.; Nisolle, M.; Foidart, J.M. Localization of laminin, fibronectin, E-cadherin, and integrins in endometrium and endometriosis. Fertil. Steril. 1997, 67, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, A.; Gmyrek, G.B.; Sozanski, R.; Sieradzka, U.; Paprocka, M.; Gabrys, M.; Jerzak, M. The influence of extracellular matrix proteins on T-cell proliferation and apoptosis in women with endometriosis or uterine leiomyoma. Am. J. Reprod. Immunol. 2004, 51, 123–129. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef]
- Lindgren, M.; Jansson, M.; Tavelin, B.; Dirix, L.; Vermeulen, P.; Nystrom, H. Type IV collagen as a potential biomarker of metastatic breast cancer. Clin. Exp. Metastasis 2021, 38, 175–185. [Google Scholar] [CrossRef]
- Giannelli, G.; Sgarra, C.; Di Naro, E.; Lavopa, C.; Angelotti, U.; Tartagni, M.; Simone, O.; Trerotoli, P.; Antonaci, S.; Loverro, G. Endometriosis is characterized by an impaired localization of laminin-5 and alpha3beta1 integrin receptor. Int. J. Gynecol. Cancer 2007, 17, 242–247. [Google Scholar] [CrossRef]
- Lee, S.Y.; Koo, Y.J.; Lee, D.H. Classification of endometriosis. Yeungnam Univ. J. Med. 2021, 38, 10–18. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Lukaszewski, Z.; Trojanowska, K.; Gorodkiewicz, E. Determination of collagen type IV by Surface Plasmon Resonance Imaging using a specific biosensor. Anal. Biochem. 2016, 515, 40–46. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Romanowicz, L.; Pyc, M.; Hermanowicz, A.; Gorodkiewicz, E. SPR imaging biosensor for the quantitation of fibronectin concentration in blood samples. J. Pharm. Biomed. Anal. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Falkowski, P.; Mrozek, P.; Lukaszewski, Z.; Oldak, L.; Gorodkiewicz, E. An Immunosensor for the Determination of Cathepsin S in Blood Plasma by Array SPRi—A Comparison of Analytical Properties of Silver-Gold and Pure Gold Chips. Biosensors 2021, 11, 298. [Google Scholar] [CrossRef] [PubMed]
| Variable | Study Group, n = 49 (SD) | Control Group, n = 35 (SD) | p-Value |
|---|---|---|---|
| Age [years] n = 74 | 32 (±4.8) n = 46 | 31.3 (±5.9) n = 28 | 0.55 |
| BMI [kg/m2] n = 70 | 22.3 (±2.8) n = 45 | 22.4 (±3.7) n = 25 | 0.97 |
| Any gestation in the past n = 72 | 0.19 (±0.40) n = 46 | 0.31 (±0.47) n = 26 | 0.29 |
| Biomarker | Study Group (SD) | Control Group (SD) | p-Value |
|---|---|---|---|
| Fibronectin plasma [mg/L] n = 69 | 329.3 (±98.5) n = 41 | 251.2 (±84.0) n = 28 | 0.001 |
| Fibronectin peritoneal fluid [μg/L] n = 78 | 26.8 (±11.1) n = 47 | 7.0 (±5.9) n = 31 | <0.001 |
| Collagen IV plasma [ng/L] n = 69 | 559.1 (±88.6) n = 41 | 540.6 (±82.6) n = 28 | 0.385 |
| Collagen IV peritoneal fluid [ng/L] n = 76 | 572.6 (±72.0) n = 45 | 583.6 (±50.9) n = 31 | 0.465 |
| Biomarker | I (SD) | II (SD) | III (SD) | IV (SD) | p-Value |
|---|---|---|---|---|---|
| Fibronectin plasma [mg/L] n = 41 | 318.0 (±102.6) n = 10 | 326.5 (±121.3) n = 7 | 341.1 (±76.4) n = 15 | 324.1 (±122.3) n = 9 | 0.029 |
| Fibronectin peritoneal fluid [μg/L] n = 47 | 25.2 (±13.3) n = 13 | 27.4 (±11.5) n = 7 | 29.5 (±8.6) n = 18 | 22.9 (±12.5) n = 9 | 0.001 |
| Collagen IV plasma [ng/L] n = 41 | 539.8 (±99.7) n = 10 | 568.2 (±81.9) n = 7 | 562.1 (±68.9) n = 15 | 568.6 (±118.6) n = 9 | 0.702 |
| Collagen IV peritoneal fluid [ng/L] n = 45 | 595.4 (±58.6) n = 13 | 582.2 (±124.0) n = 7 | 575.3 (±49.0) n = 16 | 527.2 (±66.8) n = 9 | 0.109 |
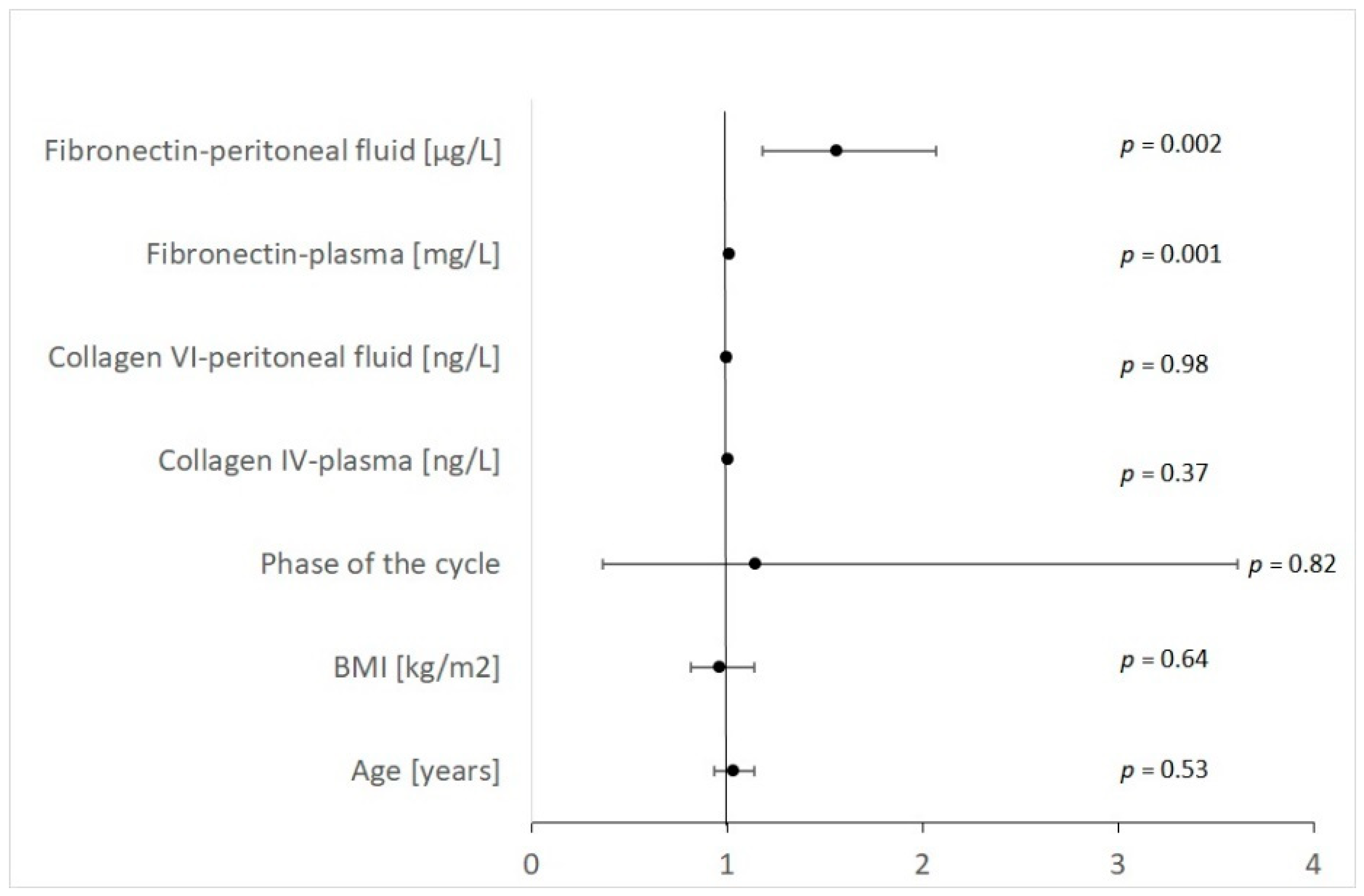
| Variable | OR | −95%CI | +95%CI | Wald Chi-Square | p |
|---|---|---|---|---|---|
| Age [years] | 1.03 | 0.93 | 1.14 | 0.40 | 0.528 |
| BMI [kg/m2] | 0.96 | 0.81 | 1.14 | 0.22 | 0.638 |
| Phase of the cycle | 1.14 | 0.36 | 3.61 | 0.05 | 0.816 |
| Collagen IV-plasma [ng/L] | 1.00 | 1.00 | 1.01 | 0.82 | 0.366 |
| Collagen VI-peritoneal fluid [ng/L] | 1.00 | 0.99 | 1.01 | 0.00 | 0.983 |
| Fibronectin—plasma [mg/L] | 1.01 | 1.01 | 1.02 | 11.06 | 0.001 |
| Fibronectin—peritoneal fluid [μg/L] | 1.56 | 1.18 | 2.07 | 9.99 | 0.002 |
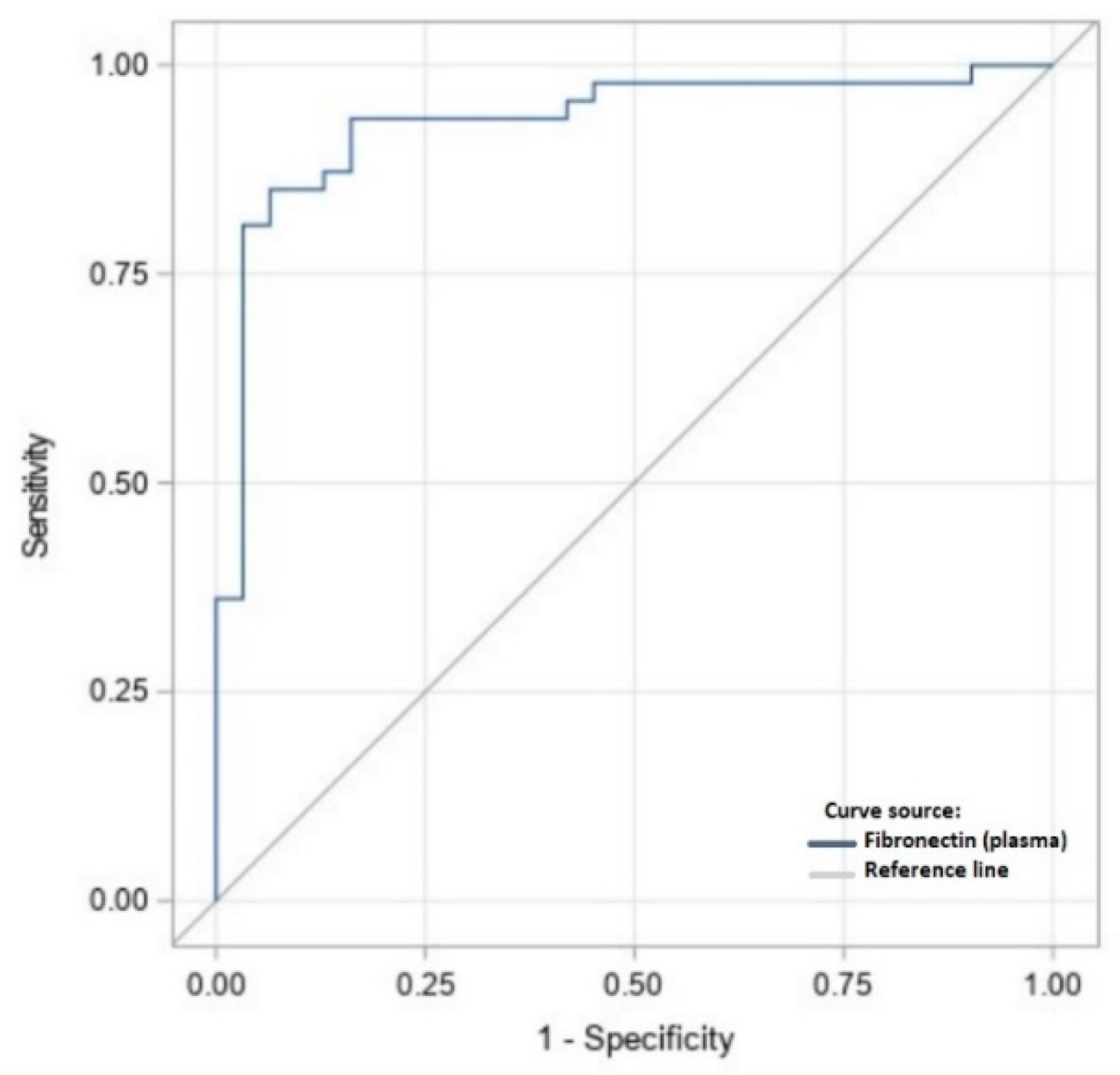
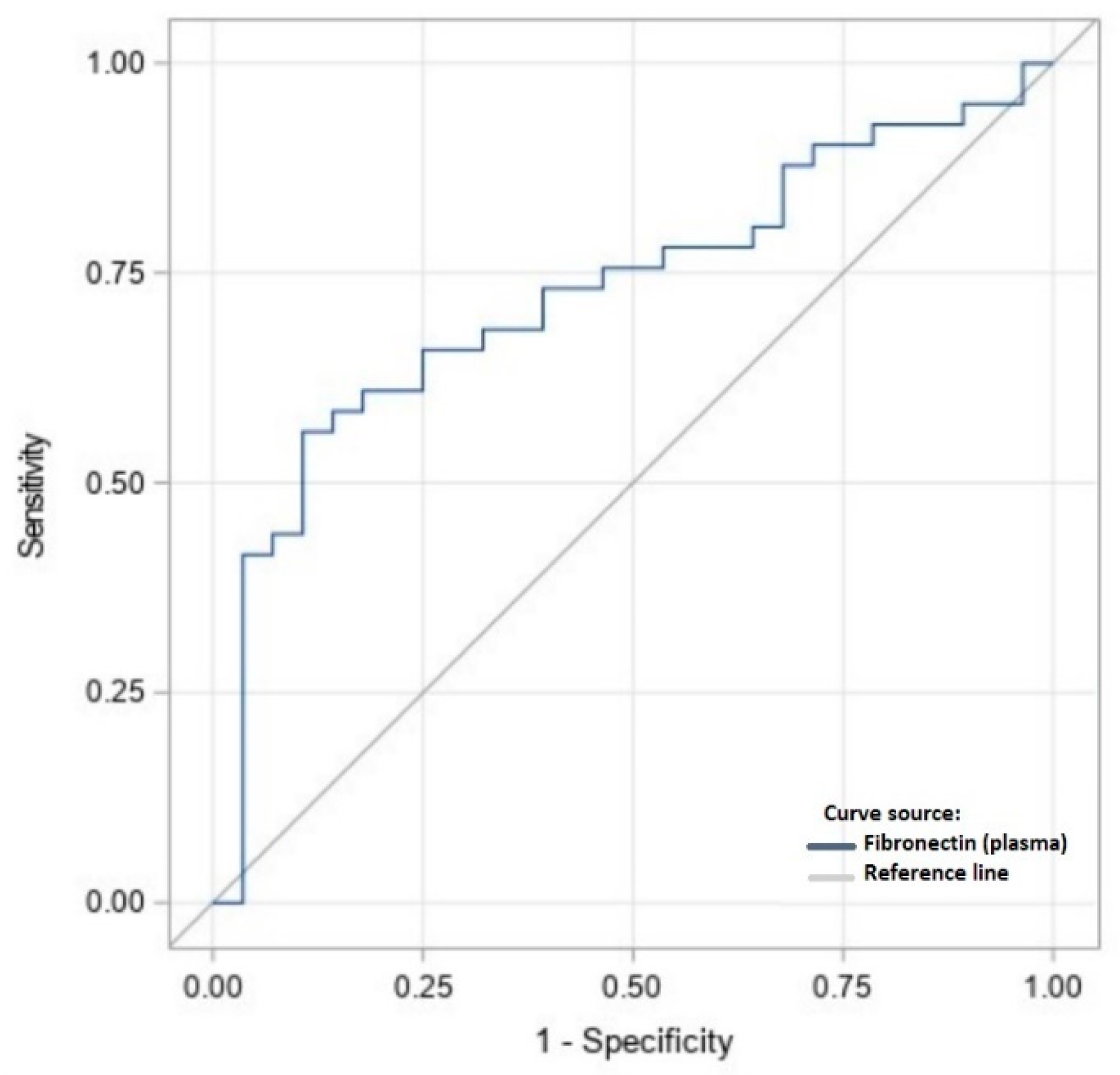
| Variable | Plasma [mg/L] | Peritoneal Fluid [μg/L] |
|---|---|---|
| Suggested cut-off value | 320.1 | 13.15 |
| Specificity | 0.73 | 0.93 |
| Sensitivity | 0.61 | 0.87 |
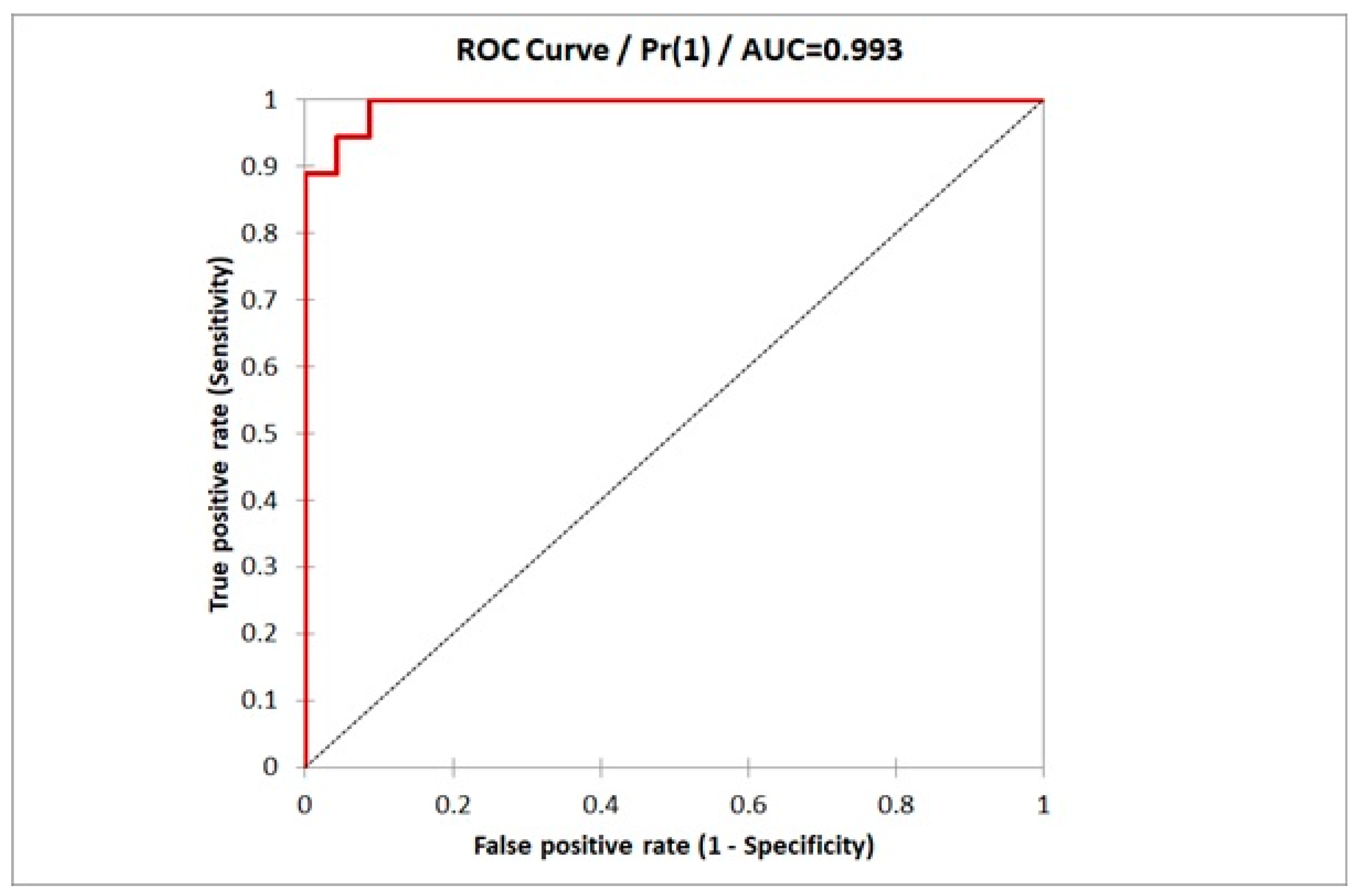
| Sensitivity | −95% CI | +95 CI | Specificity | −95% CI | +95 CI |
|---|---|---|---|---|---|
| 1.000 | 0.882 | 1.000 | 0.913 | 0.718 | 0.986 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warzecha, D.; Załęcka, J.; Mańka, G.; Kiecka, M.; Lipa, M.; Spaczyński, R.; Piekarski, P.; Banaszewska, B.; Jakimiuk, A.; Issat, T.; et al. Plasma and Peritoneal Fluid Fibronectin and Collagen IV Levels as Potential Biomarkers of Endometriosis. Int. J. Mol. Sci. 2022, 23, 15669. https://doi.org/10.3390/ijms232415669
Warzecha D, Załęcka J, Mańka G, Kiecka M, Lipa M, Spaczyński R, Piekarski P, Banaszewska B, Jakimiuk A, Issat T, et al. Plasma and Peritoneal Fluid Fibronectin and Collagen IV Levels as Potential Biomarkers of Endometriosis. International Journal of Molecular Sciences. 2022; 23(24):15669. https://doi.org/10.3390/ijms232415669
Chicago/Turabian StyleWarzecha, Damian, Julia Załęcka, Grzegorz Mańka, Mariusz Kiecka, Michał Lipa, Robert Spaczyński, Piotr Piekarski, Beata Banaszewska, Artur Jakimiuk, Tadeusz Issat, and et al. 2022. "Plasma and Peritoneal Fluid Fibronectin and Collagen IV Levels as Potential Biomarkers of Endometriosis" International Journal of Molecular Sciences 23, no. 24: 15669. https://doi.org/10.3390/ijms232415669
APA StyleWarzecha, D., Załęcka, J., Mańka, G., Kiecka, M., Lipa, M., Spaczyński, R., Piekarski, P., Banaszewska, B., Jakimiuk, A., Issat, T., Rokita, W., Młodawski, J., Szubert, M., Sieroszewski, P., Raba, G., Szczupak, K., Kluz, T., Kluza, M., Wielgoś, M., ... Laudański, P. (2022). Plasma and Peritoneal Fluid Fibronectin and Collagen IV Levels as Potential Biomarkers of Endometriosis. International Journal of Molecular Sciences, 23(24), 15669. https://doi.org/10.3390/ijms232415669






