Different Molecular Forms of TFF3 in the Human Respiratory Tract: Heterodimerization with IgG Fc Binding Protein (FCGBP) and Proteolytic Cleavage in Bronchial Secretions
Abstract
:1. Introduction
2. Results
2.1. Characterization of TFF3 Forms in Human Bronchial Tissue Specimens
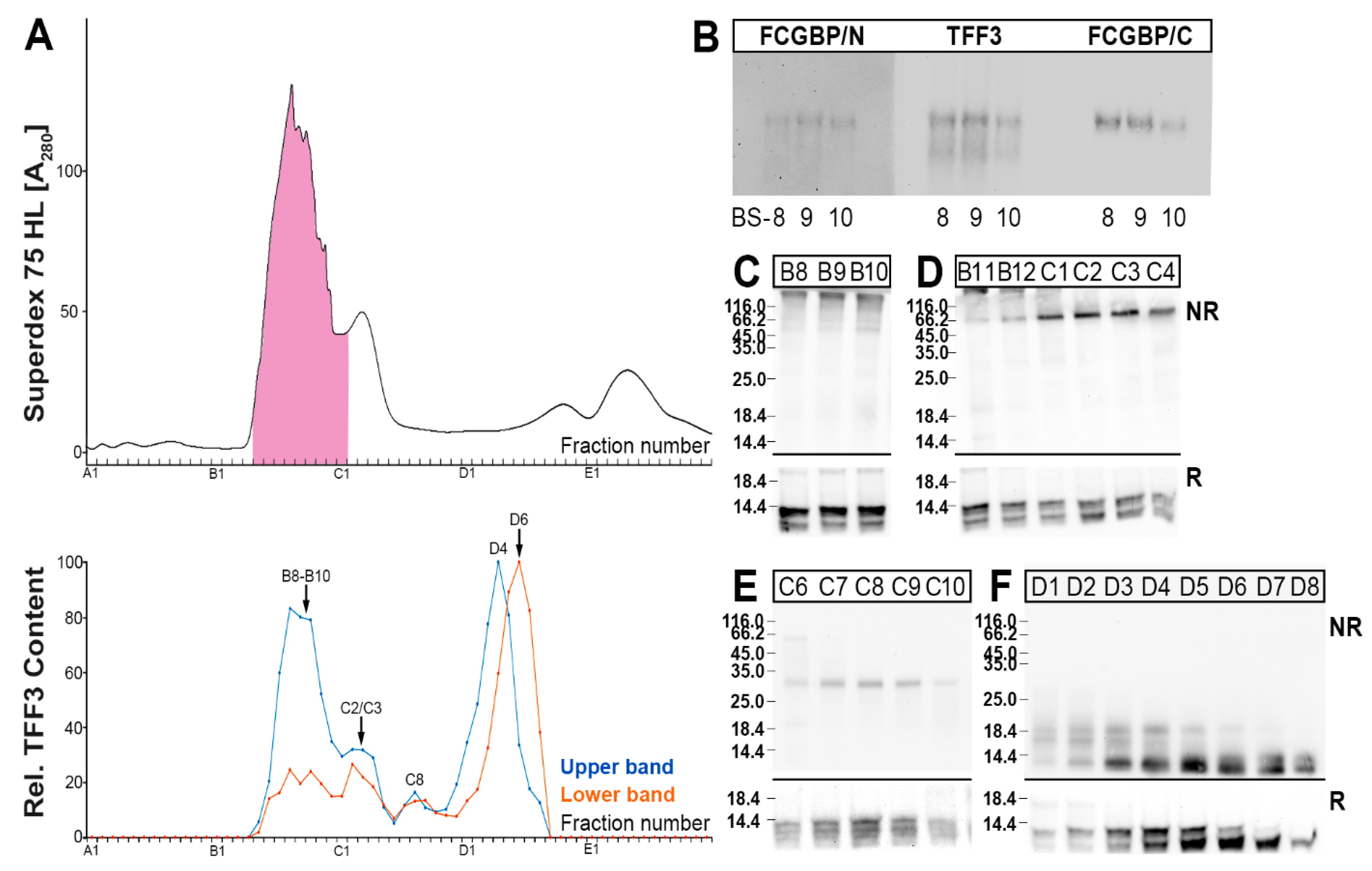
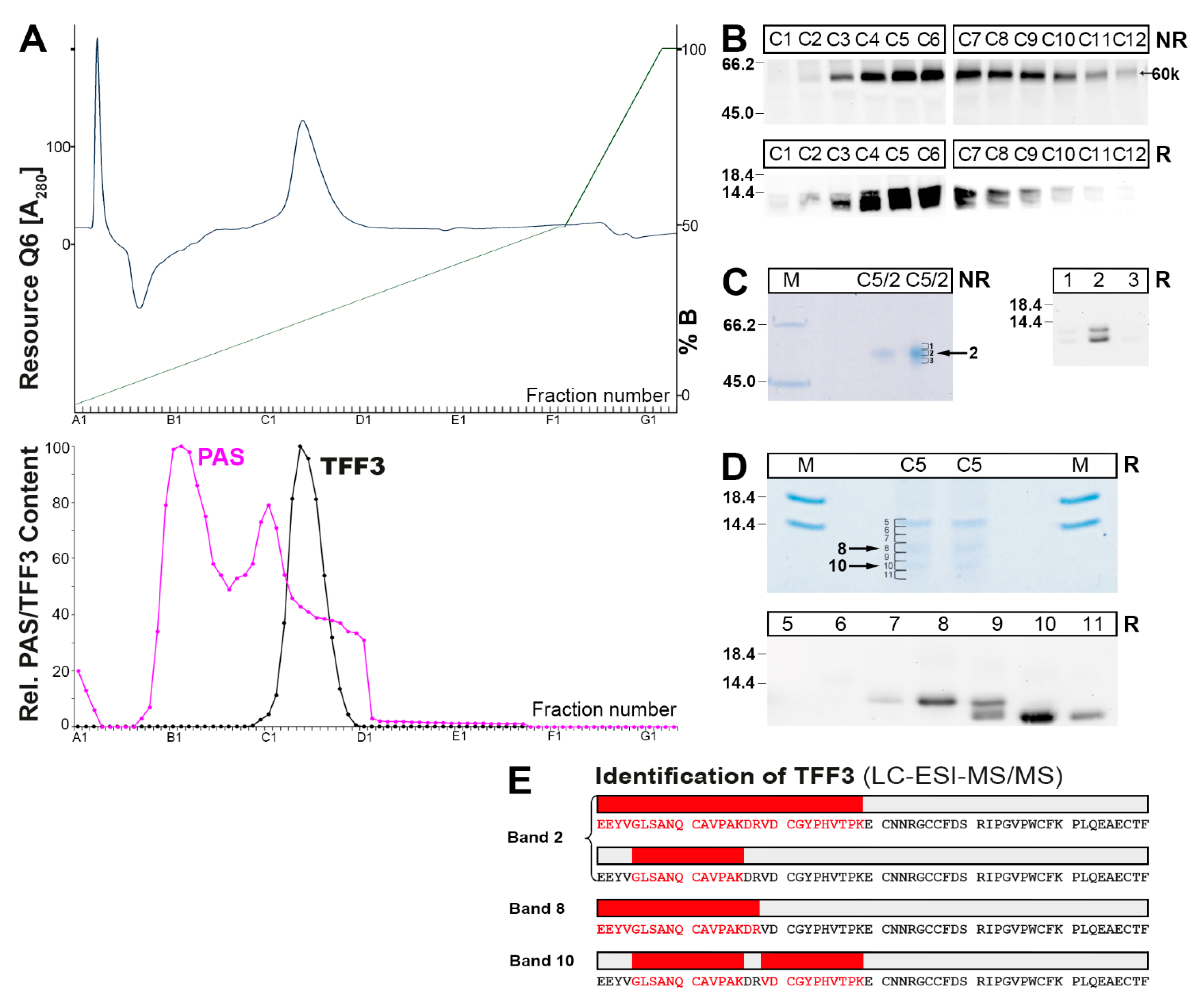
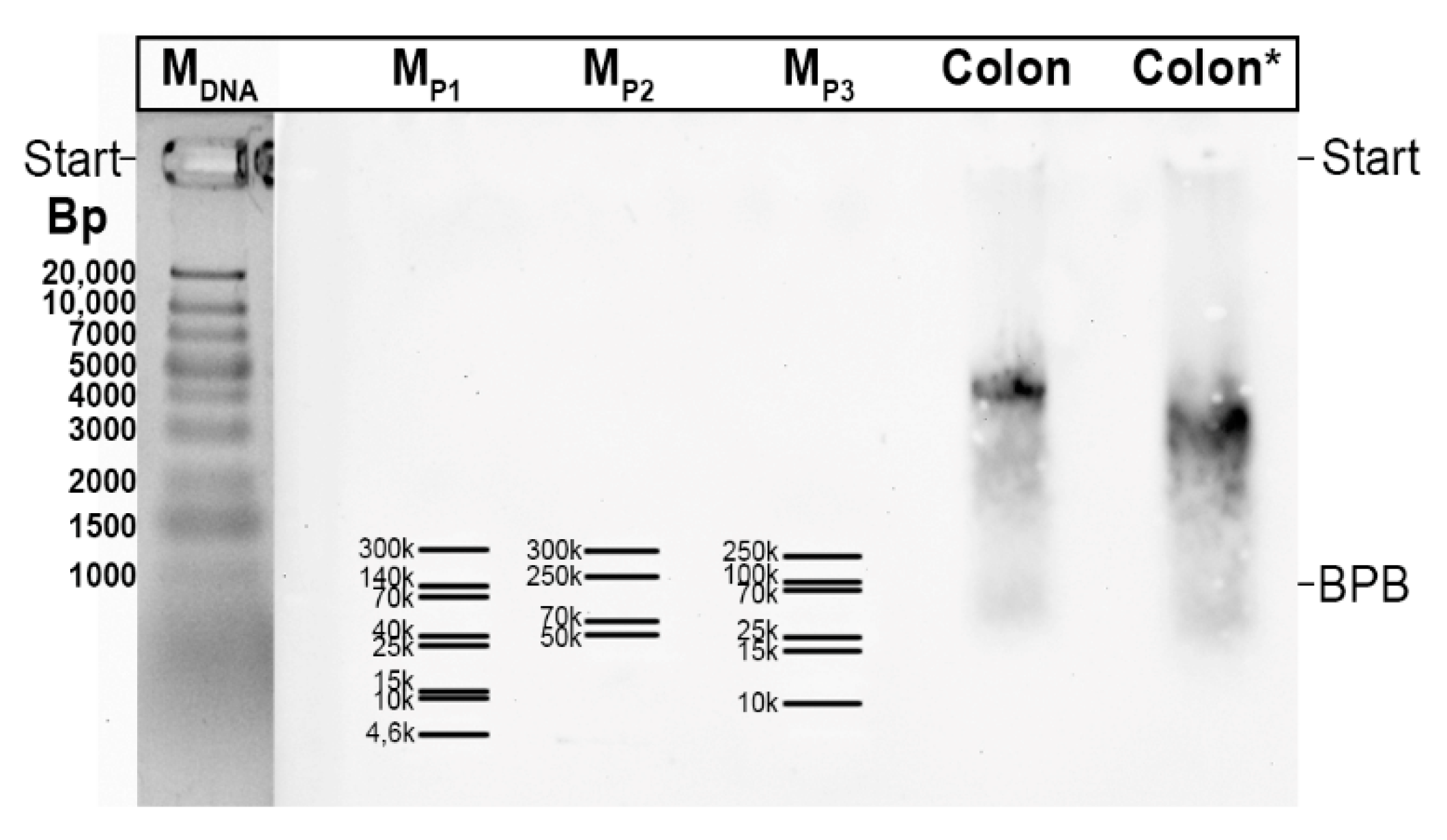
2.2. Characterization of Multiple TFF3 Forms in Human Bronchial Secretions
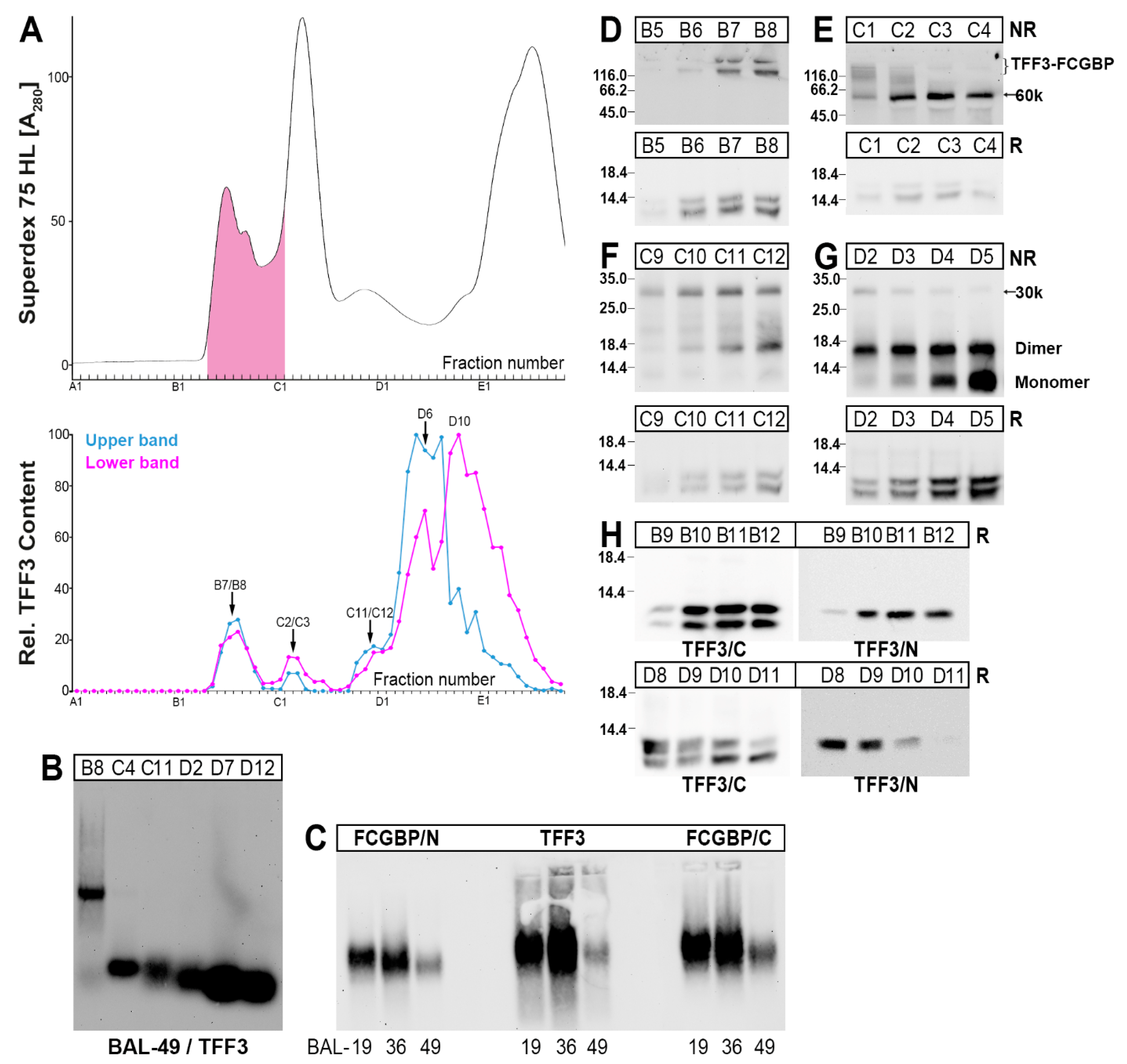
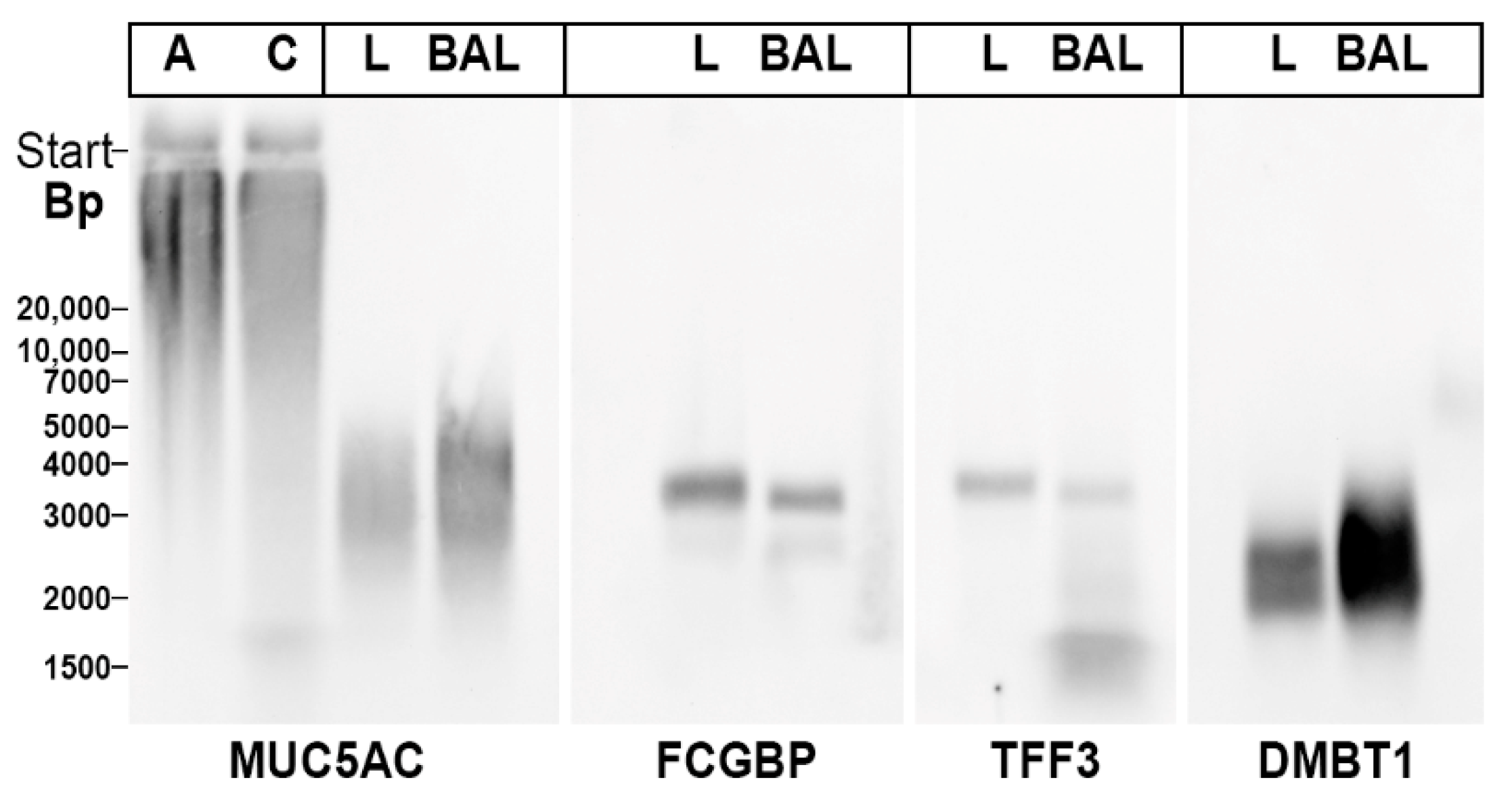
2.3. Characterization of Multiple TFF3 Forms in Human BAL Fluid
2.4. Interactions of TFF3-FCGBP with DMBT1gp340
3. Discussion
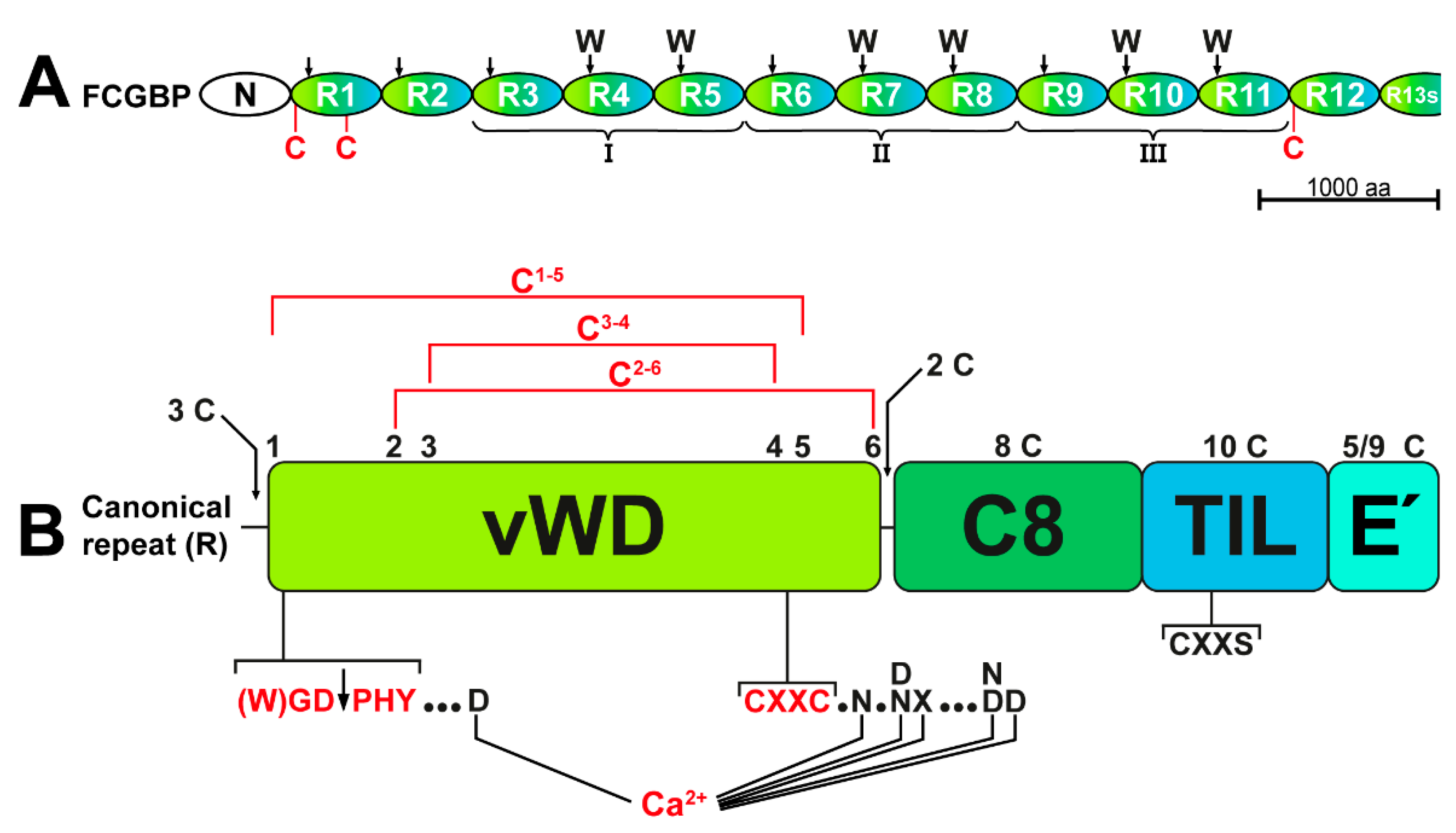
3.1. TFF3 from the Human Respiratory Tract Forms High-Molecular-Mass Heterodimers with FCGBP
3.2. TFF3-FCGBP Forms Oligomers and Does Not Bind to DMBT1gp340
3.3. Respiratory Tract Secretory Specimens Contain 60k and 30k TFF3 Heterodimers
3.4. Degradation of TFF3 in Bronchial Secretions
4. Materials and Methods
4.1. Human Specimens
4.2. Protein Purification by SEC and Anion-Exchange Chromatography
4.3. SDS-PAGE, Agarose Gel Electrophoresis, and Western Blot Analysis
4.4. Identification of Proteins by Bottom-Up Proteomics
4.4.1. Tryptic In-Gel Digestion
4.4.2. LC-MS/MS Analysis of the Tryptic Peptides
4.4.3. LC-MS/MS Data Processing and Protein Identification
5. Conclusions and Medical Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgGE | Agarose gel electrophoresis |
| BAL | Bronchoalveolar lavage |
| BS | Bronchial secretion |
| COPD | Chronic obstructive pulmonary disease |
| FCGBP | IgG Fc binding protein |
| PAS | Periodic acid Schiff |
| ROS | Reactive oxygen species |
| TFF | Trefoil factor family |
| vWF | von Willebrand factor |
References
- Hauser, F.; Poulsom, R.; Chinery, R.; Rogers, L.A.; Hanby, A.M.; Wright, N.A.; Hoffmann, W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc. Natl. Acad. Sci. USA 1993, 90, 6961–6965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjellev, S. The trefoil factor family–small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides. Encyclopedia 2021, 1, 974–987. [Google Scholar] [CrossRef]
- Wiede, A.; Jagla, W.; Welte, T.; Kohnlein, T.; Busk, H.; Hoffmann, W. Localization of TFF3, a new mucus-associated peptide of the human respiratory tract. Am. J. Respir. Crit. Care Med. 1999, 159, 1330–1335. [Google Scholar] [CrossRef] [Green Version]
- Jagla, W.; Wiede, A.; Hinz, M.; Dietzmann, K.; Gülicher, D.; Gerlach, K.L.; Hoffmann, W. Secretion of TFF-peptides by human salivary glands. Cell Tissue Res. 1999, 298, 161–166. [Google Scholar] [CrossRef]
- Wiede, A.; Hinz, M.; Canzler, E.; Franke, K.; Quednow, C.; Hoffmann, W. Synthesis and localization of the mucin-associated TFF-peptides in the human uterus. Cell Tissue Res. 2001, 303, 109–115. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Gerlach, K.L.; Zahl, C.; Hoffmann, W. Expression analysis of human salivary glands by laser microdissection: Differences between submandibular and labial glands. Cell. Physiol. Biochem. 2010, 26, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.; Nielsen, O.; Tornøe, I.; Thim, L.; Holmskov, U. Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 2007, 55, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–181. [Google Scholar] [PubMed]
- Probst, J.C.; Skutella, T.; Müller-Schmid, A.; Jirikowski, G.F.; Hoffmann, W. Molecular and cellular analysis of rP1.B in the rat hypothalamus: In situ hybridization and immunohistochemistry of a new P-domain neuropeptide. Mol. Brain Res. 1995, 33, 269–276. [Google Scholar] [CrossRef]
- Carraro, G.; Langerman, J.; Sabri, S.; Lorenzana, Z.; Purkayastha, A.; Zhang, G.; Konda, B.; Aros, C.J.; Calvert, B.A.; Szymaniak, A.; et al. Transcriptional analysis of cystic fibrosis airways at single-cell resolution reveals altered epithelial cell states and composition. Nat. Med. 2021, 27, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.H.; Oh, B.H.; Lee, H.M.; Choi, J.O.; Jung, K.Y. Expression of mRNA of trefoil factor peptides in human nasal mucosa. Acta Otolaryngol. 2001, 121, 849–853. [Google Scholar]
- dos Santos Silva, E.; Ulrich, M.; Döring, G.; Botzenhart, K.; Gött, P. Trefoil factor family domain peptides in the human respiratory tract. J. Pathol. 2000, 190, 133–142. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Chwieralski, C.E.; Bälder, R.; Hinz, M.; Braun, A.; Krug, N.; Hoffmann, W. Induced trefoil factor family 1 expression by trans-differentiating Clara cells in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 2007, 36, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viby, N.E.; Nexø, E.; Kissow, H.; Andreassen, H.; Clementsen, P.; Thim, L.; Poulsen, S.S. Trefoil factors (TFFs) are increased in bronchioalveolar lavage fluid from patients with chronic obstructive lung disease (COPD). Peptides 2015, 63, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, J.A.; Fakih, D.; Arike, L.; Rodríguez-Piñeiro, A.M.; Martínez-Abad, B.; Skansebo, E.; Jackson, S.; Root, J.; Singh, D.; McCrae, C.; et al. Attached stratified mucus separates bacteria from the epithelial cells in COPD lungs. JCI Insight 2018, 3, e120994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viby, N.E.; Pedersen, L.; Lund, T.K.; Kissow, H.; Backer, V.; Nexø, E.; Thim, L.; Poulsen, S.S. Trefoil factor peptides in serum and sputum from subjects with asthma and COPD. Clin. Respir. J. 2015, 9, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Bastholm, S.K.; Samson, M.H.; Becher, N.; Hansen, L.K.; Stubbe, P.R.; Chronakis, I.S.; Nexo, E.; Uldbjerg, N. Trefoil factor peptide 3 is positively correlated with the viscoelastic properties of the cervical mucus plug. Acta Obstetr. Gynecol. Scand. 2017, 96, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, E.P.; Ali, T.; Leonard, P.; Hearty, S.; O’Kennedy, R.; May, F.E.; Westley, B.R.; Josenhans, C.; Rust, M.; Suerbaum, S.; et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 2008, 135, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Järvå, M.A.; Lingford, J.P.; John, A.; Soler, N.M.; Scott, N.E.; Goddard-Borger, E.D. Trefoil factors share a lectin activity that defines their role in mucus. Nat. Commun. 2020, 11, 2265. [Google Scholar] [CrossRef]
- LeSimple, P.; van Seuningen, I.; Buisine, M.P.; Copin, M.C.; Hinz, M.; Hoffmann, W.; Hajj, R.; Brody, S.L.; Coraux, C.; Puchelle, E. Trefoil factor family 3 peptide promotes human airway epithelial ciliated cell differentiation. Am. J. Respir. Cell Mol. Biol. 2007, 36, 296–303. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF (trefoil factor family) peptides and their potential roles for differentiation processes during airway remodeling. Curr. Med. Chem. 2007, 14, 2716–2719. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil factor family (TFF) peptides and chemokine receptors: A promising relationship. J. Med. Chem. 2009, 52, 6505–6510. [Google Scholar] [CrossRef] [PubMed]
- Riemer, J.; Bulleid, N.; Herrmann, J.M. Disulfide formation in the ER and mitochondria: Two solutions to a common process. Science 2009, 324, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Sparvoli, A.; Fagioli, C.; Fassina, G.; Sitia, R. Formation of reversible disulfide bonds with the protein matrix of the endoplasmic reticulum correlates with the retention of unassembled Ig light chains. EMBO J. 1996, 15, 2077–2085. [Google Scholar] [CrossRef]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef]
- Houben, T.; Harder, S.; Schlüter, H.; Kalbacher, H.; Hoffmann, W. Different Forms of TFF3 in the Human Saliva: Heterodimerization with IgG Fc Binding Protein (FCGBP). Int. J. Mol. Sci. 2019, 20, 5000. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Salivary Trefoil Factor Family (TFF) Peptides and Their Roles in Oral and Esophageal Protection: Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 12221. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Kouznetsova, I.; Kalinski, T.; Meyer, F.; Hoffmann, W. Self-renewal of the human gastric epithelium: New insights from expression profiling using laser microdissection. Mol. Biosyst. 2011, 7, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. von Willebrand factor, Jedi knight of the bloodstream. Blood 2014, 124, 1412–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joba, W.; Hoffmann, W. Similarities of integumentary mucin B.1 from Xenopus laevis and prepro-von Willebrand factor at their amino-terminal regions. J. Biol. Chem. 1997, 272, 1805–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javitt, G.; Khmelnitsky, L.; Albert, L.; Bigman, L.S.; Elad, N.; Morgenstern, D.; Ilani, T.; Levy, Y.; Diskin, R.; Fass, D. Assembly Mechanism of Mucin and von Willebrand Factor Polymers. Cell 2020, 183, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Mucins and the Microbiome. Annu. Rev. Biochem. 2020, 89, 769–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, J.; Heuer, F.; Stürmer, R.; Harder, S.; Schlüter, H.; Braga Emidio, N.; Muttenthaler, M.; Jechorek, D.; Meyer, F.; Hoffmann, W. The Tumor Suppressor TFF1 Occurs in Different Forms and Interacts with Multiple Partners in the Human Gastric Mucus Barrier: Indications for Diverse Protective Functions. Int. J. Mol. Sci. 2020, 21, 2508. [Google Scholar] [CrossRef] [Green Version]
- Znalesniak, E.B.; Salm, F.; Hoffmann, W. Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 644. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Klasson, S.; Larsson, E.; Johansson, M.E.; Hansson, G.C.; Samuelsson, T. Searching the Evolutionary Origin of Epithelial Mucus Protein Components-Mucins and FCGBP. Mol. Biol. Evol. 2016, 33, 1921–1936. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and their Different Roles in the Mucosal Innate Immune Defense and More: An Update. Curr. Med. Chem. 2021, 28, 7387–7399. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, X.; Li, Y.; Zhang, J.R.; Zhu, S.J.; Yang, Q.Y.; Zhang, W.; Gong, L. Role of the mucin-like glycoprotein FCGBP in mucosal immunity and cancer. Front. Immunol. 2022, 13, 863317. [Google Scholar] [CrossRef]
- Li, C.; Wang, R.; Su, B.; Luo, Y.; Terhune, J.; Beck, B.; Peatman, E. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 2013, 39, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ehrencrona, E.; van der Post, S.; Gallego, P.; Recktenwald, C.V.; Rodriguez-Pineiro, A.M.; Garcia-Bonete, M.J.; Trillo-Muyo, S.; Bäckström, M.; Hansson, G.C.; Johansson, M.E.V. The IgGFc-binding protein FCGBP is secreted with all GDPH sequences cleaved but maintained by interfragment disulfide bonds. J. Biol. Chem. 2021, 297, 100871. [Google Scholar] [CrossRef]
- Carpenter, J.; Wang, Y.; Gupta, R.; Li, Y.; Haridass, P.; Subramani, D.B.; Reidel, B.; Morton, L.; Ridley, C.; O’Neal, W.K.; et al. Assembly and organization of the N-terminal region of mucin MUC5AC: Indications for structural and functional distinction from MUC5B. Proc. Natl. Acad. Sci. USA 2021, 118, e2104490118. [Google Scholar] [CrossRef] [PubMed]
- Ermund, A.; Meiss, L.N.; Rodriguez-Pineiro, A.M.; Bähr, A.; Nilsson, H.E.; Trillo-Muyo, S.; Ridley, C.; Thornton, D.J.; Wine, J.J.; Hebert, H.; et al. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem. Biophys. Res. Commun. 2017, 492, 331–337. [Google Scholar] [CrossRef]
- Batson, B.D.; Zorn, B.T.; Radicioni, G.; Livengood, S.S.; Kumagai, T.; Dang, H.; Ceppe, A.; Clapp, P.W.; Tunney, M.; Elborn, J.S.; et al. Cystic Fibrosis Airway Mucus Hyperconcentration Produces a Vicious Cycle of Mucin, Pathogen, and Inflammatory Interactions that Promotes Disease Persistence. Am. J. Respir. Cell Mol. Biol. 2022, 67, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Iijima, S.; Kobayashi, K.; Yoshida, T.; Brown, W.R.; Hibi, T.; Oshima, A.; Morikawa, M. Human IgGFc binding protein (FcgammaBP) in colonic epithelial cells exhibits mucin-like structure. J. Biol. Chem. 1997, 272, 15232–15241. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Leksa, N.C.; Chhabra, E.S.; Arndt, J.W.; Lu, Q.; Knockenhauer, K.E.; Peters, R.T.; Springer, T.A. The von Willebrand factor D’D3 assembly and structural principles for factor VIII binding and concatemer biogenesis. Blood 2019, 133, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Chivers, P.T.; Prehoda, K.E.; Raines, R.T. The CXXC motif: A rheostat in the active site. Biochemistry 1997, 36, 4061–4066. [Google Scholar] [CrossRef]
- Ellgaard, L.; Ruddock, L.W. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005, 6, 28–32. [Google Scholar] [CrossRef]
- Shishkin, S.S.; Eremina, L.S.; Kovalev, L.I.; Kovaleva, M.A. AGR2, ERp57/GRP58, and some other human protein disulfide isomerases. Biochemistry 2013, 78, 1415–1430. [Google Scholar] [CrossRef]
- Ilani, T.; Reznik, N.; Yeshaya, N.; Feldman, T.; Vilela, P.; Lansky, Z.; Javitt, G.; Shemesh, M.; Brenner, O.; Elkis, Y.; et al. The disulfide catalyst QSOX1 maintains the colon mucosal barrier by regulating Golgi glycosyltransferases. EMBO J. 2022, e111869. [Google Scholar] [CrossRef]
- Wilhelm, B.; Keppler, C.; Henkeler, A.; Schilli-Westermann, M.; Linder, D.; Aumüller, G.; Seitz, J. Identification and characterization of an IgG binding protein in the secretion of the rat coagulating gland. Biol. Chem. 2002, 383, 1959–1965. [Google Scholar] [CrossRef]
- Lidell, M.E.; Johansson, M.E.; Hansson, G.C. An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J. Biol. Chem. 2003, 278, 13944–13951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lidell, M.E.; Hansson, G.C. Cleavage in the GDPH sequence of the C-terminal cysteine-rich part of the human MUC5AC mucin. Biochem. J. 2006, 399, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osička, R.; Procházková, K.; Sulc, M.; Linhartová, I.; Havlíček, V.; Šebo, P. A novel “clip-and-link” activity of repeat in toxin (RTX) proteins from gram-negative pathogens. Covalent protein cross-linking by an Asp-Lys isopeptide bond upon calcium-dependent processing at an Asp-Pro bond. J. Biol. Chem. 2004, 279, 24944–24956. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.H.; Healey, E.; van Erp, S.; Bishop, B.; Tang, C.; Gilbert, R.J.C.; Radu Aricescu, A.; Pasterkamp, R.J.; Siebold, C. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science 2013, 341, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Vilar, J. Mucin granule intraluminal organization. Am. J. Respir. Cell Mol. Biol. 2007, 36, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Pestov, N.B.; Korneenko, T.V.; Shakhparonov, M.I.; Shull, G.E.; Modyanov, N.N. Loss of acidification of anterior prostate fluids in Atp12a-null mutant mice indicates that nongastric H-K-ATPase functions as proton pump in vivo. Am. J. Physiol. Cell Physiol. 2006, 291, C366–C374. [Google Scholar] [CrossRef] [Green Version]
- Ehrencrona, E. The Role of FCGBP in Mucus: Structure, Processing and Function. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2021. [Google Scholar]
- Blanchard, C.; Durual, S.; Estienne, M.; Bouzakri, K.; Heim, M.H.; Blin, N.; Cuber, J.C. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: Requirement for STAT6 and de novo protein synthesis. J. Immunol. 2004, 172, 3775–3783. [Google Scholar] [CrossRef] [Green Version]
- Zhen, G.; Park, S.W.; Nguyenvu, L.T.; Rodriguez, M.W.; Barbeau, R.; Paquet, A.C.; Erle, D.J. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am. J. Respir. Cell Mol. Biol. 2007, 36, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef] [PubMed]
- Nexoe, A.B.; Pedersen, A.A.; von Huth, S.; Detlefsen, S.; Hansen, P.L.; Holmskov, U. Immunohistochemical localization of deleted in malignant brain tumors I in normal human tissues. J. Histochem. Cytochem. 2020, 68, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.; Sorensen, G.L.; Nielsen, O.; Tornoe, I.; Thim, L.; Fenger, C.; Mollenhauer, J.; Holmskov, U. A variant of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increase expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PLoS ONE 2013, 8, e64441. [Google Scholar] [CrossRef] [Green Version]
- Kelly, E.; Greene, C.M.; McElvaney, N.G. Targeting neutrophil elastase in cystic fibrosis. Expert Opin. Ther. Targets 2008, 12, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Shinbashi, M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules 2021, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, M.C.; Brown, R.; Ryan, S.; Mall, M.A.; Weldon, S.; Taggart, C.C. Proteases, Mucus, and Mucosal Immunity in Chronic Lung Disease. Int. J. Mol. Sci. 2021, 22, 5018. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kim, S.Y.; Shin, S.; Jung, S.-H.; Yim, S.-H.; Lee, J.Y.; Lee, S.-H.; Chung, Y.-J. Overexpression of TFF3 is involved in prostate carcinogenesis via blocking mitochondria-mediated apoptosis. Exper. Mol. Med. 2018, 50, 110. [Google Scholar] [CrossRef] [Green Version]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.C.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The history and mystery of alveolar epithelial type II cells: Focus on their physiologic and pathologic role in lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef]
- Rösler, S.; Haase, T.; Claassen, H.; Schulze, U.; Schicht, M.; Riemann, D.; Brandt, J.; Wohlrab, D.; Müller-Hilke, B.; Goldring, M.B.; et al. Trefoil factor 3 is induced during degenerative and inflammatory joint disease, activates matrix metalloproteinases, and enhances apoptosis of articular cartilage chondrocytes. Arthritis Rheum. 2010, 62, 815–825. [Google Scholar] [CrossRef]
- Stürmer, R.; Reising, J.; Hoffmann, W. The TFF peptides xP1 and xP4 appear in distinctive forms in the Xenopus laevis gastric mucosa: Indications for different protective functions. Int. J. Mol. Sci. 2019, 20, 6052. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.G.; Ragge, H.; Kalinski, T.; Meyer, F.; Kalbacher, H.; Hoffmann, W. Human gastric TFF2 peptide contains an N-linked fucosylated N,N′-diacetyllactosediamine (LacdiNAc) oligosaccharide. Glycobiology 2013, 23, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, F.; Stürmer, R.; Heuer, J.; Kalinski, T.; Lemke, A.; Meyer, F.; Hoffmann, W. Different Forms of TFF2, A Lectin of the Human Gastric Mucus Barrier: In Vitro Binding Studies. Int. J. Mol. Sci. 2019, 20, 5871. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, R.; Müller, S.; Hanisch, F.G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial Porcine Gastric Mucin Preparations, also Used as Artificial Saliva, are a Rich Source for the Lectin TFF2: In Vitro Binding Studies. Chembiochem 2018, 19, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, I.; Laubinger, W.; Kalbacher, H.; Kalinski, T.; Meyer, F.; Roessner, A.; Hoffmann, W. Biosynthesis of gastrokine-2 in the human gastric mucosa: Restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins. Cell. Physiol. Biochem. 2007, 20, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagla, W.; Wiede, A.; Kölle, S.; Hoffmann, W. Differential expression of the TFF-peptides xP1 and xP4 in the gastrointestinal tract of Xenopus laevis. Cell Tissue Res. 1998, 291, 13–18. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Peitz, U.; Vieth, M.; Meyer, F.; Vestergaard, E.M.; Malfertheiner, P.; Roessner, A.; Lippert, H.; Hoffmann, W. A gradient of TFF3 (trefoil factor family 3) peptide synthesis within the normal human gastric mucosa. Cell Tissue Res. 2004, 316, 155–165. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Schwartz, J.L. Fcgbp—A potential viral trap in RV144. Open AIDS J. 2014, 8, 21–24. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weste, J.; Houben, T.; Harder, S.; Schlüter, H.; Lücke, E.; Schreiber, J.; Hoffmann, W. Different Molecular Forms of TFF3 in the Human Respiratory Tract: Heterodimerization with IgG Fc Binding Protein (FCGBP) and Proteolytic Cleavage in Bronchial Secretions. Int. J. Mol. Sci. 2022, 23, 15359. https://doi.org/10.3390/ijms232315359
Weste J, Houben T, Harder S, Schlüter H, Lücke E, Schreiber J, Hoffmann W. Different Molecular Forms of TFF3 in the Human Respiratory Tract: Heterodimerization with IgG Fc Binding Protein (FCGBP) and Proteolytic Cleavage in Bronchial Secretions. International Journal of Molecular Sciences. 2022; 23(23):15359. https://doi.org/10.3390/ijms232315359
Chicago/Turabian StyleWeste, Jens, Till Houben, Sönke Harder, Hartmut Schlüter, Eva Lücke, Jens Schreiber, and Werner Hoffmann. 2022. "Different Molecular Forms of TFF3 in the Human Respiratory Tract: Heterodimerization with IgG Fc Binding Protein (FCGBP) and Proteolytic Cleavage in Bronchial Secretions" International Journal of Molecular Sciences 23, no. 23: 15359. https://doi.org/10.3390/ijms232315359
APA StyleWeste, J., Houben, T., Harder, S., Schlüter, H., Lücke, E., Schreiber, J., & Hoffmann, W. (2022). Different Molecular Forms of TFF3 in the Human Respiratory Tract: Heterodimerization with IgG Fc Binding Protein (FCGBP) and Proteolytic Cleavage in Bronchial Secretions. International Journal of Molecular Sciences, 23(23), 15359. https://doi.org/10.3390/ijms232315359










