Efficient Construction of Symmetrical Diaryl Sulfides via a Supported Pd Nanocatalyst-Catalyzed C-S Coupling Reaction
Abstract
1. Introduction
2. Results and Discussion
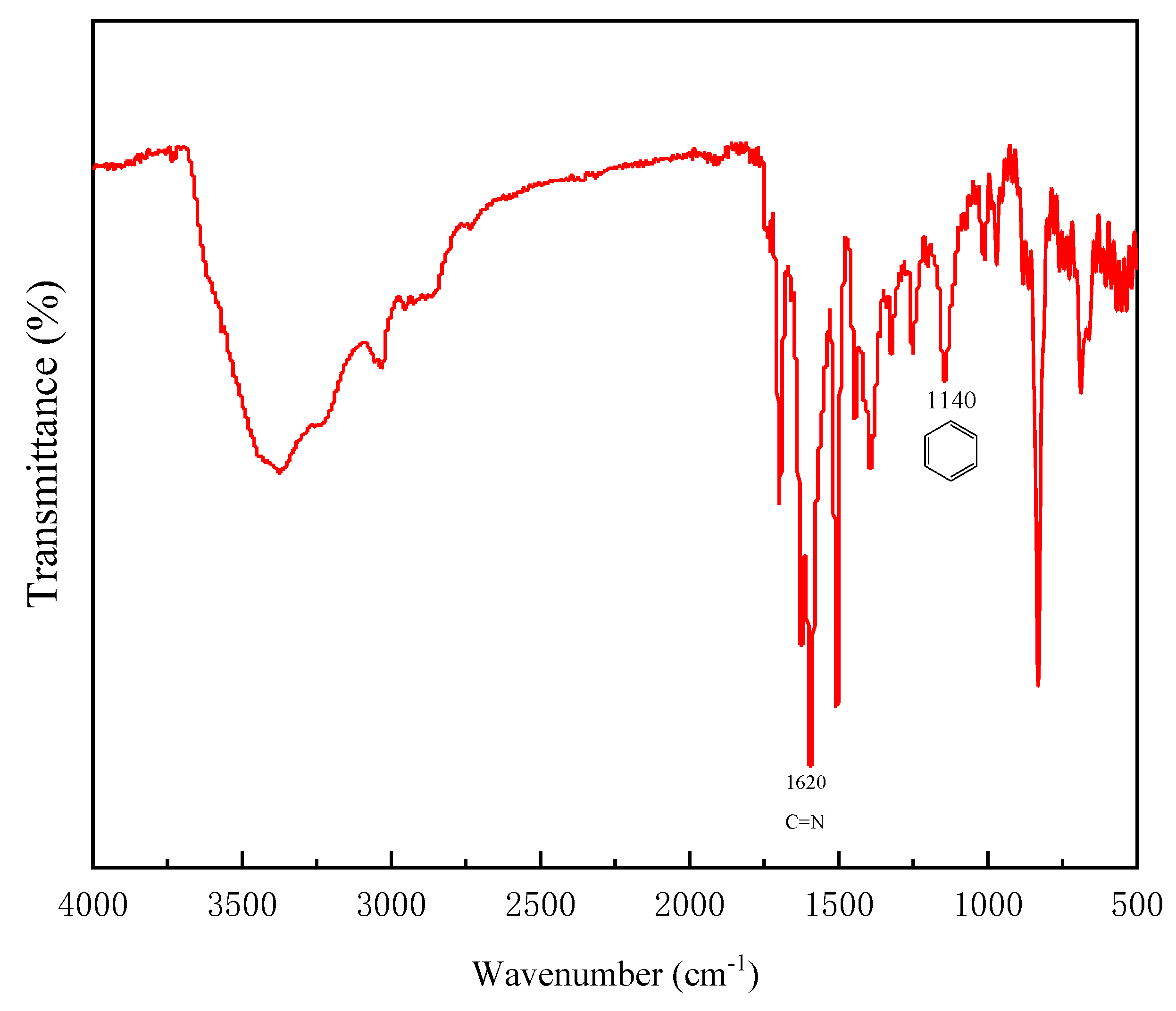
2.1. Preparation of Pd@COF-TB Nanocatalyst
2.2. Determination of Pd Loading in Pd@COF-TB Nanocatalyst
2.3. Optimization of Reaction Conditions
2.4. Substrate Expansion under Optimal Reaction Conditions
2.5. Gram-Scale Synthesis Reaction of Iodobenzene with Na2S2O3
2.6. Catalyst Reuse
2.7. Mechanism Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Pd@COF-TB Nanocatalyst
3.3. Synthesis of Sodium S-phenyl Sulfurothioate (3a)
3.4. Synthesis of 4-Methyldiphenyl Sulfide (4a)
3.5. General Procedure for the Synthesis of Symmetrical Diaryl Sulfide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poon, S.Y.; Wong, W.Y.; Cheah, K.W.; Shi, J.X. Spatial extent of the singlet and triplet excitons in luminescent angular-shaped transition-metal diynes and polyynes comprising non-π-conjugated group 16 main group elements. Chem. Eur. J. 2006, 12, 2550–2563. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Mancheño, O.G.; Bolm, C. Iron-catalysed carbon–heteroatom and heteroatom–heteroatom bond forming processes. Chem. Soc. Rev. 2008, 37, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, X. Transfer of sulfur: From simple to diverse. Chem. Asian J. 2013, 8, 2546–2563. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Link, J.T.; Pei, Z.; Reilly, E.B.; Leitza, S.; Nguyen, B.; Marsh, K.C.; Okasinski, G.F.; von Geldern, T.W.; Ormes, M. Discovery of novel p-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction. 1. Identification of an additional binding pocket based on an anilino diaryl sulfide lead. J. Med. Chem. 2000, 43, 4025–4040. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Fumo, M.G.; Menichetti, S.; Mugnaini, V.; Pedulli, G.F. Electronic and hydrogen bonding effects on the chain-breaking activity of sulfur-containing phenolic antioxidants. J. Org. Chem. 2006, 71, 6325–6332. [Google Scholar] [CrossRef]
- Denes, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl radicals in organic synthesis. Chem. Rev. 2014, 114, 2587–2693. [Google Scholar] [CrossRef]
- Migler, B.M.; Warawa, E.J.; Malick, J.B. Seroquel: Behavioral effects in conventional and novel tests for atypical antipsychotic drug. Psychopharmacology 1993, 112, 299–307. [Google Scholar] [CrossRef]
- Kim, W.; Steele, A.D.; Zhu, W.; Csatary, E.E.; Fricke, N.; Dekarske, M.M.; Jayamani, E.; Pan, W.; Kwon, B.; Sinitsa, I.F. Discovery and optimization of nTZDpa as an antibiotic effective against bacterial persisters. ACS Infect. Dis. 2018, 4, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Kaldor, S.W.; Kalish, V.J.; Davies, J.F.; Shetty, B.V.; Fritz, J.E.; Appelt, K.; Burgess, J.A.; Campanale, K.M.; Chirgadze, N.Y.; Clawson, D.K. Viracept (nelfinavir mesylate, AG1343): A potent, orally bioavailable inhibitor of HIV-1 protease. J. Med. Chem. 1997, 40, 3979–3985. [Google Scholar] [CrossRef]
- Holden, J.M.; Itil, T.; Keskiner, A.; Gannon, P. A clinical trial of an antiserotonin compound, cinanserin, in chronic schizophrenia. J. Clin. Pharmacol. 1971, 11, 220–226. [Google Scholar] [CrossRef]
- Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Enantioselective trifluoromethylthiolating lactonization catalyzed by an indane-based chiral sulfide. Angew. Chem. Int. Ed. 2016, 55, 5846–5850. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, P.; Li, Y.; Yu, X.; Shi, B.-F. Atroposelective synthesis of conjugated diene-based axially chiral styrenes via Pd (II)-catalyzed thioether-directed alkenyl C–H olefination. J. Am. Chem. Soc. 2021, 143, 12335–12344. [Google Scholar] [CrossRef]
- Ranu, B.C.; Jana, R. Ionic liquid as catalyst and reaction medium: A simple, convenient and green procedure for the synthesis of thioethers, thioesters and dithianes using an inexpensive ionic liquid, [pmIm]Br. Adv. Synth. Catal. 2005, 347, 1811–1818. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. A highly active and magnetically retrievable nanoferrite–DOPA–copper catalyst for the coupling of thiophenols with aryl halides. Chem. Commun. 2012, 48, 2582–2584. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, M.A.; Shen, Q.; Hartwig, J.F. A general and long-lived catalyst for the palladium-catalyzed coupling of aryl halides with thiols. J. Am. Chem. Soc. 2006, 128, 2180–2181. [Google Scholar] [CrossRef]
- Zong, C.; Liu, J.; Chen, S.; Zeng, R.; Zou, J. Efficient C-S cross-coupling of thiols with aryl iodides catalyzed by Cu(OAc)2· H2O and 2,2′-Biimidazole. Chin. J. Chem. 2014, 32, 212–218. [Google Scholar] [CrossRef]
- Kwong, F.Y.; Buchwald, S.L. A general, efficient, and inexpensive catalyst system for the coupling of aryl iodides and thiols. Org. Lett. 2002, 4, 3517–3520. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karimzadeh, M.; Azizi, G. Highly efficient and magnetically separable nano-CuFe2O4 catalyzed S-arylation of thiourea by aryl/heteroaryl halides. Chin. Chem. Lett. 2014, 25, 1382–1386. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Peng, W.-X. Trisaminomethane-cobalt complex supported on Fe3O4 magnetic nanoparticles as an efficient recoverable nanocatalyst for oxidation of sulfides and C–S coupling reactions. Appl. Organomet. Chem. 2020, 34, e5260. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Liu, L.; Yuan, T.; Yi, F. Efficient copper-catalyzed double S-arylation of aryl halides with sodium sulfide in PEG-400. Phosphorus Sulfur 2012, 187, 1284–1290. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Wang, H.; Li, X.; Verpoort, F.; Duan, C. A highly efficient method for the copper-catalyzed selective synthesis of diaryl chalcogenides from easily available chalcogen sources. Eur. J. Org. Chem. 2011, 2011, 7331–7338. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Seydyosefi, Z.; Tahmasbi, B. Zirconium oxide complex anchored on boehmite nanoparticles as highly reusable organometallic catalyst for C–S and C–O coupling reactions. Appl. Organomet. Chem. 2018, 32, e4396. [Google Scholar] [CrossRef]
- Ke, F.; Qu, Y.; Jiang, Z.; Li, Z.; Wu, D.; Zhou, X. An efficient copper-catalyzed carbon−sulfur bond formation protocol in water. Org. Lett. 2011, 13, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Xiao, R.; Yan, T.; Zhao, H. A simple and green synthesis of diaryl sulfides catalyzed by an MCM-41-immobilized copper (I) complex in neat water. J. Organomet. Chem. 2014, 749, 55–60. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, H.; Gao, H.; Xi, C. Cu-catalyzed synthesis of diaryl thioethers and S-cycles by reaction of aryl iodides with carbon disulfide in the presence of DBU. J. Org. Chem. 2013, 78, 5001–5006. [Google Scholar] [CrossRef] [PubMed]
- Azadi, G.; Taherinia, Z.; Naghipour, A.; Ghorbani-Choghamarani, A. Synthesis of sulfides via reaction of aryl/alkyl halides with S8 as a sulfur-transfer reagent catalyzed by Fe3O4-magnetic-nanoparticles-supported L-Histidine-Ni (II). J. Sulfur Chem. 2017, 38, 303–313. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Taherinia, Z. The first report on the preparation of peptide nanofibers decorated with zirconium oxide nanoparticles applied as versatile catalyst for the amination of aryl halides and synthesis of biaryl and symmetrical sulfides. New J. Chem. 2017, 41, 9414–9423. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, L.; Qiu, H.; Yi, W. Bunte salt CH2FSSO3Na: An efficient and odorless reagent for monofluoromethylthiolation. Org. Lett. 2018, 20, 6270–6273. [Google Scholar] [CrossRef]
- Yi, J.; Fu, Y.; Xiao, B.; Cui, W.-C.; Guo, Q.-X. Palladium catalyzed synthesis of aryl thiols: Sodium thiosulfate as a cheap and nontoxic mercapto surrogate. Tetrahedron Lett. 2011, 52, 205–208. [Google Scholar] [CrossRef]
- Qiao, Z.; Liu, H.; Xiao, X.; Fu, Y.; Wei, J.; Li, Y.; Jiang, X. Efficient access to 1,4-benzothiazine: Palladium-catalyzed double C–S bond formation using Na2S2O3 as sulfurating reagent. Org. Lett. 2013, 15, 2594–2597. [Google Scholar] [CrossRef]
- Qiao, Z.; Wei, J.; Jiang, X. Direct cross-coupling access to diverse aromatic sulfide: Palladium-catalyzed double C–S bond construction using Na2S2O3 as a sulfurating reagent. Org. Lett. 2014, 16, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Mohammadizadeh, M.R.; Saeedi, N. The synthesis of symmetrical disulfides by reacting organic halides with Na2S2O3· 5H2O in DMSO. New J. Chem. 2016, 40, 89–92. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Hu, M.; Li, C.; Li, M.; He, D.; Jiang, H. A palladium-catalyzed three-component cascade S-transfer reaction in ionic liquids. Green Chem. 2019, 21, 4084–4089. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, C.; Liu, P.; Ge, X.; Zhou, S. Covalent organic framework-supported Pd nanoparticles: An efficient and reusable heterogeneous catalyst for Suzuki–Miyaura coupling reactions. Appl. Organomet. Chem. 2022, 36, e6642. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Wang, X.; Lv, J.-J.; Feng, J.-J.; Xu, X.; Wang, A.-J.; Liang, Z. Bimetallic Au-Pd nanochain networks: Facile synthesis and promising application in biaryl synthesis. New J. Chem. 2017, 41, 3894–3899. [Google Scholar] [CrossRef]
- Gong, X.; Wu, J.; Meng, Y.; Zhang, Y.; Ye, L.-W.; Zhu, C. Ligand-free palladium catalyzed Ullmann biaryl synthesis: “household” reagents and mild reaction conditions. Green Chem. 2019, 21, 995–999. [Google Scholar] [CrossRef]
- Vasconcelos, S.N.S.; Reis, J.S.; de Oliveira, I.M.; Balfour, M.N.; Stefani, H.A. Synthesis of symmetrical biaryl compounds by homocoupling reaction. Tetrahedron 2019, 75, 1865–1959. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, J.; Zhang, S.; Cheng, H.; Zang, C.; Bian, F. Efficient photocatalytic chemoselective and stereoselective C-C bond formation over AuPd@N-rich carbon nitride. Catal. Sci. Technol. 2021, 11, 219–229. [Google Scholar] [CrossRef]
- Inkster, J.A.H.; Guerin, B.; Ruth, T.J.; Adam, M.J. Radiosynthesis and bioconjugation of [18F]FPy5yne, a prosthetic group for the 18F labeling of bioactive peptides. J. Labelled Compd. Rad. 2008, 51, 444–452. [Google Scholar] [CrossRef]
- Huo, J.-P.; Xiong, J.-F.; Mo, G.-Z.; Peng, P.; Wang, Z.-Y. Synthesis of chiral 2(5H)-furanone derivatives with 1,3-butadiyne structure. Res. Chem. Intermed. 2013, 39, 4321–4335. [Google Scholar] [CrossRef]
- Jia, H.; Li, Q.; Bayaguud, A.; Huang, Y.; She, S.; Chen, K.; Wei, Y. Diversified polyoxovanadate derivatives obtained by copper(I)-catalysed azide-alkyne cycloaddition reaction: Their synthesis and structural characterization. Dalton Trans. 2018, 47, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pu, J.; Jiang, X. A highly efficient Cu-Catalyzed S-transfer reaction: From amine to sulfide. Org. Lett. 2014, 16, 2692–2695. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, J.; Yan, R.; Yan, M.; Xu, Q. Promoting effect of crystal water leading to catalyst-free synthesis of heteroaryl thioether from heteroaryl chloride, sodium thiosulfate pentahydrate, and alcohol. J. Org. Chem. 2019, 84, 11294–11300. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Jänsch, N.; Öhlmann, T.; Meyer-Almes, F.-J.; Jiang, X. Thiocarbonyl surrogate via combination of potassium sulfide and chloroform for dithiocarbamate construction. Org. Lett. 2019, 21, 7484–7488. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, F.; Sun, L.; Huang, M.; Jiang, J.; Wang, K.; Ouyang, D.; Lu, L.; Lei, A. Electrochemical reductive cross-coupling of acyl chlorides and sulfinic acids towards the synthesis of thioesters. Green Chem. 2022, 24, 7350–7354. [Google Scholar] [CrossRef]
- Ghodsinia, S.S.E.; Akhlaghinia, B. Cu I anchored onto mesoporous SBA-16 functionalized by aminated 3-glycidyloxypropyltrimethoxysilane with thiosemicarbazide (SBA-16/GPTMS-TSC-Cu I): A heterogeneous mesostructured catalyst for S-arylation reaction under solvent-free conditions. Green Chem. 2019, 21, 3029–3049. [Google Scholar]
- Li, X.; Du, J.; Zhang, Y.; Chang, H.; Gao, W.; Wei, W. Synthesis and nano-Pd catalyzed chemoselective oxidation of symmetrical and unsymmetrical sulfides. Org. Biomol. Chem. 2019, 17, 3048–3055. [Google Scholar]
- Kollár, L.; Rao, Y.V.R.; Zugό, A.; Pongrácz, P. Palladium-catalysed thioetherification of aryl and alkenyl iodides using 1, 3, 5-trithiane as sulfur source. Tetrahedron 2022, 104, 132602. [Google Scholar]
- Liu, Y.; Kim, J.; Seo, H.; Park, S.; Chae, J. Copper (II)‐Catalyzed Single‐Step Synthesis of Aryl Thiols from Aryl Halides and 1, 2‐Ethanedithiol. Adv. Synth. Catal. 2015, 357, 2205–2212. [Google Scholar]
- Zhang, Y.; Liu, L.; Chen, J. Efficient synthesis of diaryl sulfides by copper-catalysed coupling of aryl halides and thioacetate in water. J. Chem. Res. 2013, 37, 19–21. [Google Scholar]
- Csokai, V.; Gruen, A.; Balázs, B.; Tόth, G.; Horváth, G.; Bitter, I. Unprecedented cyclizations of calix [4] arenes with glycols under the mitsunobu protocol, part 2.1 O, O-and O, S-bridged calixarenes. Org. Lett. 2004, 6, 477–480. [Google Scholar]
- May, L.; Müller, T.J.J. Electron-Rich Phenothiazine Congeners and Beyond: Synthesis and Electronic Properties of Isomeric Dithieno [1, 4] thiazines. Chem. Eur. J. 2020, 26, 12111–12118. [Google Scholar]






| Entry | Content of Pd Tested by ICP-OES | Load of Pd in the Nanocatalyst (wt%) |
|---|---|---|
| 1 | 0.126 ppm | 4.72 |
| 2 | 0.123 ppm | 4.61 |
| 3 | 0.127 ppm | 4.76 |
 | ||||||
| Entry | Solvent | Base | Pd@COF-TB (mg) | Temp (°C) | Yield 2 | |
|---|---|---|---|---|---|---|
| 1a | 2a | |||||
| 1 | DMF | KOH | 20 | 100 | 36% | 50% |
| 2 | DMSO | KOH | 20 | 100 | 21% | 67% |
| 3 | NMP | KOH | 20 | 100 | 33% | 52% |
| 4 | H2O | KOH | 20 | 100 | 12% | 35% |
| 5 | EtOH | KOH | 20 | 100 | 28% | 70% |
| 6 | PEG200 | KOH | 20 | 100 | 32% | 59% |
| 7 | DMF | NaOH | 20 | 100 | 30% | 57% |
| 8 | DMF | K2CO3 | 20 | 100 | 16% | 66% |
| 9 | DMF | Cs2CO3 | 20 | 100 | 24% | 58% |
| 10 | DMF | NaOMe | 20 | 100 | 33% | 60% |
| 11 | DMF | Et3N | 20 | 100 | 59% | 17% |
| 12 | DMF | DBU | 20 | 100 | 33% | 0 |
| 13 | DMF | DIPEA | 20 | 100 | 80% | 12% |
| 14 | DMF | DIPEA | 0 | 100 | 0 | 0 |
| 15 | DMF | DIPEA | 10 | 100 | 61% | 14% |
| 16 | DMF | DIPEA | 15 | 100 | 70% | 12% |
| 17 | DMF | DIPEA | 25 | 100 | 80% | 14% |
| 18 | DMF | DIPEA | 30 | 100 | 80% | 16% |
| 19 3 | DMF | DIPEA | Pd(OAc)2, 2 mg | 100 | 51% | 35% |
| 20 | DMF | DIPEA | 20 | 40 | 43% | 18% |
| 21 | DMF | DIPEA | 20 | 60 | 56% | 15% |
| 22 | DMF | DIPEA | 20 | 80 | 63% | 16% |
| 23 | DMF | DIPEA | 20 | 120 | 94% | 3% |
| 24 | DMF | DIPEA | 20 | 140 | 86% | 4% |
 |
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Liu, P.; Teng, Q.; Wang, Y.; Meng, Q.; Qian, C. Efficient Construction of Symmetrical Diaryl Sulfides via a Supported Pd Nanocatalyst-Catalyzed C-S Coupling Reaction. Int. J. Mol. Sci. 2022, 23, 15360. https://doi.org/10.3390/ijms232315360
Jin H, Liu P, Teng Q, Wang Y, Meng Q, Qian C. Efficient Construction of Symmetrical Diaryl Sulfides via a Supported Pd Nanocatalyst-Catalyzed C-S Coupling Reaction. International Journal of Molecular Sciences. 2022; 23(23):15360. https://doi.org/10.3390/ijms232315360
Chicago/Turabian StyleJin, Hao, Penghao Liu, Qiaoqiao Teng, Yuxiang Wang, Qi Meng, and Chao Qian. 2022. "Efficient Construction of Symmetrical Diaryl Sulfides via a Supported Pd Nanocatalyst-Catalyzed C-S Coupling Reaction" International Journal of Molecular Sciences 23, no. 23: 15360. https://doi.org/10.3390/ijms232315360
APA StyleJin, H., Liu, P., Teng, Q., Wang, Y., Meng, Q., & Qian, C. (2022). Efficient Construction of Symmetrical Diaryl Sulfides via a Supported Pd Nanocatalyst-Catalyzed C-S Coupling Reaction. International Journal of Molecular Sciences, 23(23), 15360. https://doi.org/10.3390/ijms232315360







