Gallium (III) Complexes Based on Aminobisphenolate Ligands: Extremely High Active ROP-Initiators from Well-Known and Easily Accessible Compounds
Abstract
1. Introduction
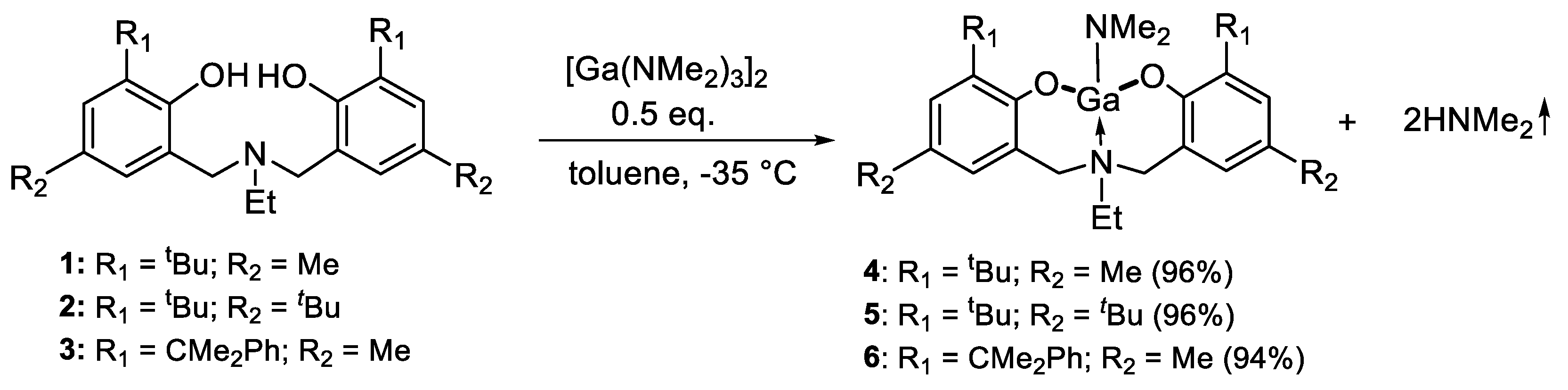
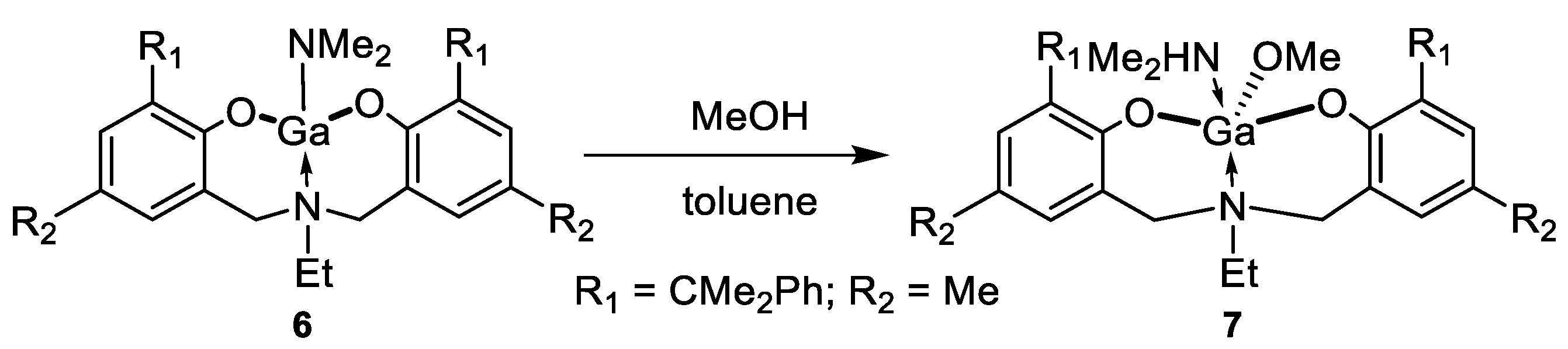
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Ligand 3
3.2. General Synthesis of Ga Complexes (4–6)
3.3. Synthesis of Complex 4
3.4. Synthesis of Complex 5
3.5. Synthesis of Complex 6
3.6. Typical Polymerization Procedure in Bulk
3.7. Single Crystal X-ray Diffraction Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, N.P.D.; Muthuvelu, K.S.; Arumugasamy, S.K. Recent Advancement in Biomedical Applications of Polycaprolactone and Polycaprolactone-Based Materials. In Encyclopedia of Materials: Plastics and Polymers; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2022; pp. 795–809. [Google Scholar]
- Mandal, P.; Shunmugam, R. Polycaprolactone: A biodegradable polymer with its application in the field of self-assembly study. J. Macromol. Sci. Part A 2021, 58, 111–129. [Google Scholar]
- Rani, G.U.; Sharma, S. Biopolymers, Bioplastics and Biodegradability: An Introduction. In Encyclopedia of Materials: Plastics and Polymers; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2022; pp. 474–486. [Google Scholar]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, D.; Zhang, W.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent progress in the application of group 1, 2 & 13 metal complexes as catalysts for the ring opening polymerization of cyclic esters. Inorg. Chem. Front. 2019, 6, 2619–2652. [Google Scholar]
- Dagorne, S.; Normand, M.; Kirillov, E.; Carpentier, J.-F. Gallium and indium complexes for ring-opening polymerization of cyclic ethers, esters and carbonates. Coord. Chem. Rev. 2013, 257, 1869–1886. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate, 1. Polymerization of ε-caprolactone. Macromol. Rapid Commun. 1998, 19, 567–572. [Google Scholar]
- Punyodom, W.; Limwanich, W.; Meepowpan, P.; Thapsukhon, B. Ring-opening polymerization of ε-caprolactone initiated by tin(II) octoate/n-hexanol: DSC isoconversional kinetics analysis and polymer synthesis. Des. Monomers Polym. 2021, 24, 89–97. [Google Scholar] [CrossRef]
- Giram, P.S.; Garnaik, B. Evaluation of biocompatibility of synthesized low molecular weight PLGA copolymers using zinc L-proline through green route for biomedical application. Polym. Adv. Technol. 2021, 32, 4502–4515. [Google Scholar]
- Wichmann, O.; Sillanpää, R.; Lehtonen, A. Structural properties and applications of multidentate [O,N,O,X′] aminobisphenolate metal complexes. Coord. Chem. Rev. 2012, 256, 371–392. [Google Scholar] [CrossRef]
- Akintayo, D.C.; Munzeiwa, W.A.; Jonnalagadda, S.B.; Omondi, B. Zn(II) pyridinyl amine complexes, synthesis and crystal structure studies: A comparative study of the effect of nuclearity and benzoate type on the ring-opening polymerization of cyclic esters. Polyhedron 2022, 213, 115589. [Google Scholar] [CrossRef]
- Cross, E.D.; Tennekone, G.K.; Decken, A.; Shaver, M.P. Aluminum amine-(bis)phenolate complexes for ring-opening polymerization of rac-lactide and ε-caprolactone. Green Mater. 2013, 1, 79–86. [Google Scholar] [CrossRef]
- Alcazar-Roman, L.M.; O’Keefe, B.J.; Hillmyer, M.A.; Tolman, W.B. Electronic influence of ligand substituents on the rate of polymerization of ε-caprolactone by single-site aluminium alkoxide catalysts. Dalton Trans. 2003, 15, 3082–3087. [Google Scholar]
- Chen, C.-T.; Huang, C.-A.; Huang, B.-H. Aluminium metal complexes supported by amine bis-phenolate ligands as catalysts for ring-opening polymerization of ε-caprolactone. Dalton Trans. 2003, 19, 3799–3803. [Google Scholar] [CrossRef]
- Chen, C.-T.; Huang, C.-A.; Huang, B.-H. Aluminum Complexes Supported by Tridentate Aminophenoxide Ligand as Efficient Catalysts for Ring-Opening Polymerization of ε-Caprolactone. Macromolecules 2004, 37, 7968–7973. [Google Scholar] [CrossRef]
- Phomphrai, K.; Chumsaeng, P.; Sangtrirutnugul, P.; Kongsaeree, P.; Pohmakotr, M. Reverse orders of reactivities in the polymerization of cyclic esters using N2O2 aluminium alkoxide complexes. Dalton Trans. 2010, 39, 1865–1871. [Google Scholar]
- Kuchuk, E.A.; Zaitsev, K.V.; Mamedova, F.A.; Churakov, A.V.; Zaitseva, G.S.; Lemenovsky, D.A.; Karlov, S.S. Synthesis, structure, and catalytic activity of new aluminum and titanium complexes based on aminobisphenolate ligands containing bulky substituents. Russ. Chem. Bull. 2016, 65, 1743–1749. [Google Scholar]
- Li, X.; Jia, Z.; Pan, X.; Wu, J. Isoselective Ring-Opening Polymerization of rac-Lactide Catalyzed by Sodium/potassium Tetradentate Aminobisphenolate Ion-paired Complexes. Chem. Asian J. 2019, 14, 662–669. [Google Scholar] [CrossRef]
- Devaine-Pressing, K.; Oldenburg, F.J.; Menzel, J.P.; Springer, M.; Dawe, L.N.; Kozak, C.M. Lithium, sodium, potassium and calcium amine-bis(phenolate) complexes in the ring-opening polymerization of rac-lactide. Dalton Trans. 2020, 49, 1531–1544. [Google Scholar] [CrossRef]
- Yao, C.; Yang, Y.; Xu, S.; Ma, H. Potassium complexes supported by monoanionic tetradentate amino-phenolate ligands: Synthesis, structure and catalysis in the ring-opening polymerization of rac-lactide. Dalton Trans. 2017, 46, 6087–6097. [Google Scholar]
- Devaine-Pressing, K.; Lehr, J.H.; Pratt, M.E.; Dawe, L.N.; Sarjeant, A.A.; Kozak, C.M. Magnesium amino-bis(phenolato) complexes for the ring-opening polymerization of rac-lactide. Dalton Trans. 2015, 44, 12365–12375. [Google Scholar] [CrossRef]
- Yuan, F.; Zhou, Y.; Li, L.; Zhu, X. Synthesis and structures of amine bis(phenolate) lanthanide thiolates and their application in the polymerization of ε-caprolactone. Inorg. Chim. Acta 2013, 408, 33–38. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Chen, J.; Yao, Y.; Luo, Y. Rare-earth metal derivatives supported by aminophenoxy ligand: Synthesis, characterization and catalytic performance in lactide polymerization. Appl. Organomet. Chem. 2020, 34, e5296. [Google Scholar] [CrossRef]
- White, S.J.O.; Shine, J.P. Exposure Potential and Health Impacts of Indium and Gallium, Metals Critical to Emerging Electronics and Energy Technologies. Curr. Environ. Health Rep. 2016, 3, 459–467. [Google Scholar] [PubMed]
- Chitambar, C.R. Medical Applications and Toxicities of Gallium Compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [PubMed]
- Hild, F.; Neehaul, N.; Bier, F.; Wirsum, M.; Gourlaouen, C.; Dagorne, S. Synthesis and Structural Characterization of Various N,O,N-Chelated Aluminum and Gallium Complexes for the Efficient ROP of Cyclic Esters and Carbonates: How Do Aluminum and Gallium Derivatives Compare ? Organometallics 2013, 32, 587–598. [Google Scholar] [CrossRef]
- Dodonov, V.A.; Morozov, A.G.; Rumyantsev, R.V.; Fukin, G.K.; Skatova, A.A.; Roesky, P.W.; Fedushkin, I.L. Synthesis and ε-Caprolactone Polymerization Activity of Electron-Deficient Gallium and Aluminum Species Containing a Charged Redox-Active dpp-Bian Ligand. Inorg. Chem. 2019, 58, 16559–16573. [Google Scholar] [CrossRef]
- Basiak, D.; Dobrzycki, Ł.; Socha, P.; Rzepiński, P.; Plichta, A.; Bujnowski, K.; Synoradzki, L.; Orłowska, N.; Ziemkowska, W. Aminophenolates of aluminium, gallium and zinc: Synthesis, characterization and polymerization activity. Appl. Organomet. Chem. 2017, 31, e3748. [Google Scholar] [CrossRef]
- Bakewell, C.; White, A.J.P.; Long, N.J.; Williams, C.K. 8-Quinolinolato Gallium Complexes: Iso-selective Initiators for rac-Lactide Polymerization. Inorg. Chem. 2013, 52, 12561–12567. [Google Scholar]
- Beament, J.; Mahon, M.F.; Buchard, A.; Jones, M.D. Salan group 13 complexes—Structural study and lactide polymerisation. New J. Chem. 2017, 41, 2198–2203. [Google Scholar] [CrossRef]
- Specklin, D.; Fliedel, C.; Hild, F.; Mameri, S.; Karmazin, L.; Bailly, C.; Dagorne, S. Mononuclear salen-gallium complexes for iso-selective ring-opening polymerization (ROP) of rac-lactide. Dalton Trans. 2017, 46, 12824–12834. [Google Scholar] [PubMed]
- Horeglad, P.; Cybularczyk, M.; Trzaskowski, B.; Żukowska, G.Z.; Dranka, M.; Zachara, J. Dialkylgallium Alkoxides Stabilized with N-Heterocyclic Carbenes: Opportunities and Limitations for the Controlled and Stereoselective Polymerization of rac-Lactide. Organometallics 2015, 34, 3480–3496. [Google Scholar]
- Ghosh, S.; Gowda, R.R.; Jagan, R.; Chakraborty, D. Gallium and indium complexes containing the bis(imino)phenoxide ligand: Synthesis, structural characterization and polymerization studies. Dalton Trans. 2015, 44, 10410–10422. [Google Scholar] [CrossRef] [PubMed]
- Cybularczyk-Cecotka, M.; Zaremba, R.; Hurko, A.; Plichta, A.; Dranka, M.; Horeglad, P. Dialkylgallium alkoxides—A tool for facile and stereoselective synthesis of PLA–drug conjugates. New J. Chem. 2017, 41, 14851–14854. [Google Scholar] [CrossRef]
- Kremer, A.B.; Andrews, R.J.; Milner, M.J.; Zhang, X.R.; Ebrahimi, T.; Patrick, B.O.; Diaconescu, P.L.; Mehrkhodavandi, P. A Comparison of Gallium and Indium Alkoxide Complexes as Catalysts for Ring-Opening Polymerization of Lactide. Inorg. Chem. 2017, 56, 1375–1385. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Martell, A.E.; Koch, S.A.; Hwang, J.; Quarless, D.A.; Welch, M.J. The Gallium(III) and Indium(III) Complexes of Tris(2-mercaptobenzyl)amine and Tris(2-hydroxybenzyl)amine. Inorg. Chem. 1998, 37, 5902–5911. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Lettenbauer, J.; Kumberger, O.; Lachmann, J.; Müller, G. Modellsysteme für die Gallium-Extraktion, II/Model Systems for Gallium Extraction, II. Zeitschrift Naturforschung B 1991, 46, 1065–1076. [Google Scholar] [CrossRef]
- Lanznaster, M.; Hratchian, H.P.; Heeg, M.J.; Hryhorczuk, L.M.; McGarvey, B.R.; Schlegel, H.B.; Verani, C.N. Structural and Electronic Behavior of Unprecedented Five-Coordinate Iron(III) and Gallium(III) Complexes with a New Phenol-Rich Electroactive Ligand. Inorg. Chem. 2006, 45, 955–957. [Google Scholar]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Ershova, I.V.; Bogomyakov, A.S.; Fukin, G.K.; Piskunov, A.V. Features of Magnetic Behavior in the Row of Pentacoordinated Bis-o-Iminobenzosemiquinonato Metal (Al, Ga, In) Complexes. Ber. Dtsch. Chem. Ges. 2019, 2019, 938–948. [Google Scholar]
- Ajellal, N.; Carpentier, J.-F.; Guillaume, C.; Guillaume, S.M.; Helou, M.; Poirier, V.; Sarazin, Y.; Trifonov, A. Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 2010, 39, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Liu, D.-C.; Ko, B.-T. Synthesis, characterization and reactivity of single-site aluminium amides bearing benzotriazole phenoxide ligands: Catalysis for ring-opening polymerization of lactide and carbon dioxide/propylene oxide coupling. Dalton Trans. 2013, 42, 11488–11496. [Google Scholar] [PubMed]
- Ikpo, N.; Saunders, L.N.; Walsh, J.L.; Smith, J.M.B.; Dawe, L.N.; Kerton, F.M. Zinc Complexes of Piperazinyl-Derived Aminephenolate Ligands: Synthesis, Characterization and Ring–Opening Polymerization Activity. Ber. Dtsch. Chem. Ges. 2011, 2011, 5347–5359. [Google Scholar] [CrossRef]
- Stirling, E.; Champouret, Y.; Visseaux, M. Catalytic metal-based systems for controlled statistical copolymerisation of lactide with a lactone. Polym. Chem. 2018, 9, 2517–2531. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Kuchuk, E.A.; Churakov, A.V.; Navasardyan, M.A.; Egorov, M.P.; Zaitseva, G.S.; Karlov, S.S. Synthesis and structural characterization of low-valent group 14 metal complexes based on aminobisphenol ligands. Inorg. Chim. Acta 2017, 461, 213–220. [Google Scholar] [CrossRef]
- Safaei, E.; Rasouli, M.; Weyhermüller, T.; Bill, E. Synthesis and characterization of binuclear [ONXO]-type amine-bis(phenolate) copper(II) complexes. Inorg. Chim. Acta 2011, 375, 158–165. [Google Scholar] [CrossRef]
- Waggoner, K.M.; Olmstead, M.M.; Power, P.P. Structural and spectroscopic characterization of the compounds [Al(NMe2)3]2, [Ga(NMe2)3]2, [(Me2N)2Al{μ-N(H)1-Ad}]2 (1-Ad = 1-adamantanyl) and [{Me(μ-NPh2)Al}2NPh(μ-C6H4)]. Polyhedron 1990, 9, 257–263. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS. In Program for Scaling and Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]

| Entry | Catalyst, [cat] | t, [min] | Conversion, [%] | Mn a (calc), [g/mol] | Mn b (exp), [g/mol] | PDI |
|---|---|---|---|---|---|---|
| ε-caprolactone (80 °C, [M]0/[cat] = 200:1) | ||||||
| 1 | 4 | 15 | 100 | 22,800 | 9172 | 1.80 |
| 2 | 5 | 15 | 100 | 22,800 | 15,407 | 1.51 |
| 3 | 6 | 15 | 100 | 22,800 | 28,538 | 1.47 |
| ε-caprolactone (100 °C, [M]0/[cat] = 500:1) | ||||||
| 4 | 5 | 15 | 86 | - | - | - |
| 5 | 30 | 100 | 72,000 | 42,023 | 1.79 | |
| ε-caprolactone (25 °C, [M]0/[cat] = 200:1) | ||||||
| 6 | 5 | 15 | 100 | 22,800 | 12,270 | 1.38 |
| L-lactide (100 °C, [M]0/[cat] = 200:1) | ||||||
| 7 | 4 | 15 | 75 | - | - | - |
| 8 | 30 | 100 | 28,800 | 28,620 | 1.36 | |
| 9 | 5 | 15 | 79 | - | - | - |
| 10 | 30 | 100 | 28,800 | 21,595 | 1.17 | |
| 11 | 6 | 15 | 71 | - | - | - |
| 12 | 30 | 100 | 28,800 | 21,834 | 1.26 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mankaev, B.N.; Hasanova, L.F.; Churakov, A.V.; Egorov, M.P.; Karlov, S.S. Gallium (III) Complexes Based on Aminobisphenolate Ligands: Extremely High Active ROP-Initiators from Well-Known and Easily Accessible Compounds. Int. J. Mol. Sci. 2022, 23, 15649. https://doi.org/10.3390/ijms232415649
Mankaev BN, Hasanova LF, Churakov AV, Egorov MP, Karlov SS. Gallium (III) Complexes Based on Aminobisphenolate Ligands: Extremely High Active ROP-Initiators from Well-Known and Easily Accessible Compounds. International Journal of Molecular Sciences. 2022; 23(24):15649. https://doi.org/10.3390/ijms232415649
Chicago/Turabian StyleMankaev, Badma N., Leyla F. Hasanova, Andrei V. Churakov, Mikhail P. Egorov, and Sergey S. Karlov. 2022. "Gallium (III) Complexes Based on Aminobisphenolate Ligands: Extremely High Active ROP-Initiators from Well-Known and Easily Accessible Compounds" International Journal of Molecular Sciences 23, no. 24: 15649. https://doi.org/10.3390/ijms232415649
APA StyleMankaev, B. N., Hasanova, L. F., Churakov, A. V., Egorov, M. P., & Karlov, S. S. (2022). Gallium (III) Complexes Based on Aminobisphenolate Ligands: Extremely High Active ROP-Initiators from Well-Known and Easily Accessible Compounds. International Journal of Molecular Sciences, 23(24), 15649. https://doi.org/10.3390/ijms232415649








