Sex-Specific Multiparameter Blood Test for the Early Diagnosis of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
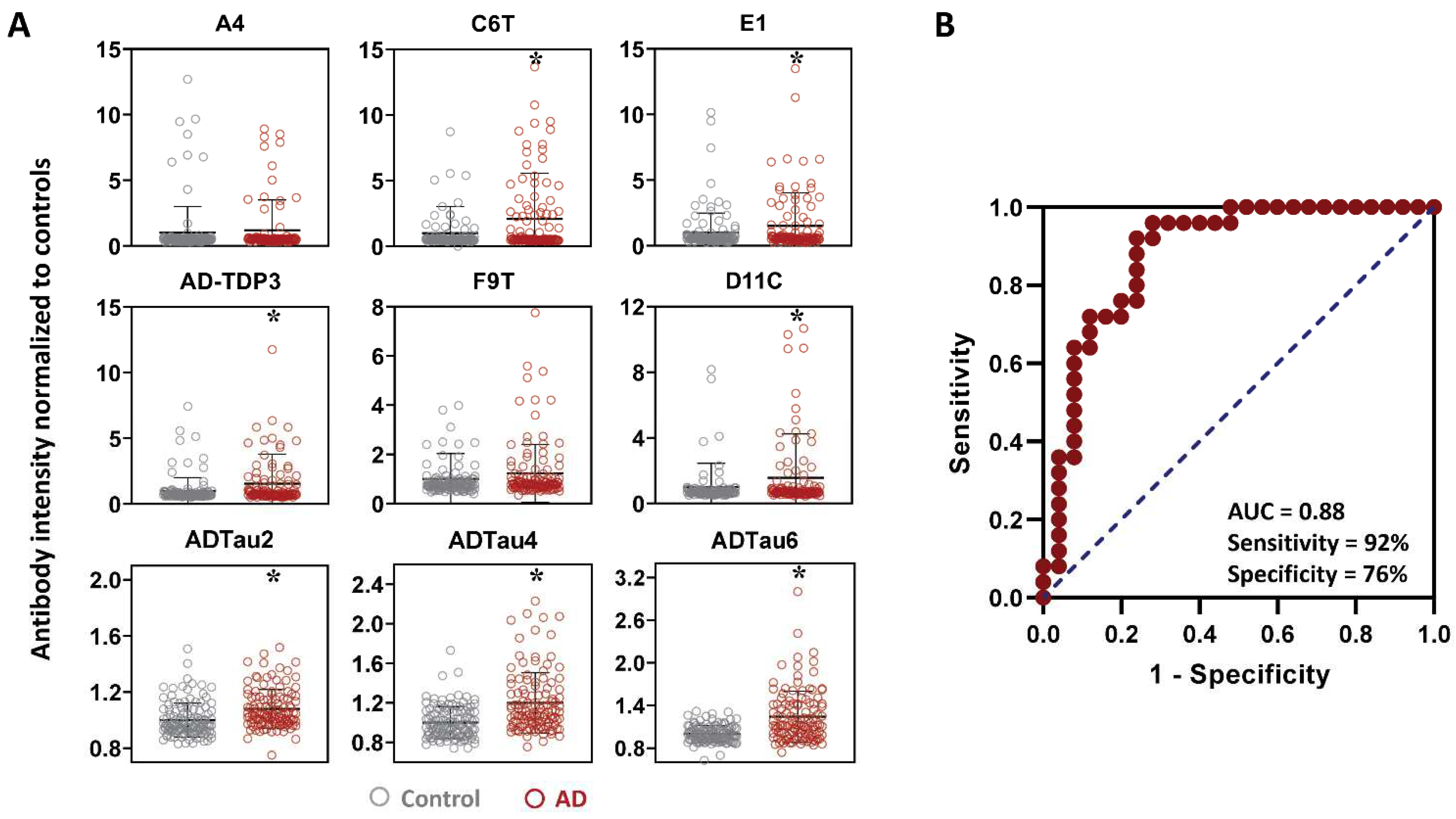
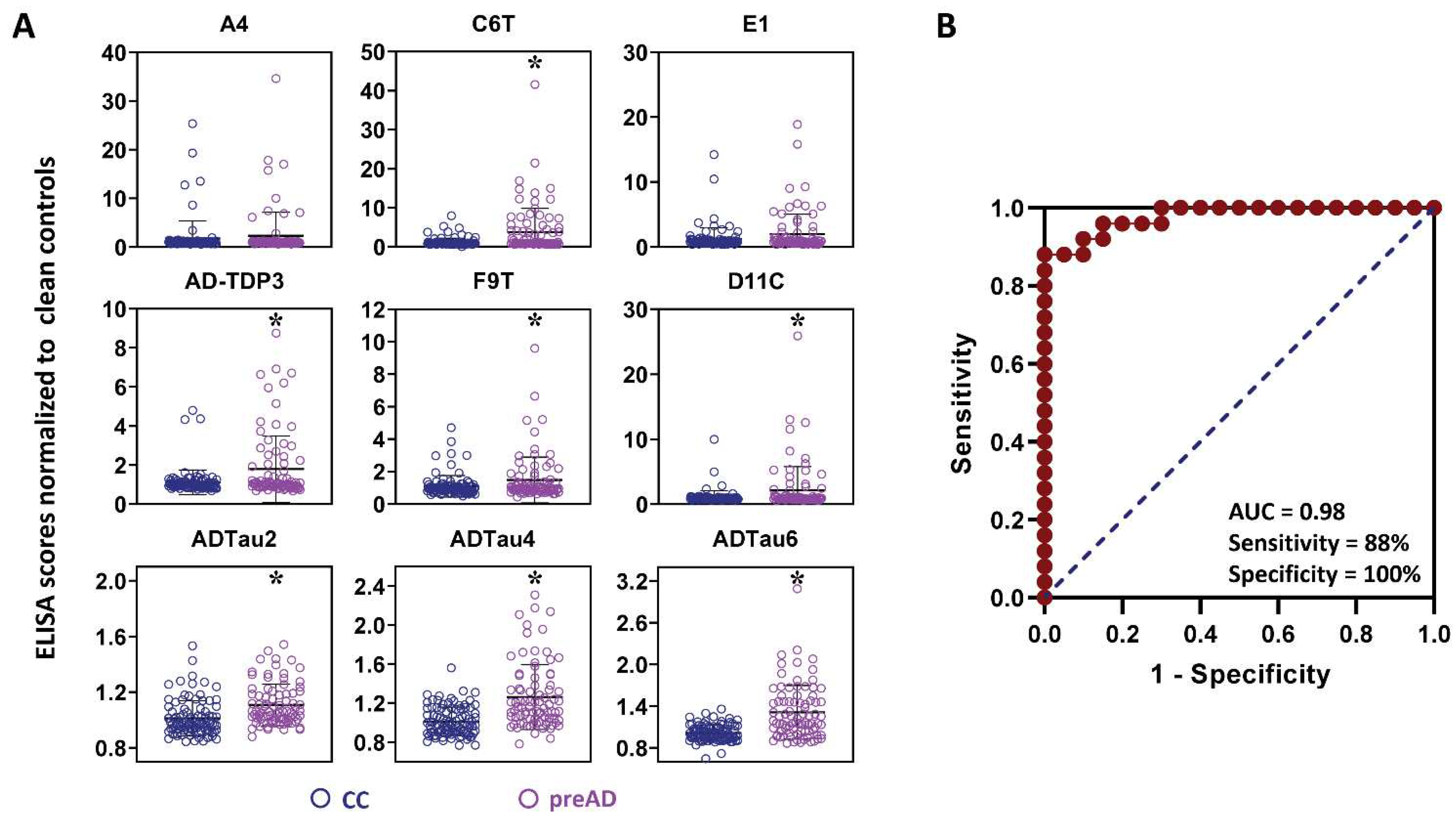
2.1. Protein Variant Profiles (AD vs. Controls)
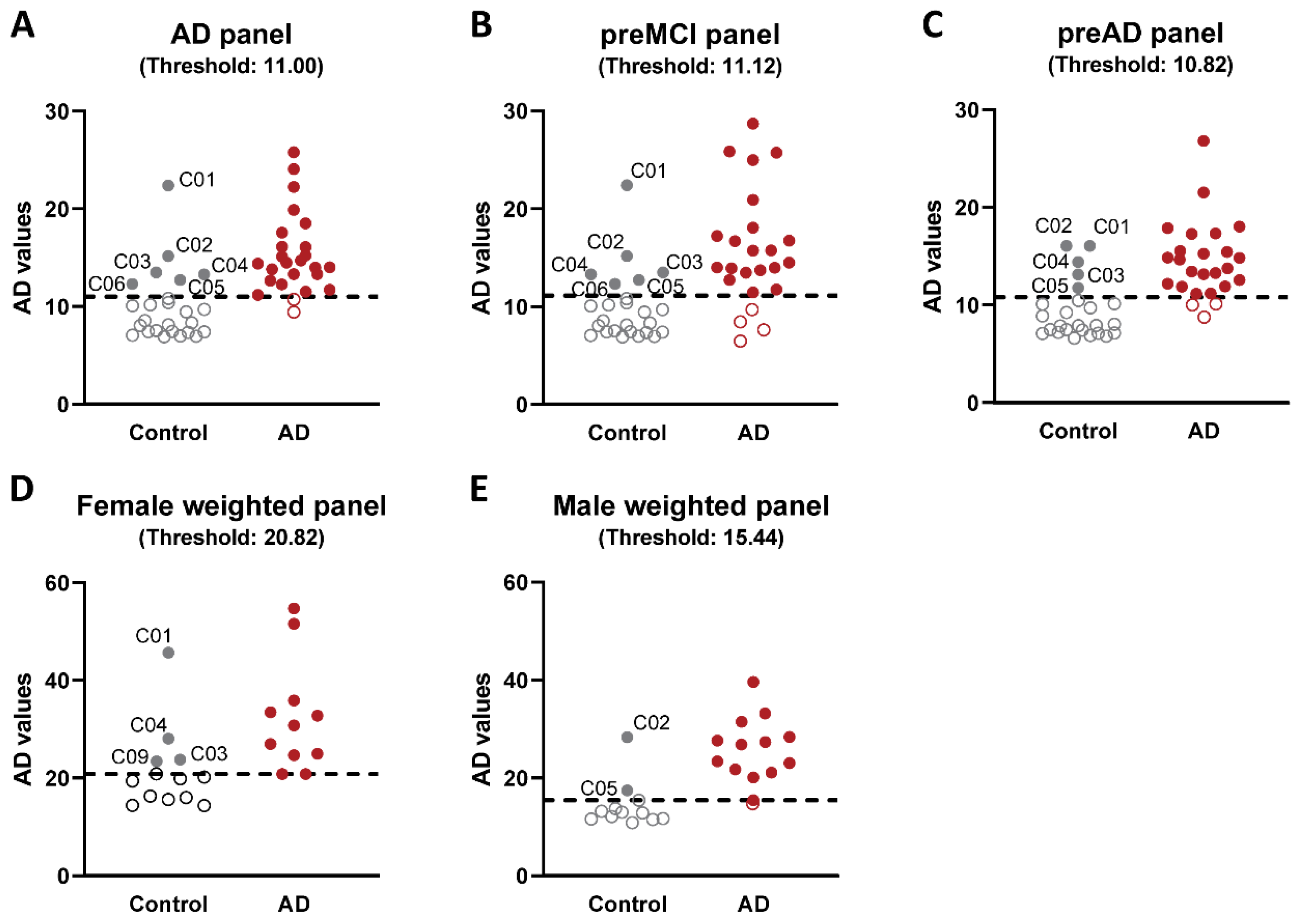
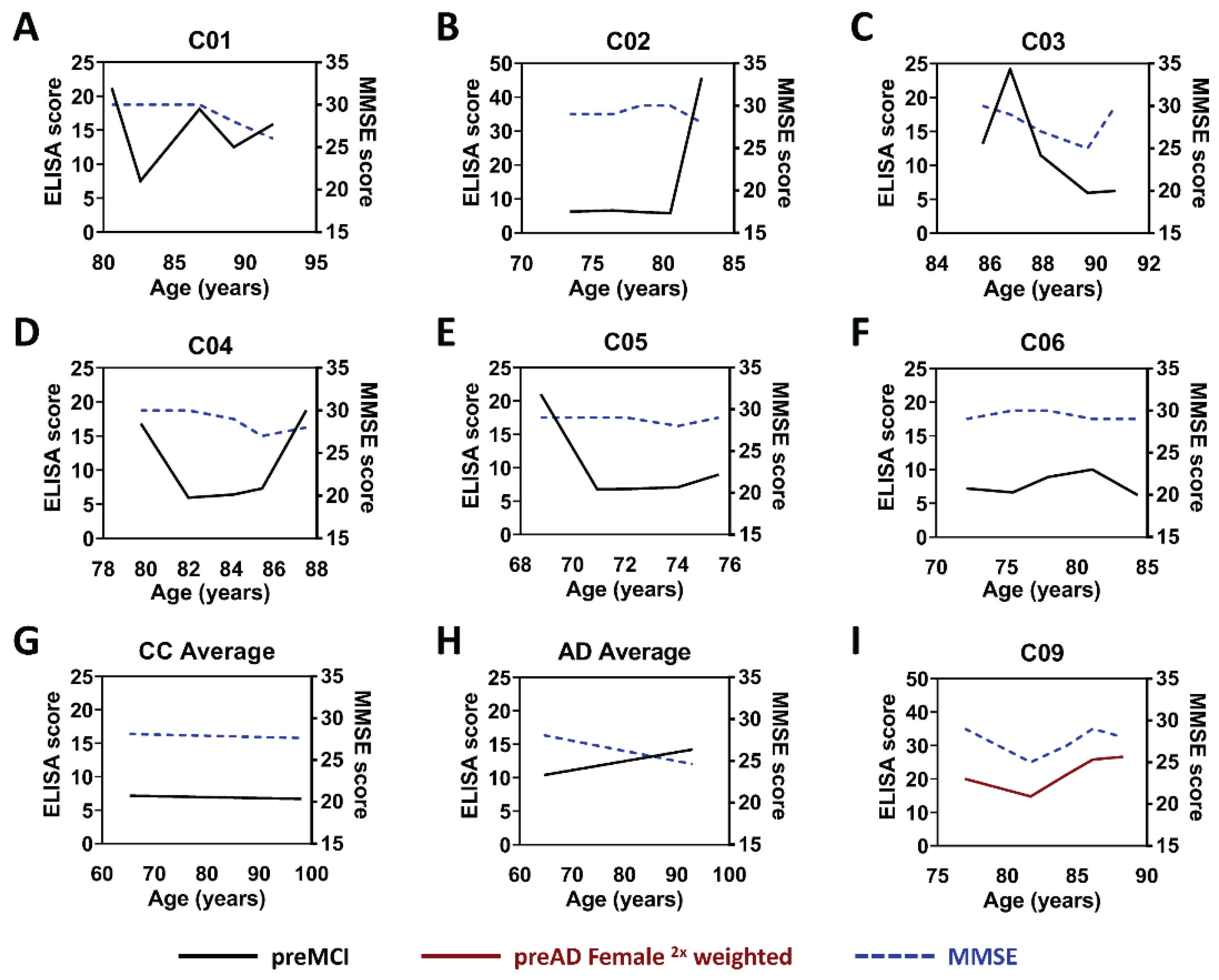
2.2. Analysis of Flagged Control Cases as Potential Pre-Symptomatic AD Cases
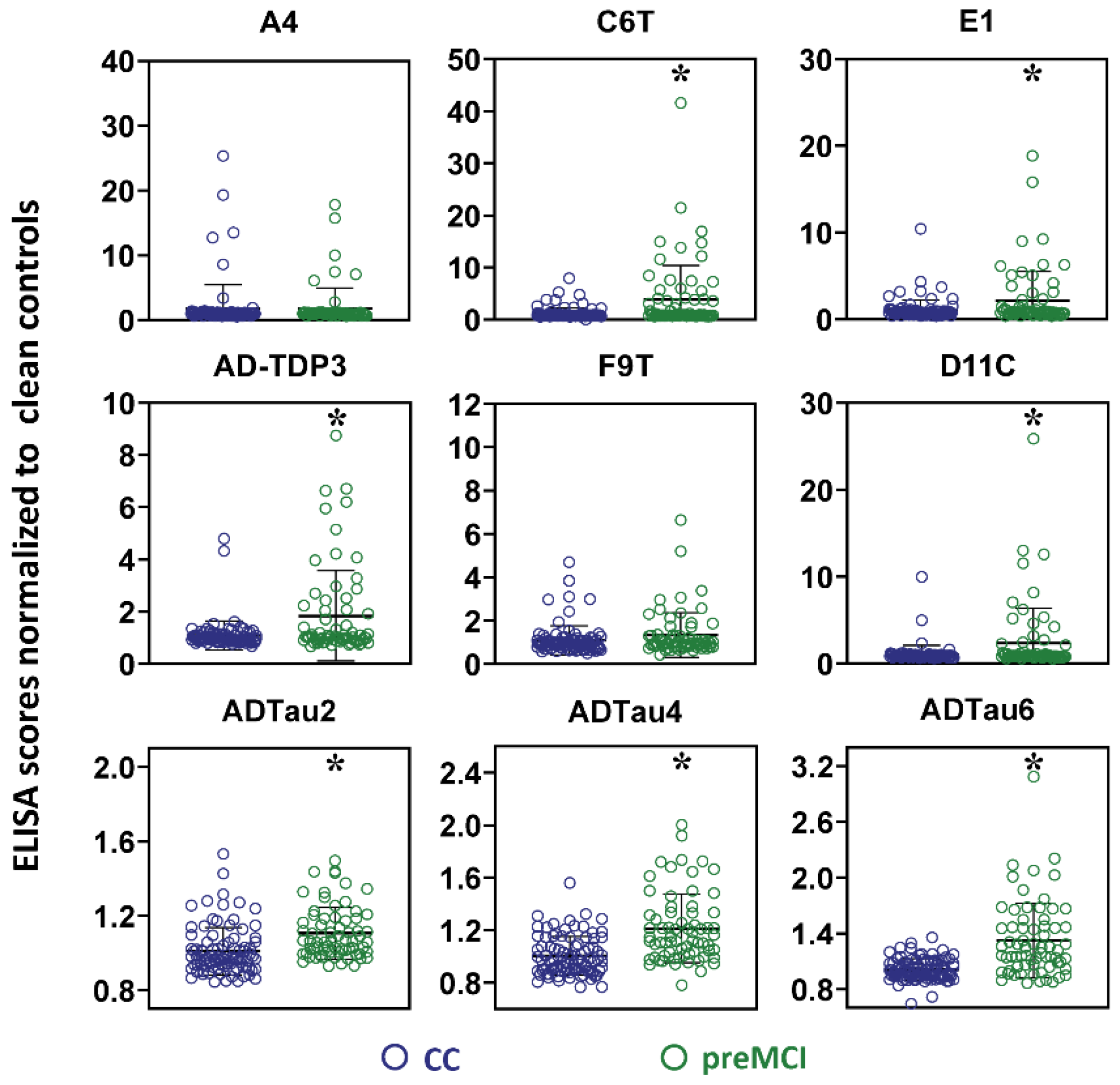
2.3. Analysis of Samples from AD Cases before Clinical Diagnosis of AD
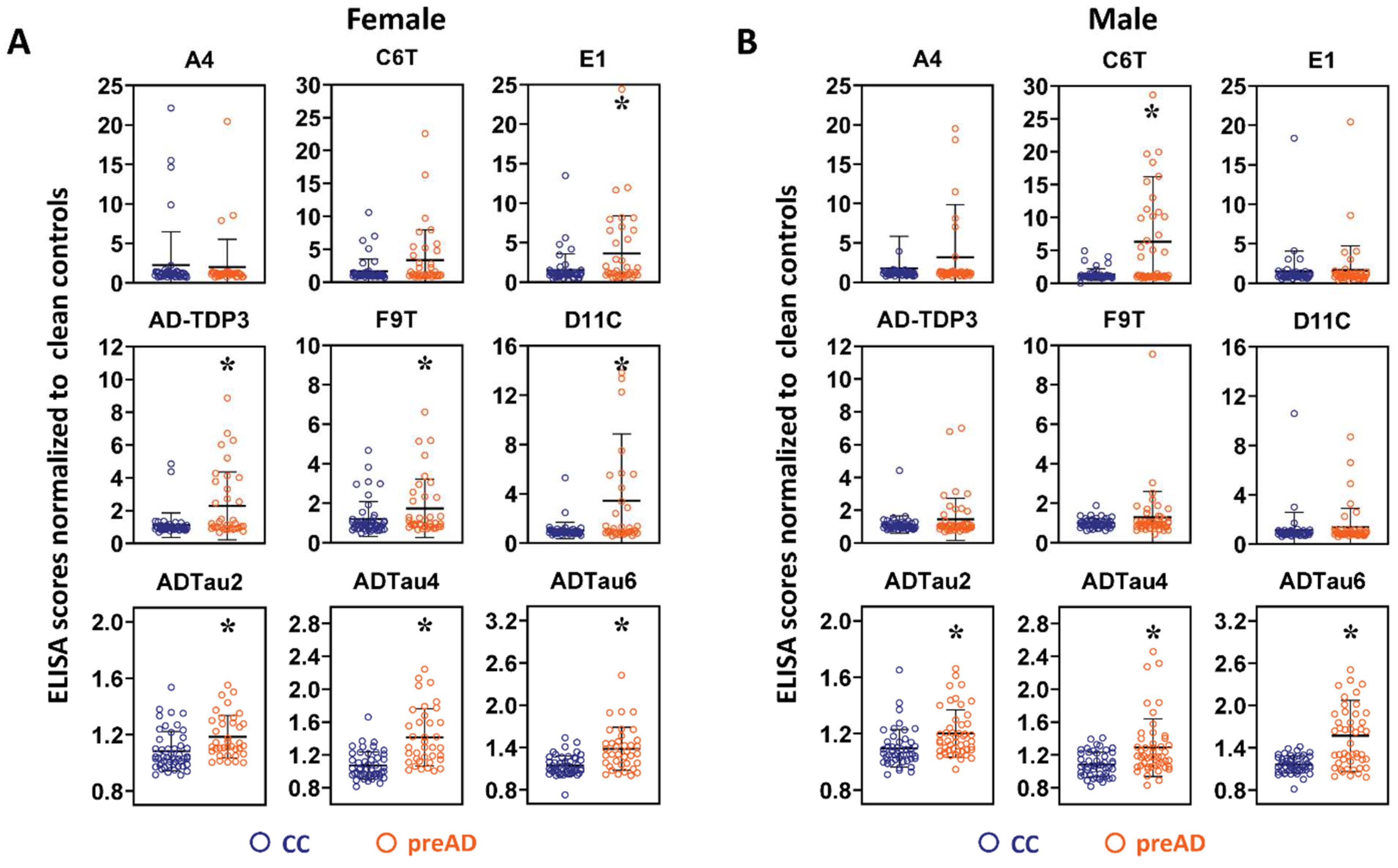
2.4. Sex Differences in Protein Variant Biomarkers
2.5. Early Diagnosis Time Frame
3. Discussion
4. Material and Methods
4.1. Study Population
4.2. Capture ScFvs
4.3. Detection Phages
4.4. Sandwich ELISA
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachurin, S.O.; Bovina, E.V.; Ustyugov, A.A. Drugs in Clinical Trials for Alzheimer’s Disease: The Major Trends. Med. Res. Rev. 2017, 37, 1186–1225. [Google Scholar] [CrossRef] [PubMed]
- Rygiel, K. Novel strategies for Alzheimer’s disease treatment: An overview of anti-amyloid beta monoclonal antibodies. Indian J. Pharmacol. 2016, 48, 629–636. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Maarouf, C.L.; Sue, L.I.; Hu, Y.; Wilson, J.; Beach, T.G. Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer’s disease. Biomarkers 2009, 14, 493–501. [Google Scholar] [CrossRef]
- Cedazo-Minguez, A.; Winblad, B. Biomarkers for Alzheimer’s disease and other forms of dementia: Clinical needs, limitations and future aspects. Exp. Gerontol. 2010, 45, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Henchcliffe, C.; Dodel, R.; Beal, M.F. Biomarkers of Parkinson’s disease and Dementia with Lewy bodies. Prog. Neurobiol. 2011, 95, 601–613. [Google Scholar] [CrossRef]
- Kirmess, K.M.; Meyer, M.R.; Holubasch, M.S.; Knapik, S.S.; Hu, Y.; Jackson, E.N.; Harpstrite, S.E.; Verghese, P.B.; West, T.; Fogelman, I.; et al. The PrecivityAD™ test: Accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin. Chim. Acta 2021, 519, 267–275. [Google Scholar] [CrossRef]
- Renner, M.; Lacor, P.N.; Velasco, P.T.; Xu, J.; Contractor, A.; Klein, W.L.; Triller, A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 2010, 66, 739–754. [Google Scholar] [CrossRef]
- Usenovic, M.; Niroomand, S.; Drolet, R.E.; Yao, L.; Gaspar, R.C.; Hatcher, N.G.; Schachter, J.; Renger, J.J.; Parmentier-Batteur, S. Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J. Neurosci. 2015, 35, 14234–14250. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.H. TDP-43: The relationship between protein aggregation and neurodegeneration in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. FEBS J. 2011, 278, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Uryu, K.; Nakashima-Yasuda, H.; Forman, M.S.; Kwong, L.K.; Clark, C.M.; Grossman, M.; Miller, B.L.; Kretzschmar, H.A.; Lee, V.M.; Trojanowski, J.Q.; et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J. Neuropathol. Exp. Neurol. 2008, 67, 555–564. [Google Scholar] [CrossRef]
- Dickson, D.W. TDP-43 immunoreactivity in neurodegenerative disorders: Disease versus mechanism specificity. Acta Neuropathol. 2008, 115, 147–149. [Google Scholar] [CrossRef]
- Amador-Ortiz, C.; Lin, W.L.; Ahmed, Z.; Personett, D.; Davies, P.; Duara, R.; Graff-Radford, N.R.; Hutton, M.L.; Dickson, D.W. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann. Neurol. 2007, 61, 435–445. [Google Scholar] [CrossRef]
- Nelson, P.T.; Brayne, C.; Flanagan, M.E.; Abner, E.L.; Agrawal, S.; Attems, J.; Castellani, R.J.; Corrada, M.M.; Cykowski, M.D.; Di, J.; et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer’s disease neuropathology: Combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol. 2022, 144, 27–44. [Google Scholar] [CrossRef]
- Johnson, B.S.; Snead, D.; Lee, J.J.; McCaffery, J.M.; Shorter, J.; Gitler, A.D. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J. Biol. Chem. 2009, 284, 20329–20339. [Google Scholar] [CrossRef]
- Barkhordarian, H.; Emadi, S.; Schulz, P.; Sierks, M.R. Isolating recombinant antibodies against specific protein morphologies using atomic force microscopy and phage display technologies. Protein Eng. Des. Sel. 2006, 19, 497–502. [Google Scholar] [CrossRef]
- Emadi, S.; Barkhordarian, H.; Wang, M.S.; Schulz, P.; Sierks, M.R. Isolation of a human single chain antibody fragment against oligomeric α-synuclein that inhibits aggregation and prevents α-synuclein induced toxicity. J. Mol. Biol. 2007, 368, 1132–1144. [Google Scholar] [CrossRef]
- Emadi, S.; Kasturirangan, S.; Wang, M.S.; Schulz, P.; Sierks, M.R. Detecting morphologically distinct oligomeric forms of α-synuclein. J. Biol. Chem. 2009, 284, 11048–11058. [Google Scholar] [CrossRef] [PubMed]
- Emadi, S.; Liu, R.; Yuan, B.; Schulz, P.; McAllister, C.; Lyubchenko, Y.; Messer, A.; Sierks, M.R. Inhibiting aggregation of α-synuclein with human single chain antibody fragments. Biochemistry 2004, 43, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Emadi, S.; Sierks, M.R.; Messer, A. A human single-chain Fv intrabody blocks aberrant cellular effects of overexpressed α-synuclein. Mol. Ther. 2004, 10, 1023–1031. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, B.; Emadi, S.; Zameer, A.; Schulz, P.; McAllister, C.; Lyubchenko, Y.; Goud, G.; Sierks, M.R. Single chain variable fragments against β-Amyloid (Aβ) can inhibit Aβ aggregation and prevent Aβ-induced neurotoxicity. Biochemistry 2004, 43, 6959–6967. [Google Scholar] [CrossRef]
- Zameer, A.; Schulz, P.; Wang, M.S.; Sierks, M.R. Single chain Fv antibodies against the 25–35 Aβ fragment inhibit aggregation and toxicity of Aβ42. Biochemistry 2006, 45, 11532–11539. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M.; Venkataraman, L.; Tian, H.; Khan, G.; Harris, B.T.; Sierks, M.R. Novel atomic force microscopy based biopanning for isolation of morphology specific reagents against TDP-43 variants in amyotrophic lateral sclerosis. J. Vis. Exp. 2015, 96, e52584. [Google Scholar] [CrossRef]
- Williams, S.M.; Khan, G.; Harris, B.T.; Ravits, J.; Sierks, M.R. TDP-43 protein variants as biomarkers in amyotrophic lateral sclerosis. BMC Neurosci. 2017, 18, 20. [Google Scholar] [CrossRef]
- Zameer, A.; Kasturirangan, S.; Emadi, S.; Nimmagadda, S.V.; Sierks, M.R. Anti-oligomeric Aβ single-chain variable domain antibody blocks Aβ-induced toxicity against human neuroblastoma cells. J. Mol. Biol. 2008, 384, 917–928. [Google Scholar] [CrossRef]
- Kasturirangan, S.; Li, L.; Emadi, S.; Boddapati, S.; Schulz, P.; Sierks, M.R. Nanobody specific for oligomeric beta-amyloid stabilizes nontoxic form. Neurobiol. Aging 2012, 33, 1320–1328. [Google Scholar] [CrossRef]
- Kasturirangan, S.; Reasoner, T.; Schulz, P.; Boddapati, S.; Emadi, S.; Valla, J.; Sierks, M.R. Isolation and characterization of antibody fragments selective for specific protein morphologies from nanogram antigen samples. Biotechnol. Prog. 2013, 29, 463–471. [Google Scholar] [CrossRef]
- Tian, H.; Davidowitz, E.; Lopez, P.; He, P.; Schulz, P.; Moe, J.; Sierks, M.R. Isolation and characterization of antibody fragments selective for toxic oligomeric tau. Neurobiol. Aging. 2015, 36, 1342–1355. [Google Scholar] [CrossRef][Green Version]
- Venkataraman, L.; He, P.; Schulz, P.; Sierks, M.R. Isolation and characterization of antibody fragment selective for human Alzheimer’s disease brain-derived tau variants. Neurobiol. Aging 2020, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M.; Schulz, P.; Rosenberry, T.L.; Caselli, R.J.; Sierks, M.R. Blood-based oligomeric and other protein variant biomarkers to facilitate pre-symptomatic diagnosis and staging of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 58, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M.; Schulz, P.; Sierks, M.R. Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson’s and Alzheimer’s diseases. Eur. J. Neurosci. 2016, 43, 3–16. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Facts and Figures. Available online: https://www.alz.org/alzheimers-dementia/facts-figures (accessed on 20 October 2022).
- Jansen, W.J.; Janssen, O.; Tijms, B.M.; Vos, S.J.B.; Ossenkoppele, R.; Visser, P.J.; Aarsland, D.; Alcolea, D.; Altomare, D.; von Arnim, C.; et al. Prevalence estimates of amyloid abnormality across the Alzheimer Disease Clinical Spectrum. JAMA Neurol. 2022, 79, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Meyer, P.F.; Pichet Binette, A.; Gonneaud, J.; Breitner, J.C.S.; Villeneuve, S. Characterization of Alzheimer disease biomarker discrepancies using cerebrospinal fluid phosphorylated tau and AV1451 positron emission tomography. JAMA Neurol. 2020, 77, 508–516. [Google Scholar] [CrossRef]
- McDade, E.; Bednar, M.M.; Brashear, H.R.; Miller, D.S.; Maruff, P.; Randolph, C.; Ismail, Z.; Carrillo, M.C.; Weber, C.J.; Bain, L.J.; et al. The pathway to secondary prevention of Alzheimer’s disease. Alzheimer’s Dement. 2020, 6, e12069. [Google Scholar] [CrossRef]
- Amjad, H.; Roth, D.L.; Sheehan, O.C.; Lyketsos, C.G.; Wolff, J.L.; Samus, Q.M. Underdiagnosis of Dementia: An observational study of patterns in diagnosis and awareness in US older adults. J. Gen. Intern. Med. 2018, 33, 1131–1138. [Google Scholar] [CrossRef]
- Levine, D.A.; Gross, A.L.; Briceño, E.M.; Tilton, N.; Giordani, B.J.; Sussman, J.B.; Hayward, R.A.; Burke, J.F.; Hingtgen, S.; Elkind, M.S.V.; et al. Sex differences in cognitive decline among US adults. JAMA Netw. Open 2021, 4, e210169. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.T.; Klein, W.L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 96, 529–543. [Google Scholar] [CrossRef]
- Tian, H.; Davidowitz, E.; Lopez, P.; Emadi, S.; Moe, J.; Sierks, M. Trimeric tau is toxic to human neuronal cells at low nanomolar concentrations. Int. J. Cell Biol. 2013, 2013, 260787. [Google Scholar] [CrossRef]
- Salvadores, N.; Shahnawaz, M.; Scarpini, E.; Tagliavini, F.; Soto, C. Detection of misfolded Aβ oligomers for sensitive biochemical diagnosis of Alzheimer’s disease. Cell Rep. 2014, 7, 261–268. [Google Scholar] [CrossRef]
- Yang, T.; Hong, S.; O’Malley, T.; Sperling, R.A.; Walsh, D.M.; Selkoe, D.J. New ELISAs with high specificity for soluble oligomers of amyloid β-protein detect natural Aβ oligomers in human brain but not CSF. Alzheimer’s Dement. 2013, 9, 99–112. [Google Scholar] [CrossRef]
- Josephs, K.A.; Whitwell, J.L.; Weigand, S.D.; Murray, M.E.; Tosakulwong, N.; Liesinger, A.M.; Petrucelli, L.; Senjem, M.L.; Knopman, D.S.; Boeve, B.F.; et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014, 127, 811–824. [Google Scholar] [CrossRef]
- Fang, Y.-S.; Tsai, K.-J.; Chang, Y.-J.; Kao, P.; Woods, R.; Kuo, P.-H.; Wu, C.-C.; Liao, J.-Y.; Chou, S.-C.; Lin, V.; et al. Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia-TDP patients. Nat. Commun. 2014, 5, 4824. [Google Scholar] [CrossRef]
- Venkataraman, L.; He, P.; Khan, G.; Harris, B.T.; Sierks, M.R. Isolation and characterization of antibody fragments selective for human FTD brain derived TDP-43 variants. BMC Neurosci. 2020, 21, 36. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.; Howieson, D.; Loring, D. Neuropsychological Assessment, 4th ed.; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Schulz, P.; Sierks, M.R. A sensitive phage-based capture ELISA for sub-femtomolar detection of protein variants directly from biological samples. Biotechnol. Prog. 2015, 31, 289–298. [Google Scholar] [CrossRef]
- Yuan, B.; Schulz, P.; Liu, R.; Sierks, M.R. Improved affinity selection using phage display technology and off-rate based selection. Electron. J. Biotechnol. 2006, 9, 171–175. [Google Scholar] [CrossRef]
- Williams, S.M.; Peltz, C.; Yaffe, K.; Schulz, P.; Sierks, M.R. CNS disease-related protein variants as blood-based biomarkers in traumatic brain injury. Neurology 2018, 91, 702–709. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 22 June 2022).
| scFv | Target Antigen Description |
|---|---|
| Aβ variants | |
| A4 | Synthetically generated oligomeric Aβ |
| E1 | Synthetically generated oligomeric Aβ |
| C6T | Human AD brain-derived oligomeric Aβ |
| Tau variants | |
| F9T | Synthetically generated trimeric tau variant |
| D11C | Synthetically generated trimeric tau variant |
| ADTau2 | Human AD brain-derived tau variant |
| ADTau4 | Human AD brain-derived tau variant |
| ADTau6 | Human AD brain-derived tau variant |
| TDP-43 variants | |
| AD-TDP3 | Human AD and ALS brain-derived TDP-43 variant |
| Control | AD | |
|---|---|---|
| Patients (n) | 25 | 25 |
| Average age (years) | 82.1 ± 7.7 | 80.5 ± 6.1 |
| Average age at final draw (years) | 85.9 ± 7.5 | 83.9 ± 5.4 |
| Sex (n) | M (12), F (13) | M (14), F (11) |
| Average Participation span (years) | 7.7 | 7.3 |
| Average MMSE * | 28.1 ± 1.83 | 26.1 ± 2.8 |
| Biomarker Panel | scFvs in Panel |
|---|---|
| AD/preMCI | C6T, E1, D11C, ADTau2, ADTau4, ADTau6, AD-TDP3 |
| preAD | C6T, D11C, F9T, ADTau2, ADTau4, ADTau6, AD-TDP3 |
| Female preAD | C6T, E1, D11C, F9T, ADTau2, ADTau4, ADTau6, AD-TDP3 |
| Male preAD | C6T, D11C, F9T, ADTau2, ADTau4, ADTau6, AD-TDP3 |
| Description | Cases Detected /Total Cases | Average Years before Clinical AD Diagnosis |
|---|---|---|
| preAD biomarker panel | ||
| Diagnosis of AD cases from preMCI/MCI samples | 22/25 | 6.0 |
| Detection in 1st available preMCI/MCI sample | 14/25 | 6.7 |
| Diagnosis of AD cases from preMCI samples | 19/24 | 6.2 |
| Detection in 1st available preMCI sample | 13/24 | 6.8 |
| Female-biased biomarker panel | ||
| Diagnosis of female AD cases from preMCI/MCI samples | 11/11 | 6.5 |
| Detected in 1st available female preMCI/MCI sample | 7/11 | 7.1 |
| Diagnosis of female AD cases from preMCI samples | 10/11 | 6.5 |
| Detected in 1st available female preMCI sample | 7/11 | 7.1 |
| Male-biased biomarker panel | ||
| Diagnosis of male AD cases from preMCI/MCI samples | 13/14 | 6.1 |
| Detection in 1st available male preMCI/MCI sample | 9/14 | 6.6 |
| Diagnosis of male AD cases from preMCI samples | 11/13 | 6.6 |
| Detection in 1st available male preMCI sample | 8/13 | 6.8 |
| Combined female/male biomarker panel data | ||
| Diagnosis of female and male AD cases from preMCI/MCI samples | 24/25 | 6.3 |
| Combined female and male detection in 1st available preMCI/MCI sample | 16/25 | 6.8 |
| Diagnosis of female and male AD cases from preMCI samples | 21/24 | 6.5 |
| Combined female and male detection in 1st available preMCI sample | 15/24 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.J.; Schulz, P.; Venkataraman, L.; Caselli, R.J.; Sierks, M.R. Sex-Specific Multiparameter Blood Test for the Early Diagnosis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 15670. https://doi.org/10.3390/ijms232415670
Cho HJ, Schulz P, Venkataraman L, Caselli RJ, Sierks MR. Sex-Specific Multiparameter Blood Test for the Early Diagnosis of Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(24):15670. https://doi.org/10.3390/ijms232415670
Chicago/Turabian StyleCho, Hyung Joon, Philip Schulz, Lalitha Venkataraman, Richard J. Caselli, and Michael R. Sierks. 2022. "Sex-Specific Multiparameter Blood Test for the Early Diagnosis of Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 24: 15670. https://doi.org/10.3390/ijms232415670
APA StyleCho, H. J., Schulz, P., Venkataraman, L., Caselli, R. J., & Sierks, M. R. (2022). Sex-Specific Multiparameter Blood Test for the Early Diagnosis of Alzheimer’s Disease. International Journal of Molecular Sciences, 23(24), 15670. https://doi.org/10.3390/ijms232415670






