Correlation between Adrenoceptor Expression and Clinical Parameters in Degenerated Lumbar Intervertebral Discs
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. AR Gene Expression and Modic Classification
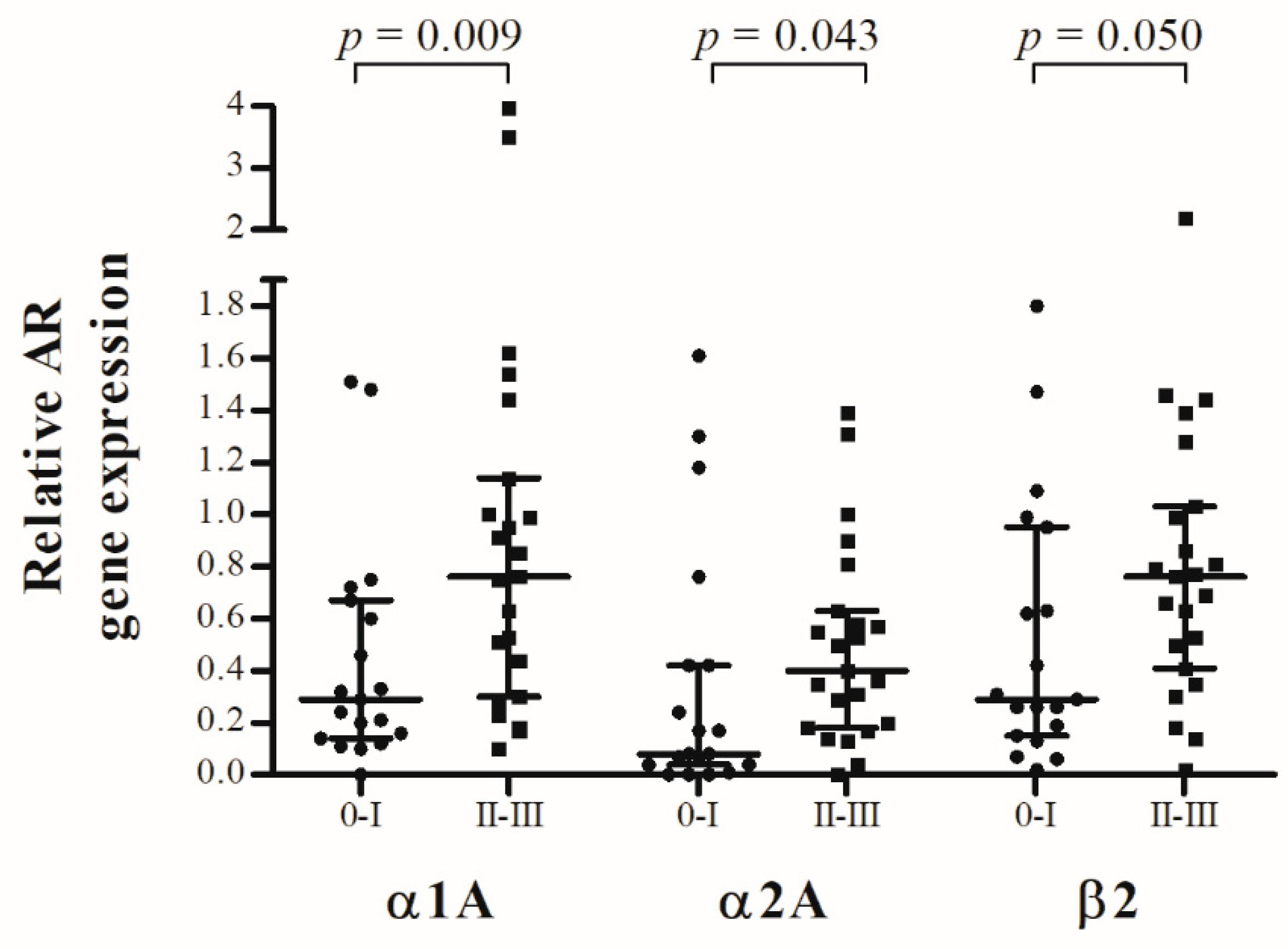
2.3. AR Gene Expression and Pfirrmann Classification
2.4. AR Gene Expression and Clinical Parameters
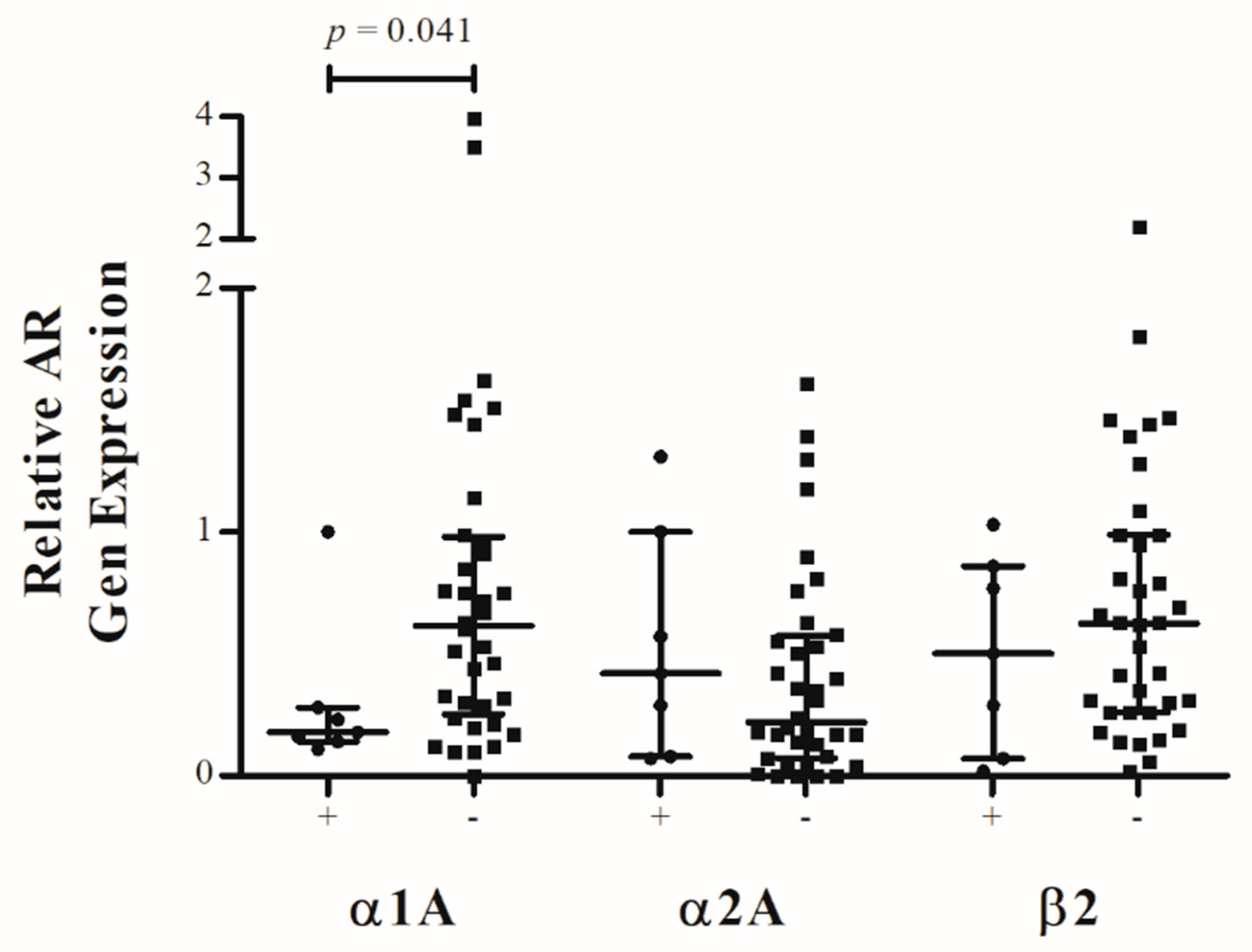
2.5. Adjacent Segment Disease
3. Discussion
4. Materials and Methods
4.1. Human IVD Tissue Samples and Patient Characteristics
4.2. AR Gene Expression
4.3. Radiological Classification of IVDD and Patient Characteristics
4.4. Ethical Approval
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.-P.A.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.W.; Welting, T.J.; van Royen, B.J.; van Dieën, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf-Yde, C.; Kyvik, K.O.; Bruun, N.H. Low back pain and lifestyle. Part II—Obesity. Information from a population-based sample of 29,424 twin subjects. Spine 1999, 24, 779–783, discussion 783-4. [Google Scholar] [CrossRef]
- Cheung, K.M.C.; Karppinen, J.; Chan, D.; Ho, D.W.H.; Song, Y.-Q.; Sham, P.; Cheah, K.S.E.; Leong, J.C.Y.; Luk, K.D.K. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Luoma, K.; Riihimäki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A. Low back pain in relation to lumbar disc degeneration. Spine 2000, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Russo, F.; Di Martino, A.; Denaro, V. Intervertebral disc regeneration: From the degenerative cascade to molecular therapy and tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 679–690. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef]
- Dowdell, J.; Erwin, M.; Choma, T.; Vaccaro, A.; Iatridis, J.; Cho, S.K. Intervertebral Disk Degeneration and Repair. Neurosurgery 2017, 80, S46–S54. [Google Scholar] [CrossRef]
- Adams, M.A.; Dolan, P.; McNally, D.S. The internal mechanical functioning of intervertebral discs and articular cartilage, and its relevance to matrix biology. Matrix Biol. 2009, 28, 384–389. [Google Scholar] [CrossRef]
- Adams, M.A.; Lama, P.; Zehra, U.; Dolan, P. Why do some intervertebral discs degenerate, when others (in the same spine) do not? Clin. Anat. 2015, 28, 195–204. [Google Scholar] [CrossRef]
- Mayer, J.E.; Iatridis, J.C.; Chan, D.; Qureshi, S.A.; Gottesman, O.; Hecht, A.C. Genetic polymorphisms associated with intervertebral disc degeneration. Spine J. 2013, 13, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Sampara, P.; Banala, R.R.; Vemuri, S.K.; Av, G.R.; Gpv, S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018, 25, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Handa, T.; Ishihara, H.; Ohshima, H.; Osada, R.; Tsuji, H.; Obata, K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine 1997, 22, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.-P.A.; van der Veen, A.J.; van Royen, B.J.; Kingma, I.; Smit, T.H. Intradiscal pressure depends on recent loading and correlates with disc height and compressive stiffness. Eur. Spine J. 2014, 23, 2359–2368. [Google Scholar] [CrossRef]
- Grässel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef]
- Sohn, R.; Rösch, G.; Junker, M.; Meurer, A.; Zaucke, F.; Jenei-Lanzl, Z. Adrenergic signalling in osteoarthritis. Cell. Signal. 2021, 82, 109948. [Google Scholar] [CrossRef]
- Jiao, K.; Zeng, G.; Niu, L.-N.; Yang, H.-X.; Ren, G.-T.; Xu, X.-Y.; Li, F.-F.; Tay, F.R.; Wang, M.-Q. Activation of α2A-adrenergic signal transduction in chondrocytes promotes degenerative remodelling of temporomandibular joint. Sci. Rep. 2016, 6, 30085. [Google Scholar] [CrossRef]
- Jiao, K.; Niu, L.-N.; Li, Q.-h.; Ren, G.-T.; Zhao, C.-m.; Liu, Y.-d.; Tay, F.R.; Wang, M.-Q. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci. Rep. 2015, 5, 12593. [Google Scholar] [CrossRef]
- Taheri, S.; Winkler, T.; Schenk, L.S.; Neuerburg, C.; Baumbach, S.F.; Zustin, J.; Lehmann, W.; Schilling, A.F. Developmental Transformation and Reduction of Connective Cavities within the Subchondral Bone. Int. J. Mol. Sci. 2019, 20, 770. [Google Scholar] [CrossRef]
- Rustenburg, C.M.E.; Emanuel, K.S.; Peeters, M.; Lems, W.F.; Vergroesen, P.-P.A.; Smit, T.H. Osteoarthritis and intervertebral disc degeneration: Quite different, quite similar. JOR Spine 2018, 1, e1033. [Google Scholar] [CrossRef]
- Barczewska, M.; Juranek, J.; Wojtkiewicz, J. Origins and Neurochemical Characteristics of Porcine Intervertebral Disc Sympathetic Innervation: A Preliminary Report. J. Mol. Neurosci. 2017, 63, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kupka, J.; Kohler, A.; El Bagdadi, K.; Bostelmann, R.; Brenneis, M.; Fleege, C.; Chan, D.; Zaucke, F.; Meurer, A.; Rickert, M.; et al. Adrenoceptor Expression during Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2020, 21, 2085. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Freeman, B.J.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urban, J.P.G. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Matsusue, Y. Neuronal regulation of bone metabolism and anabolism: Calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc. Res. Tech. 2002, 58, 61–69. [Google Scholar] [CrossRef]
- Lorenz, J.; Schäfer, N.; Bauer, R.; Jenei-Lanzl, Z.; Springorum, R.H.; Grässel, S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthr. Cartil. 2016, 24, 325–334. [Google Scholar] [CrossRef]
- Edgar, M.A. The nerve supply of the lumbar intervertebral disc. J. Bone Joint Surg. Br. 2007, 89, 1135–1139. [Google Scholar] [CrossRef]
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef]
- Johnson, W.E.B.; Patterson, A.M.; Eisenstein, S.M.; Roberts, S. The presence of pleiotrophin in the human intervertebral disc is associated with increased vascularization: An immunohistologic study. Spine 2007, 32, 1295–1302. [Google Scholar] [CrossRef]
- Rutges, J.P.H.J.; van der Jagt, O.P.; Oner, F.C.; Verbout, A.J.; Castelein, R.J.M.; Kummer, J.A.; Weinans, H.; Creemers, L.B.; Dhert, W.J.A. Micro-CT quantification of subchondral endplate changes in intervertebral disc degeneration. Osteoarthr. Cartil. 2011, 19, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Videman, T.; Battié, M.C. Lumbar vertebral endplate lesions: Prevalence, classification, and association with age. Spine 2012, 37, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Mok, F.P.S.; Samartzis, D.; Karppinen, J.; Luk, K.D.K.; Fong, D.Y.T.; Cheung, K.M.C. ISSLS prize winner: Prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: A population-based study of 2449 individuals. Spine 2010, 35, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.F.; Hukkanen, M.V.; McCarthy, I.D.; Redfern, D.R.; Batten, J.J.; Crock, H.V.; Hughes, S.P.; Polak, J.M. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J. Bone Jt. Surg. Br. 1997, 79, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Storch, U.; Mederos y Schnitzler, M.; Gudermann, T. G protein-mediated stretch reception. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1241–H1249. [Google Scholar] [CrossRef]
- Nishida, T.; Kubota, S. Roles of CCN2 as a mechano-sensing regulator of chondrocyte differentiation. Jpn. Dent. Sci. Rev. 2020, 56, 119–126. [Google Scholar] [CrossRef]
- Sato, K.; Kikuchi, S.; Yonezawa, T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine 1999, 24, 2468–2474. [Google Scholar] [CrossRef]
- Pitsillides, A.A.; Beier, F. Cartilage biology in osteoarthritis—Lessons from developmental biology. Nat. Rev. Rheumatol. 2011, 7, 654–663. [Google Scholar] [CrossRef]
- Mooney, V. Where is the lumbar pain coming from? Ann. Med. 1989, 21, 373–379. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Kang, J.; Kandel, R.; Risbud, M.V. New Horizons in Spine Research: Disc biology, spine biomechanics and pathomechanisms of back pain. J. Orthop. Res. 2016, 34, 1287–1288. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hoelscher, G.L.; Bullock, L.; Ingram, J.A.; Norton, H.J.; Hanley, E.N. Human annulus signaling cues for nerve outgrowth: In vitro studies. J. Orthop. Res. 2016, 34, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.H.I.; Lozito, T.P.; Cuperman, T.; Yurube, T.; Moon, H.J.; Ngo, K.; Tuan, R.S.; St Croix, C.; Sowa, G.A.; Rodrigues, L.M.R.; et al. Catabolic effects of endothelial cell-derived microparticles on disc cells: Implications in intervertebral disc neovascularization and degeneration. J. Orthop. Res. 2016, 34, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- White, J.C.; Sweet, W.H. Pain: Its Mechanisms and Neurosurgical Control. Br. J. Surg. 1955, 43, 108. [Google Scholar] [CrossRef]
- El-Mahdi, M.A.; Abdel Latif, F.Y.; Janko, M. The spinal nerve root “innervation”, and a new concept of the clinicopathological interrelations in back pain and sciatica. Neurochirurgia 1981, 24, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Cavanaugh, J.M.; Kallakuri, S.; Chen, C.; Yamashita, T. Sympathetic afferent units from lumbar intervertebral discs. J. Bone Jt. Surg. Br. 2006, 88, 554–557. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, J. Sympathetic facilitation of sustained discharges of polymodal nociceptors. Pain 1989, 38, 85–90. [Google Scholar] [CrossRef]
- Roberts, W.J.; Elardo, S.M. Sympathetic activation of A-delta nociceptors. Somatosens. Res. 1985, 3, 33–44. [Google Scholar] [CrossRef]
- Bahari, Z.; Meftahi, G.H. Spinal α2 -adrenoceptors and neuropathic pain modulation; therapeutic target. Br. J. Pharmacol. 2019, 176, 2366–2381. [Google Scholar] [CrossRef]
- Eisenach, J.C.; de Kock, M.; Klimscha, W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995). Anesthesiology 1996, 85, 655–674. [Google Scholar] [CrossRef]
- Bán, E.G.; Brassai, A.; Vizi, E.S. The role of the endogenous neurotransmitters associated with neuropathic pain and in the opioid crisis: The innate pain-relieving system. Brain Res. Bull. 2020, 155, 129–136. [Google Scholar] [CrossRef]
- Rodrigues, L.L.F.R.; Oliveira, M.C.G.; Pelegrini-da-Silva, A.; de Arruda Veiga, M.C.F.; Parada, C.A.; Tambeli, C.H. Peripheral sympathetic component of the temporomandibular joint inflammatory pain in rats. J. Pain 2006, 7, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.; Mayor, F.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Esp. Cardiol. 2019, 72, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Abhishek, A.; Muir, K.; Zhang, W.; Maciewicz, R.A.; Doherty, M. Association of Beta-Blocker Use with Less Prevalent Joint Pain and Lower Opioid Requirement in People with Osteoarthritis. Arthritis Care Res. 2017, 69, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Driban, J.B.; Lo, G.H.; Eaton, C.B.; Lapane, K.L.; Nevitt, M.; Harvey, W.F.; McCulloch, C.E.; McAlindon, T.E. Exploratory analysis of osteoarthritis progression among medication users: Data from the Osteoarthritis Initiative. Ther. Adv. Musculoskelet. Dis. 2016, 8, 207–219. [Google Scholar] [CrossRef]
- Pasco, J.A.; Henry, M.J.; Sanders, K.M.; Kotowicz, M.A.; Seeman, E.; Nicholson, G.C. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J. Bone Miner. Res. 2004, 19, 19–24. [Google Scholar] [CrossRef]
- Abdul-Hadi, O.; Parvizi, J.; Austin, M.S.; Viscusi, E.; Einhorn, T. Nonsteroidal Anti-Inflammatory Drugs in Orthopaedics. J. Bone Jt. Surg. Am. 2009, 91, 2020–2027. [Google Scholar]
- Huo, M.H.; Troiano, N.W.; Pelker, R.R.; Gundberg, C.M.; Friedlaender, G.E. The influence of ibuprofen on fracture repair: Biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J. Orthop. Res. 1991, 9, 383–390. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Al-Ghawas, M.; Alkhiary, Y.M.; Cullinane, D.M.; Krall, E.A.; Fitch, J.L.; Webb, E.G.; Thiede, M.A.; Einhorn, T.A. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J. Bone Joint Surg. Am. 2007, 89, 114–125. [Google Scholar] [CrossRef]
- Murnaghan, M.; Li, G.; Marsh, D.R. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: An inhibition of angiogenesis? J. Bone Jt. Surg. Am. 2006, 88 (Suppl. 3), 140–147. [Google Scholar] [CrossRef]
- Haass, M.; Kübler, W. Nicotine and sympathetic neurotransmission. Cardiovasc. Drugs Ther. 1997, 10, 657–665. [Google Scholar] [CrossRef]
- Dudli, S.; Fields, A.J.; Samartzis, D.; Karppinen, J.; Lotz, J.C. Pathobiology of Modic changes. Eur. Spine J. 2016, 25, 3723–3734. [Google Scholar] [CrossRef] [PubMed]
- Fayad, F.; Lefevre-Colau, M.-M.; Drapé, J.-L.; Feydy, A.; Chemla, N.; Quintéro, N.; Rannou, F.; Poiraudeau, S.; Fermanian, J.; Revel, M. Reliability of a modified Modic classification of bone marrow changes in lumbar spine MRI. Jt. Bone Spine 2009, 76, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, J.; Besa, P.; Campos, M.; Cikutovic, P.; Cabezon, M.; Molina, M.; Cruz, J.P. The Pfirrmann classification of lumbar intervertebral disc degeneration: An independent inter- and intra-observer agreement assessment. Eur. Spine J. 2016, 25, 2728–2733. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Balk, G.A.; Stewart, R.E. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014, 155, 2545–2550. [Google Scholar] [CrossRef]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 1991; ISBN 9780803942462. [Google Scholar]
- Hollander, M. Nonparametric Statistical Methods, 3rd ed.; Online-Ausg.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; ISBN 978-0-470-38737-5. [Google Scholar]


| Patient Characteristics | |
|---|---|
| Patients, n | 43 |
| Sex, no. female (%)/no. male (%) | 34 (79.1%)/9 (20.9%) |
| Age [years], mean (SD) | 69.23 (7.66) |
| BMI [kg/m2], mean (SD) | 30.77 (5.67) |
| Preoperative NPRS, mean (SD) | 6.81 (1.53) |
| Postoperative NPRS, mean (SD) | 2.40 (1.61) |
| Adjacent segment disease, n (%) | 7 (16.3%) |
| Nicotine abuse, n (%) | 11 (25.6%) |
| Medication: NSAID, n (%) | 20 (46.5%) |
| Medication: β-blocker, n (%) | 22 (51.2%) |
| Level of operation: | |
| L 1/2 | 2 (4.7%) |
| L 2/3 | 6 (14.0%) |
| L 3/4 | 9 (20.9%) |
| L 4/5 | 15 (34.9%) |
| L5/S1 | 11 (25.6%) |
| Total | 43 (100%) |
| Modic classification, n (%) | |
| 0 | 10 (23.3%) |
| I | 9 (20.9%) |
| II | 20 (46.5%) |
| III | 3 (7.0%) |
| Missing MRI | 1 (2.3%) |
| Total | 43 (100%) |
| Pfirrmann classification, n (%) | |
| I | 0 (0%) |
| II | 2 (4.7%) |
| III | 13 (30.2%) |
| IV | 14 (32.6%) |
| V | 13 (30.2%) |
| Missing MRI | 1 (2.3%) |
| Total | 43 (100%) |
| Gene Symbol | NCBI Reference | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| GAPDH | NM_001289745.2 | CTCCTGTTCGACAGTCAGCC | TTCCCGTTCTCAGCCTTGAC |
| ADRA1A | NM_000680.3 | CCATGCTCCAGCCAAGAGTT | TCCTGTCCTAGACTTCCTCCC |
| ADRA1B | NM_000679.3 | GTCCACCGTCATCTCCATCG | GAACAAGGAGCCAAGCGGTAG |
| ADRA1D | NM_000678.3 | TGACTTTCCGCGATCTCCTG | TTACCTGCCACGGCCATAAG |
| ADRA2A | NM_000681.3 | TGGTCATCGGAGTGTTCGTG | GCCCACTAGGAAGATGGCTC |
| ADRA2B | NM_000682.6 | GACATTTCACCGGCAACACC | GGGACTGAGAACCAGGAAGC |
| ADRA2C | NM000683.3 | CGATGTGCTGTTTTGCACCT | GGATGTACCAGGTCTCGTCG |
| ADRB1 | NM_000684.2 | TAGCAGGTGAACTCGAAGCC | ATCTTCCACTCCGGTCCTCT |
| ADRB2 | NM_000024.5 | CAGAGCCTGCTGACCAAGAA | GCCTAACGTCTTGAGGGCTT |
| ADRB3 | NM_000025.2 | GCCAATTCTGCCTTCAACCC | GCCAGAGGTTTTCCACAGGT |
| TH | NM_000360.3 | CAGGCAGAGGCCATCATGT | GTGGTCCAAGTCCAGGTCAG |
| Modic Classification | ||||
|---|---|---|---|---|
| Vertebral Endplates | Histopathology | Signal intensity | ||
| Modic I | Marrow edema | T1 hypointense, T2 hyperintense | ||
| Modic II | Fatty degeneration | T1 hyperintense, T2 iso-/hyperintense | ||
| Modic III | Subchondral bony sclerosis | T1 hypointense, T2 hypointense | ||
| Pfirrmann classification | ||||
| Grade | Structure | Signal intensity (T2) | Distinction of Nucleus and Anulus | Height |
| Pfirrmann I | homogeneous, white | hyperintense | clear | normal |
| Pfirrmann II | inhomogeneous, horizontal bands | hyperintense | clear | normal |
| Pfirrmann III | inhomogeneous, gray | intermediate | unclear | normal– slightly decreased |
| Pfirrmann IV | inhomogeneous, gray to black | intermediate to hypointense | lost | slightly– moderately decreased |
| Pfirrmann V | inhomogeneous, black | hypointense | lost | collapsed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenneis, M.; Jenei-Lanzl, Z.; Kupka, J.; Braun, S.; Junker, M.; Zaucke, F.; Rickert, M.; Meurer, A. Correlation between Adrenoceptor Expression and Clinical Parameters in Degenerated Lumbar Intervertebral Discs. Int. J. Mol. Sci. 2022, 23, 15358. https://doi.org/10.3390/ijms232315358
Brenneis M, Jenei-Lanzl Z, Kupka J, Braun S, Junker M, Zaucke F, Rickert M, Meurer A. Correlation between Adrenoceptor Expression and Clinical Parameters in Degenerated Lumbar Intervertebral Discs. International Journal of Molecular Sciences. 2022; 23(23):15358. https://doi.org/10.3390/ijms232315358
Chicago/Turabian StyleBrenneis, Marco, Zsuzsa Jenei-Lanzl, Johannes Kupka, Sebastian Braun, Marius Junker, Frank Zaucke, Marcus Rickert, and Andrea Meurer. 2022. "Correlation between Adrenoceptor Expression and Clinical Parameters in Degenerated Lumbar Intervertebral Discs" International Journal of Molecular Sciences 23, no. 23: 15358. https://doi.org/10.3390/ijms232315358
APA StyleBrenneis, M., Jenei-Lanzl, Z., Kupka, J., Braun, S., Junker, M., Zaucke, F., Rickert, M., & Meurer, A. (2022). Correlation between Adrenoceptor Expression and Clinical Parameters in Degenerated Lumbar Intervertebral Discs. International Journal of Molecular Sciences, 23(23), 15358. https://doi.org/10.3390/ijms232315358







