Mesenchymal Stem Cells in Burn Wound Management
Abstract
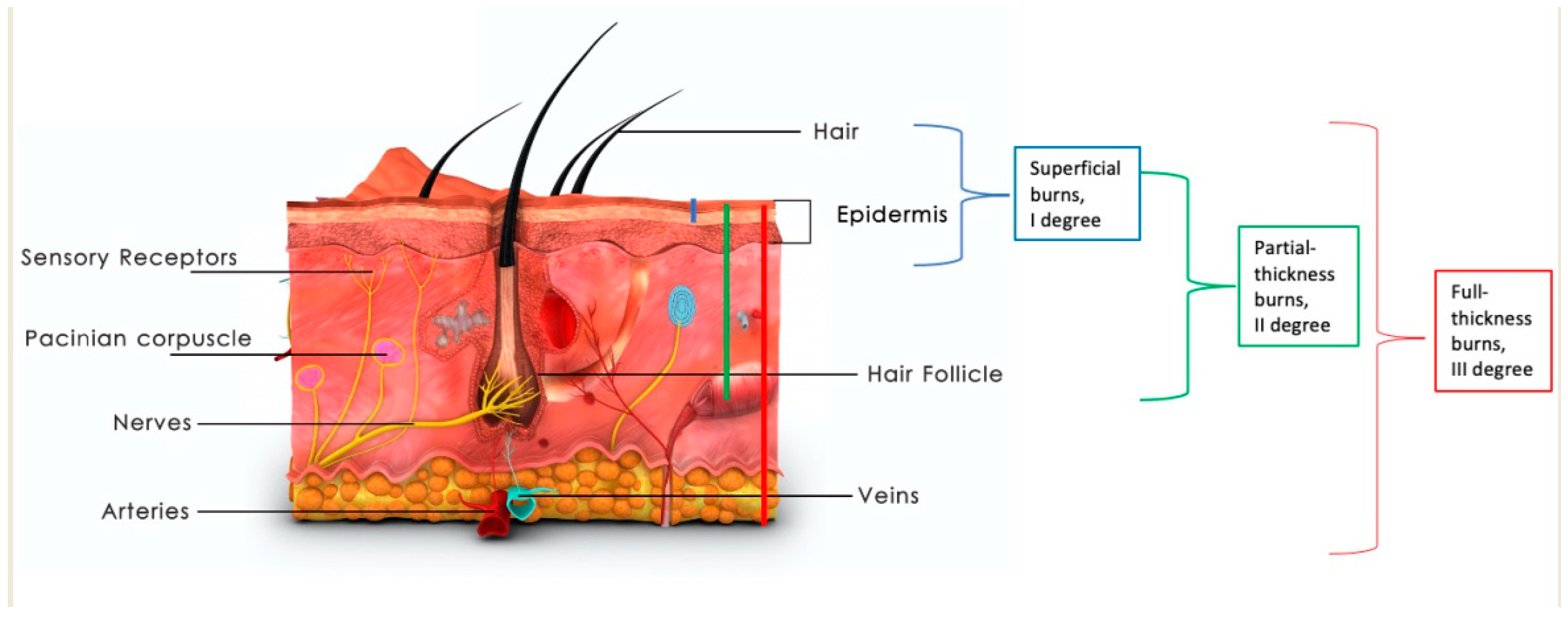
:1. Introduction
2. Methods
2.1. Residual Stem Cells’ Reaction to Thermal Injury
2.2. Potential Use of Stem Cells in Burn Wound Healing
2.3. Umbilical Cord and Placental Stem Cells
2.4. Bone-Marrow-Derived Stem Cells
2.5. Adipose-Derived Stem Cells
- (1)
- Trilineage potential presenting in the ability to differentiate into adipocytes, chondrocytes, and osteocytes in vitro;
- (2)
- Expression of the surface markers CD73, CD90, and CD105;
- (3)
- Lack of expression of hematopoietic and endothelial antigens CD14 (or CD11b), CD19 (or CD74alfa), CD 34, CD 45, and HLA-DR surface markers;
- (4)
2.6. Hair Follicle Stem Cells, Keratinocyte Progenitors, and Stem Cells from Excised Burned Skin
2.7. Stem Cells in Scar Prevention and Treatment
| Study | Study Type | Patients and Methods | Outcomes | Conclusion |
|---|---|---|---|---|
| Piejko [79] | Clinical experiment, case study | Contracting post-burn scar on the neck was qualified for excision. Autogenic AD-SCs were harvested by surgical excision of adipose tissue 3 weeks before the next stage. The cells were isolated, cultured, and seeded into the dermis substitute-matrix based on bovine collagen and sulphite-chondroitin. Four weeks after the scar excision the silicone layer of the matrix was removed and the neodermis was covered with a split-thickness skin graft. | No adverse events reported, good scar quality and texture. | Autologous stem cells can promote “scarless” healing of deep tissue wounds. |
| Li [81] | Experiment | Adipose and scar tissue was collected from subjects that underwent plastic excision of scars. Adipose-derived stem cells were isolated and cultured. Then, exosomes were isolated. BABL/c mice were randomly divided into groups; 3 days after creating a full-thickness injury, exosomes were injected subcutaneously. | Exosomes inhibited the proliferation and migration of fibroblasts, decreased the expression of Col1, Col3, α-SMA, IL-17RA, and p-Smad2/p-Smad3 and increased the levels of SIP1 in HSFs. miR-192-5p was highly expressed in ADSC-Exo and targeted the expression of IL-17RA to decrease the pro-fibrotic protein levels. | ADSC-Exo have antifibrotic features and improve wound healing. |
| Zahorec [80] | Clinical experiment | 8 patients with post-burn scars: 2 keloid, 6 hypertrophic. Adipose tissue was harvested with the Coleman technique, then ADSCs were isolated and cultured. ADSCs were injected with a 20G needle, subdermally after scar resection. | Improvement in VSS score in a 6-month observation (7.63 to 2.38), elapsing from scar incidence to correction was 79 months | Autologous ADSCs are safe and effective in preventing and treating post-burn scars. |
| Meng [82] | Experimental | Fibroblasts from hypertrophic scars were co-cultured with umbilical cord stem cells. | Umbilical cord stem cells suppressed the proliferation and migration ability of fibroblasts, with the TGF β1/Smad3 pathway inhibited as well. Additionally, levels of mRNA of collagen type Iα2 (COL1A2), collagen III α1 (COL3A1) and actin α2 smooth muscle (ACTA-2) were lower. | Umbilical cord stem cells have anti-fibrotic potential. |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, K.; Tan, Z.; Zhang, C.; Fu, X. Mesenchymal stem cells for sweat gland regeneration after burns: From possibility to reality. Burns 2016, 42, 492–499. [Google Scholar] [CrossRef]
- Daar, A.S.; Greenwood, H.L. A proposed definition of regenerative medicine. J. Tissue Eng. Regen. Med. 2007, 1, 179–184. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.Z.; Farrokhi, A.; Ghahary, A.; Jalili, R.B. Therapeutic Use of Stem Cells in Treatment of Burn Injuries. J. Burn Care Res. 2018, 39, 175–182. [Google Scholar] [CrossRef]
- O’Halloran, N.; Courtney, D.; Kerin, M.J.; Lowery, A.J. Adipose-Derived Stem Cells in Novel Approaches to Breast Reconstruction: Their Suitability for Tissue Engineering and Oncological Safety. Breast Cancer 2017, 11, 1178223417726777. [Google Scholar] [CrossRef] [Green Version]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef]
- Mikłosz, A.; Nikitiuk, B.A.; Chabowski, A. Using adipose-derived mesenchymal stem cells to fight the metabolic complications of obesity: Where do we stand? Obes. Rev. 2022, 23, e13413. [Google Scholar] [CrossRef]
- Shukla, L.; Yuan, Y.; Shayan, R.; Greening, D.W.; Karnezis, T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front. Pharmacol. 2020, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Argentati, C.; Morena, F.; Bazzucchi, F.; Armentano, I.; Emiliani, C.; Martino, S. Adipose Stem Cell Translational Applications: From Bench-to-Bedside. Int. J. Mol. Sci. 2018, 19, 3475. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.R.; Medhat, W.; El-Fakahany, H.; Abdel-Raouf, H.; Snyder, E.Y. Deriving Keratinocyte Progenitor Cells and Keratinocytes from Human-Induced Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2020, 54, e119. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.H.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef]
- Huang, L.; Burd, A. An update review of stem cell applications in burns and wound care. Indian J. Plast. Surg. 2012, 45, 229–236. [Google Scholar] [CrossRef]
- Nuutila, K. Hair Follicle Transplantation for Wound Repair. Adv. Wound Care 2021, 10, 153–163. [Google Scholar] [CrossRef]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Elloso, M.; Kambli, A.; Aijaz, A.; van de Kamp, A.; Jeschke, M.G. Burns in the Elderly: Potential Role of Stem Cells. Int. J. Mol. Sci. 2020, 21, 4604. [Google Scholar] [CrossRef]
- Maranda, E.L.; Rodriguez-Menocal, L.; Badiavas, E.V. Role of Mesenchymal Stem Cells in Dermal Repair in Burns and Diabetic Wounds. Curr. Stem Cell Res. Ther. 2017, 12, 61–70. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J. Adipose-Derived Stem Cells for Facial Rejuvenation. J. Pers. Med. 2022, 12, 117. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Staniszewska, M.; Słuczanowska-Głąbowska, S.; Drukała, J. Stem cells and skin regeneration. Folia Histochem. Cytobiol. 2011, 49, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Park, B.-S.; Jang, K.A.; Sung, J.-H.; Park, J.-S.; Kwon, Y.H.; Kim, K.J.; Kim, W.-S. Adipose-Derived Stem Cells and Their Secretory Factors as a Promising Therapy for Skin Aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar]
- Zou, M.L.; Liu, S.Y.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Teng, Y.Y.; Feng, Y.; Yuan, F.L. Insights into the role of adipose-derived stem cells: Wound healing and clinical regenerative potential. J. Cell. Physiol. 2021, 236, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wu, Y.; Wu, M. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front. Cell Dev. Biol. 2020, 8, 574223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, Z.; Peng, Z.; Lu, F. Anti-Aging Effect of Adipose-Derived Stem Cells in a Mouse Model of Skin Aging Induced by D-Galactose. PLoS ONE 2014, 9, e97573. [Google Scholar] [CrossRef] [Green Version]
- Castilho, R.M.; Squarize, C.H.; Gutkind, J.S. Exploiting PI3K/mTOR signaling to accelerate epithelial wound healing. Oral Dis. 2013, 19, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Kareem, N.; Aijaz, A.; Jeschke, M.G. Stem Cell Therapy for Burns: Story so Far. Biologics 2021, 15, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhao, F.; Zhang, Q.; Huang, X.; Wang, Z. Autophagy and skin wound healing. Burn. Trauma 2022, 10, tkac003. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, L.; Chen, P.; Zhang, C.; Tang, S.; Chen, X. Comparison of the Efficacy and Safety of Cell-Assisted Lipotransfer and Platelet-Rich Plasma Assisted Lipotransfer: What Should We Expect from a Systematic Review with Meta-Analysis? Cell Transplant. 2021, 30, 963689721989607. [Google Scholar] [CrossRef]
- Xiong, S.; Yi, C.; Pu, L. An Overview of Principles and New Techniques for Facial Fat Grafting. Clin. Plast. Surg. 2020, 47, 7–17. [Google Scholar] [CrossRef]
- Nanba, D. Human keratinocyte stem cells: From cell biology to cell therapy. J. Dermatol. Sci. 2019, 96, 66–72. [Google Scholar] [CrossRef]
- Drukała, J.; Bandura, L.; Cieślik, K.; Korohoda, W. Locomotion of human skin keratinocytes on polystyrene, fibrin, and collagen substrata and its modification by cell-to-cell contacts. Cell Transplant. 2001, 10, 765–771. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.D.; Van der Merwe, L.; Dai, W.T.; Lin, C. Efficacy of stem cell therapy for burn wounds: A systematic review and meta-analysis of preclinical studies. Stem Cell Res. Ther. 2020, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Wang, Y.; Yang, Z.; Xie, Z. Efficacy assessment of mesenchymal stem cell transplantation for burn wounds in animals: A systematic review. Stem Cell Res. Ther. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.R.; Chicco, M.; Huang, J.; Qi, L.; Burdick, J.; Williams, G.M.; Cameron, A.M.; Sun, Z. Stem cells in burn wound healing: A systematic review of the literature. Burns 2019, 45, 1014–1023. [Google Scholar] [CrossRef]
- Abbas, O.L.; Özatik, O.; Gönen, Z.B.; Öğüt, S.; Özatik, F.Y.; Salkın, H.; Musmul, A. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Dental Pulp as Sources of Cell Therapy for Zone of Stasis Burns. J. Investig. Surg. 2019, 32, 477–490. [Google Scholar] [CrossRef]
- Burd, A.; Ahmed, K.; Lam, S.; Ayyappan, T.; Huang, L. Stem cell strategies in burns care. Burns 2007, 33, 282–291. [Google Scholar] [CrossRef]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Lv, R.; Xu, J.; Hirman, A.R.; Du, L. IGF-1-Expressing Placenta-Derived Mesenchymal Stem Cells Promote Scalding Wound Healing. J. Surg. Res. 2021, 265, 100–113. [Google Scholar] [CrossRef]
- Jian-Xing, D.; Wen-Jun, L.; Yue-Qin, Z.; Wang, D.; Gao-Fei, Z.; Jia-Mei, L.; Han-Xiao, L. Umbilical Cord Mesenchymal Stem Cells for Inflammatory Regulation After Excision and Grafting of Severe Burn Wounds in Rats. J. Burn Care Res. 2021, 42, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, K.; Zhang, C.; Liu, Y.; Liang, F.; Hu, W.; Bian, X.; Yang, S.; Fu, X. Burns Impair Blood-Brain Barrier and Mesenchymal Stem Cells Can Reverse the Process in Mice. Front. Immunol. 2020, 11, 578879. [Google Scholar] [CrossRef]
- Abdel-Gawad, D.R.I.; Moselhy, W.A.; Ahmed, R.R.; Al-Muzafar, H.M.; Amin, K.A.; Amin, M.M.; El-Nahass, E.S.; Abdou, K.A.H. Therapeutic effect of mesenchymal stem cells on histopathological, immunohistochemical, and molecular analysis in second-grade burn model. Stem Cell Res. Ther. 2021, 12, 308. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Zhang, W.; Lv, H.; Li, J.; Wu, L.; Zhang, H.; Yang, B.; Zhu, M.; Jiang, J. Anti-Inflammatory Effects of Magnetically Targeted Mesenchymal Stem Cells on Laser-Induced Skin Injuries in Rats. Int. J. Nanomed. 2020, 15, 5645–5659. [Google Scholar] [CrossRef]
- Ramhormozi, P.; Mohajer Ansari, J.; Simorgh, S.; Nobakht, M. Bone Marrow-Derived Mesenchymal Stem Cells Combined With Simvastatin Accelerates Burn Wound Healing by Activation of the Akt/mTOR Pathway. J. Burn Care Res. 2020, 41, 1069–1078. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, M.; Mou, X.X.; Ye, L. Overexpressing of caveolin-1 in mesenchymal stem cells promotes deep second-degree burn wound healing. J. Biosci. Bioeng. 2021, 131, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, O.; Prasai, A.; Perez-Bello, D.; El Ayadi, A.; Petrov, I.Y.; Esenaliev, R.O.; Petrov, Y.; Herndon, D.N.; Finnerty, C.C.; Prough, D.S.; et al. Adipose-derived stem cells improve grafted burn wound healing by promoting wound bed blood flow. Burn. Trauma 2020, 8, tkaa009. [Google Scholar] [CrossRef] [PubMed]
- Hermeto, L.C.; DeRossi, R.; Oliveira, R.J.; Gomes, F.G.; Ferreira, W.R.; Galhardo, J.A.; Möck, T.B.; Basaglia, W.V.; Fernandes, D.M. The efficacy of topical insulin application on rat model with burn wounds treated with adipose-derived stem cells. Int. J. Burn. Trauma 2020, 10, 296–306. [Google Scholar]
- Costa de Oliveira Souza, C.M.; de Souza, C.F.; Mogharbel, B.F.; Irioda, A.C.; Cavichiolo Franco, C.R.; Sierakowski, M.R.; Athayde Teixeira de Carvalho, K. Nanostructured Cellulose-Gellan-Xyloglucan-Lysozyme Dressing Seeded with Mesenchymal Stem Cells for Deep Second-Degree Burn Treatment. Int. J. Nanomed. 2021, 16, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Barrera, J.A.; Trotsyuk, A.A.; Maan, Z.N.; Bonham, C.A.; Larson, M.R.; Mittermiller, P.A.; Henn, D.; Chen, K.; Mays, C.J.; Mittal, S.; et al. Adipose-Derived Stromal Cells Seeded in Pullulan-Collagen Hydrogels Improve Healing in Murine Burns. Tissue Eng. Part A 2021, 27, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Roshangar, L.; Rad, J.S.; Kheirjou, R.; Khosroshahi, A.F. Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J. Tissue Eng. Regen. Med. 2021, 15, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Yazdi, F.; Ghahary, A.; Mirdoraghi, M.; Sarvnaz, H.; Asgardoon, M.H.; Rastegar, T.; Malek, F.; Abbasi Moayyer, T.; Ghaffari Dafchahi, K.; Takzaree, N. Promotion of Burn Wound Healing by Local Application of Adipose-Derived Mesenchymal Stem Cells: An Experimental Study. Med. J. Islam. Repub. Iran 2021, 35, 172. [Google Scholar] [CrossRef]
- Franck, C.L.; Senegaglia, A.C.; Leite, L.M.B.; de Moura, S.A.B.; Francisco, N.F.; Ribas Filho, J.M. Influence of Adipose Tissue-Derived Stem Cells on the Burn Wound Healing Process. Stem Cells Int. 2019, 2019, 2340725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azam, M.; Ghufran, H.; Butt, H.; Mehmood, A.; Ashfaq, R.; Ilyas, A.M.; Ahmad, M.R.; Riazuddin, S. Curcumin preconditioning enhances the efficacy of adipose-derived mesenchymal stem cells to accelerate healing of burn wounds. Burn. Trauma 2021, 9, tkab021. [Google Scholar] [CrossRef]
- Babakhani, A.; Nobakht, M.; Pazoki Torodi, H.; Dahmardehei, M.; Hashemi, P.; Mohajer Ansari, J.; Ramhormozi, P.; Yari, A.; Heidari, F. Effects of Hair Follicle Stem Cells on Partial-Thickness Burn Wound Healing and Tensile Strength. Iran Biomed. J. 2020, 24, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini-Nik, S.; Dolp, R.; Eylert, G.; Datu, A.K.; Parousis, A.; Blakeley, C.; Jeschke, M.G. Stem cells derived from burned skin—The future of burn care. eBioMedicine 2018, 37, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitala, D.; Łabuś, W.; Klama-Baryła, A.; Kraut, M.; Maj, M.; Szapski, M. Application of Amniotic Stem Cells on an Acellular Dermal Matrix Scaffold in a Burned Patient: A Case Report. Transplant. Proc. 2020, 52, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, M.F.; Vasilchenkov, A.V.; Onishchenko, N.A.; Krasheninnikov, M.E.; Kravchenko, V.I.; Gorshenin, T.L.; Pidtsan, R.E.; Potapov, I.V. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Boil. Med. 2005, 139, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, E.; Marín, G.H.; Drago, H.; Sturla, F.; Salas, E.; Gardiner, C.; Bossi, S.; Lamonega, R.; Guzmán, A.; Nuñez, A.; et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: New evidence for their use in regenerative medicine. Transplant. Proc. 2006, 38, 967–969. [Google Scholar] [CrossRef]
- Lataillade, J.J.; Doucet, C.; Bey, E.; Carsin, H.; Huet, C.; Clairand, I.; Bottollier-Depois, J.; Chapel, A.; Ernou, I.; Gourven, M.; et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen. Med. 2007, 2, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, M.G.; Rehou, S.; McCann, M.R.; Shahrokhi, S. Allogeneic mesenchymal stem cells for treatment of severe burn injury. Stem Cell Res. Ther. 2019, 10, 337. [Google Scholar] [CrossRef]
- Alatyyat, S.M.; Alasmari, H.M.; Aleid, O.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Umbilical cord stem cells: Background, processing and applications. Tissue Cell 2020, 65, 101351. [Google Scholar] [CrossRef]
- Aghayan, H.R.; Payab, M.; Mohamadi-Jahani, F.; Aghayan, S.S.; Larijani, B.; Arjmand, B. GMP-Compliant Production of Human Placenta-Derived Mesenchymal Stem Cells. Methods Mol. Biol. 2021, 2286, 213–225. [Google Scholar] [CrossRef]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl- Moktader, M.; Elsherefy, S.; Gabr, H. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar] [PubMed]
- Alt, E.U.; Winnier, G.; Haenel, A.; Rothoerl, R.; Solakoglu, O.; Alt, C.; Schmitz, C. Towards a Comprehensive Understanding of UA-ADRCs (Uncultured, Autologous, Fresh, Unmodified, Adipose Derived Regenerative Cells, Isolated at Point of Care) in Regenerative Medicine. Cells 2020, 9, 1097. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, J.; Szóstek-Mioduchowska, A.Z.; Kopcewicz, M.; Walendzik, K.; Machcińska, S.; Gawrońska-Kozak, B. Adipose-Derived Stromal/Stem Cells from Large Animal Models: From Basic to Applied Science. Stem Cell Rev. Rep. 2021, 17, 719–738. [Google Scholar] [CrossRef]
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules 2021, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, M.; Brzezicka, A.; Skoniecka, A.; Zieliński, J.; Pikuła, M. Adipose-derived stromal cells for nonhealing wounds: Emerging opportunities and challenges. Med. Res. Rev. 2021, 41, 2130–2171. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Natesan, S.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Christy, R.J. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J. Burn Care Res. 2013, 34, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, K.L.; Goverman, J.; Ma, H.; Fischman, A.; Yu, Y.M.; Bilodeau, M.; Rad, A.M.; Bonab, A.A.; Tompkins, R.G.; Fagan, S.P. Stem cells and burns: Review and therapeutic implications. J. Burn Care Res. 2010, 31, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, B.; Zhu, H.; Yu, Q.; Xie, F.; Chen, C.; Li, Q. Adipose-derived stem cells embedded in platelet-rich plasma scaffolds improve the texture of skin grafts in a rat full-thickness wound model. Burns 2020, 46, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Heard, T.C.; Gómez, B.I.; Saathoff, M.E.; Duarte, J.; Dubick, M.A.; Bynum, J.A.; Christy, R.J.; Burmeister, D.M. Minimal Effects of Intravenous Administration of Xenogeneic Adipose Derived Stem Cells on Organ Function in a Porcine 40% TBSA Burn Model. J. Burn Care Res. 2021, 42, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Wu, Y.C.; Huang, S.H.; Wang, H.D.; Kuo, Y.R.; Lee, S.S. Autologous and not allogeneic adipose-derived stem cells improve acute burn wound healing. PLoS ONE 2018, 13, e0197744. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhou, H.; Du, W.; Huang, X.; Zheng, X.; Zhang, C.; Hu, H.; Wang, J.; Quan, R. Hair follicle stem cells combined with human allogeneic acellular amniotic membrane for repair of full thickness skin defects in nude mice. J. Tissue Eng. Regen. Med. 2020, 14, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Dolp, R.; Eylert, G.; Auger, C.; Aijaz, A.; Chen, Y.A.; Amini-Nik, S.; Parousis, A.; Datu, A.K.; Jeschke, M.G. Biological characteristics of stem cells derived from burned skin—A comparative study with umbilical cord stem cells. Stem Cell Res. Ther. 2021, 12, 137. [Google Scholar] [CrossRef]
- van der Veen, V.C.; Vlig, M.; van Milligen, F.J.; de Vries, S.I.; Middelkoop, E.; Ulrich, M.M. Stem cells in burn eschar. Cell Transplant. 2012, 21, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics. Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10, quiz 11-2. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Sun, Z.L.; Liu, S.Y.; Wu, J.-J.; Zhao, B.-H.; Lv, G.-Z.; Du, Y.; Yu, S.; Yang, M.-L.; Yuan, F.-L.; et al. Direct and Indirect Roles of Macrophages in Hypertrophic Scar Formation. Front. Physiol. 2019, 10, 1101. [Google Scholar] [CrossRef]
- Piejko, M.; Radziun, K.; Bobis-Wozowicz, S.; Waligórska, A.; Zimoląg, E.; Nessler, M.; Chrapusta, A.; Madeja, Z.; Drukała, J. Adipose-Derived Stromal Cells Seeded on Integra® Dermal Regeneration Template Improve Post-Burn Wound Reconstruction. Bioengineering 2020, 7, 67. [Google Scholar] [CrossRef]
- Zahorec, P.; Sarkozyova, N.; Ferancikova, N.; Bukovcan, P.; Danisovic, L.; Bohac, M.; Tomas, M.; Koller, J. Autologous mesenchymal stem cells application in post-burn scars treatment: A preliminary study. Cell Tissue Bank. 2021, 22, 39–46. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Gao, X.; Chen, X.; Yu, J. Umbilical cord-derived mesenchymal stem cells exert anti-fibrotic action on hypertrophic scar-derived fibroblasts in co-culture by inhibiting the activation of the TGF β1/Smad3 pathway. Exp. Ther. Med. 2021, 21, 210. [Google Scholar] [CrossRef] [PubMed]


| Study | Study Type | Methods | Outcomes | Conclusion |
|---|---|---|---|---|
| Li YK [36] | Experiment, mice | Human amniotic mesenchymal stem cells were recruited, selected, and cultured from human fetal placentas obtained from the volunteers, and injected subcutaneously into thermally injured mouse skin. | A mean number of 2 × 106 stem cells was injected. An increase in PCNA and CK19 was observed on days 7 and 14, with more tubular structures observed after the initial 6 h post-burn, as well as inhibited heat stress-induced apoptosis and promoted proliferation of dermal fibroblasts and keratinocytes and activated PI3K/AKT/mTOR signaling and GSK3β/β-catenin. | Human amniotic mesenchymal stem cells inhibit stress-induced apoptosis. |
| Cheng [37] | Experimental, mice | Human placental MCS were obtained. Using a lentivirus, overexpression of IGF-1 was obtained in MSCs; 96 mice were divided into 4 groups: control, burn, burn+unmodified MCS, burn + IGF-1modfied MCS. The cells were injected on days 1, 4, 8, 12, 16, and 20 after the burn injury with 2 × 106 of hPMSC-Lv-Vector or hPMSC-Lv-IGF-1 at 4 points around the burn wounds. | Induction of epithelial differentiation was observed. Inhibition of cell apoptosis and stimulation of cell proliferation was observed in the IGF-1 group. Modified stem cells stimulated wound healing and in the skin specimen reduced inflammatory cells in the wound bed. They also reduced pro-inflammatory cytokine levels of IL-1b, IL-6 and TNF-a, as well as TGF-β1, collagen I and collagen III expressions in vivo, and increased VEGF levels. | Modified MSCs with overexpression of IGF-1 have the potential to promote faster wound healing and reduction of scar contraction. |
| Jian-Xing [38] | Experiment, rats | A 30% third-degree burn was created. After escharotomy, human umbilical cord stem cells were injected into the tail vein of rats. | Levels of IL-6 and TNF-α were lower, phosphorylation levels of P38MAPK and NF-B P65 proteins in the liver to reduce the inflammatory response, a shift to anti-inflammatory M2 population of macrophages in the skin graft. | Stem cells administrated intravascularly reduce inflammation by regulating liver secretion of proteins and cytokines. They improve skin graft healing and reduce scarring. |
| Yang [39] | Experimental, mice | Allogenic umbilical cord mesenchymal stem cells were injected into the tail vein in severely burned mice. The Dextran model was used to evaluate blood–brain barrier permeability. | UC-MSC reduced blood–brain barrier permeability and decreased levels of IL-6 and IL-1β in serum and in the brain. | Systematic injection of UC-MCS can improve the integrity of the blood-brain barrier and prevent neurological symptoms in severe burns. |
| Abdel-Gawad [40] | Experiment, rats | 90 rats divided into three groups: control (6), burn model (42), and study (42). Bone-marrow stem cells were injected subcutaneously in the study group. | Decrease in wound contraction, downregulation of TGF-β, IL-6, TNF-α, MMP-9, and microRNA21. | Bone-marrow-derived stem cells improve healing of burn wound and reduce scar formation. |
| Li [41] | Experimental, rats | BM-MSC were harvested from rats and labeled with Fe3O4 NP. A full-thickness burn was created, and then stem cell were injected in the tail vein. | Labeled stem cells were non-toxic. Labeled stem cells migrated to the burn wound up to day 7. Increase in neoangiogenesis factors was observed: increase in CD31 and α-SMA. Reduction of systemic levels of IL-1α, IL-2, IL-6, and interferon (INF)-γ. | Intravascularly injected stem cells can migrate to the burn wound and improve healing. They reduce systemic levels of pro-inflammatory cytokines. |
| Ramhormozi [42] | Experimental, rats | Bone-marrow-derived stem cells (BMS) were obtained from adult male Wistar rats; 40 rats were burned and divided into groups: control, simvastatin iv, BMS intradermally, and BMS+ simvastatin. Wound healing, collagen, re-epithelialization were examined. Additionally, qRT-PCR for Akt/mTOR signaling pathway; CD31 and VEGF genes. | Better wound healing was observed in the group were stem cells were injected intradermally and simvastatin was administrated intravascularly. Additionally, levels of α-SMA, CD31 and VEGF genes in granulation tissues were also significantly higher. In the qRT-PCR findings, the expression levels of Akt and mTOR transcripts were higher. | A combined therapy improved healing by stimulating Akt/mTOR signaling pathway. |
| Wu [43] | Experiment, rats | Allogenic BM-CS were used. A model of a deep second-degree burn was created. A plasmid pLV-CMV-EF1-fLuc-T2A-puro was used to create a recombinant lentivirus with overexpression of caveolin-1 and transfected to BM-MSCs and injected intradermally 5 min after burn injury. | Overexpression of caveolin-1 improves the efficiency of BM-MCSs in burn wound healing and shortened the healing time to 10 days. The protein expression of TGF-b1, TGF-b3, FGF, and EGF was increased. Additionally, serum levels of IL-1b, IL-6 and TNF-a were decreased. | Application of exogenous MSCs overexpressing caveolin-1 improves burn wound healing. |
| Fujiwara [44] | Experimental, sheep | Allogenic ADSCs were obtained and administered topically; 7 sheep were enrolled into the study. After a burn and excision of a deep burn, the wound was covered with a skin graft (2 × 2 cm) and ADSCs were applied topically in the study group. | Topical use of allogenic ADSCs in sheep improved graft intake and wound blood flow, increased VEGF levels in the wound, and accelerated wound epithelialization after the excision of a full-thickness burn model. | Topical allogenic ADSCs accelerate graft intake and improve wound vascularization. A new burn model was established. |
| Hermeto [45] | Experiment, rats | Two rats were donors of adipose-derived stem cells. ADSCs were harvested, isolated, and cultures; 40 rats were divided into 4 groups: placebo gel, insulin gel, topical ADSCs, and topical ADSCs+ insulin gel applied on superficial second-degree burns. | ADSCs improved healing and reduced the wound extent in both topical ADSC and topical DSC+ insulin gel groups. | Topical use of ADSC can be useful in the treatment of superficial second-degree burns. |
| Costa de Oliveira Souza [46] | Experimental, rats | Cellulose membranes incorporated with 10% tamarind xyloglucan plus gellan gum 1:1 and 10% lysozyme, or with 10% gellan gum and 10% lysozyme were seeded with allogenic ADSCs and used in a rat burn model. The study group consisted of 40 rats. | No impairment of stem cell activity was observed. The cellulose membrane had a potential antimicrobial activity. Stem cells accelerated epithelialization. | Cellulose membrane is non-toxic for stem cells, and it enables cell proliferation and maturation, as well as migration. |
| Barrera [47] | Experimental, mice | Allogenic ADSCs were isolated, cultured, enriched with CD26+/CD55+ and seeded in a hydrogel dressing. A contact burn model was established. | Hydrogel seeded with ADSCs healed the wound faster than ADSCs injected. They improved wound vascularization and increased levels of mRNA for Vegfa and protein levels for MCP-1, SDF-1, and VEGF. Additionally, the quality of the scar was better with the lower fractional dimension of collagen architecture. | SC-seeded hydrogel significantly improves healing in murine burns. |
| Dong [48] | Experiment, mice | Hydrogel system comprised of a hyperbranched poly (ethylene glycol) diacrylate (HB-PEGDA) polymer, a commercially available thiol-functionalized hyaluronic acid (HA-SH) and a short RGD peptide enriched in xenogenic ADSC was used to treat second-degree burns in mice. | On day 3, a significant improvement in healing in the treated group was observed. The examination of the specimens showed a higher number of vessels, ratio of collagen type III to I, and reduction of active myofibroblasts. | A novel combined dressing enhanced neovascularization, promoted wound closure and reduced scar formation. |
| Roshangar [49] | Experimental, rats | Allogenic ADSCs were incorporated in 3D bioprinter-derived gel scaffold and used in scald burn model. | The scaffold was not toxic and did not interfere with ADSC capacities and viability. | 3D bioprinter-derived gel scaffold enhanced burn wound treatment. |
| Razei Yazdi [50] | Experimental, rats | Xenogeneic ADSCs were injected intradermally in 4 areas. | ADSCs reduced inflammatory cells in the wound. They promoted VEGF gene expression and secretion of TGF-β. ADSC also promoted proliferation of dermal fibroblasts. | ADSCs improve burn wound healing by affecting fibroblasts, keratinocytes, and inflammatory cells, as well as increasing the expression of the TGF-β and VEGF genes. |
| Franck [51] | Experimental, rats | 23 rats were used, and one was a donor for ADSCs. A burn model was conducted. ADSCs were injected into the burn wound just after wound cooling. An amount of 3.2 × 106 was used. | ADSCs reduced the burn wound area after 14 days. There was no difference in inflammatory infiltration between the study and control groups. The number of lymphatic vessels was reduced. The concentration of collagen type III was elevated. | ADSCs improve wound healing and reduce scar formation. |
| Azam [52] | Experimental, rats | ADSCs were isolated from 22 rats, then the isolated cells were incubated in a medium with curcumin for 24 h. An acid burn model was used, 24 h after the injury an excision was made, and the rats were divided into 3 groups. ADSCs were injected intradermally around the wound. | Curcumin improved the healing capacities of ADSCs, increased the capacity of migration, proliferation and paracrine potential, and suppressed secretion of pro-inflammatory cytokines in comparison with innate ADSCs. | Curcumin-preconditioned ADSCs may show potential in the treatment of acid burns. |
| Babakhani [53] | Experimental, rats | Hair-follicle stem cells were derived from 10 rats. A group of 45 rats was divided into three groups: treatment, control, and sham. A burn model was conducted. The stem cells were injected around the wound bed. | Stem cells improved healing and epidermal thickness. They also stimulated neovascularization and increased the expression of CD31. | Stem cells injected around a deep partial- thickness burn accelerate healing by improvement of epidermal density and neovascularization. |
| Amini-Nik [54] | Experimental, mice and pigs | Human stem cells were obtained from deeply burned skin. They were used topically in a burn model in 5 mice and 4 pigs. | No tumor formation of stem cells was observed. Stem cells accelerated healing in a mouse and pig model. After 12 days after administration, the stem cells were still present in the wound. | Stem cells obtained from burned skin may be useful in future autologous skin regeneration of deep burns. |
| Study | Study Type | Patients & Methods | Outcomes |
|---|---|---|---|
| Kitala [55] | Case report | A 40-year-old patient with deep thermal burns (IIb°/III°-36%TBSA, III°/IV°-1%TBSA). Amnio-derived stem cells were isolated by mechanical homogenization from the placenta and cultured. After bed wound debridement, the wound was covered with stem cells in a saline solution and covered with a dermal matrix substitute. | No adverse events were reported. The wound healed within 12 days. Pain reduction was observed. |
| Rasulov [56] | Case report | Allogeneic stem cells were collected from two healthy volunteers from the iliac plate. The cells were applied to the surface of the wounds and, after several days, the skin was re-transplanted, this time achieving complete wound closure. A female patient with extensive skin burn (I-II-IIIAB skin burn, total area 40%, area of IIIB degree 30%) was treated. | Rapid healing of the donor site and accelerated healing of deep burns. |
| Mansilla [57] | Clinical study | Examination of the concentration of stem cells (Flow cytometric analysis, using a large monoclonal antibody panel: CD44, CD45, CD14, DR, CD34, CD19, CD13, CD29, CD105, CD1a, CD90, CD38, CD25. MSC phenotype was considered positive for CD44, CD13, CD29, CD90, and CD105, and negative for the other monoclonals) in the peripheral blood of burned (3 days after injury) and healthy volunteers. | Positive correlation between the number of cells and the extent of the burn. Younger patients had a higher number of stem cells than older patients. |
| Lattaillade [58] | Case report | A case of severe buttock radiation burns (2000 Gy at the center of the skin surface lesion) in a 27-year-old Chilean. After primary and secondary excisions, bone-marrow-derived mesenchymal stem cells were applied. | Successful treatment of a radiation burn using autogenous myeloid stem cells and a collagen matrix |
| Jeschke [59] | Case report | A case of a patient with full-thickness burns covering 70% of the body surface, in whom allogeneic myeloid stem cells were used. After debridement of the burn wound, stem cells and fibrin glue were applied to the surface of the burn wound and covered with allografts. About half of the grafts were healed. In the next procedure, the edges of the wound were injected with a commercial suspension of allogeneic myeloid stem cells and the allografts were broken down, with about 90% of the wounds closed as a result. | Stem cells accelerate wound healing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surowiecka, A.; Chrapusta, A.; Klimeczek-Chrapusta, M.; Korzeniowski, T.; Drukała, J.; Strużyna, J. Mesenchymal Stem Cells in Burn Wound Management. Int. J. Mol. Sci. 2022, 23, 15339. https://doi.org/10.3390/ijms232315339
Surowiecka A, Chrapusta A, Klimeczek-Chrapusta M, Korzeniowski T, Drukała J, Strużyna J. Mesenchymal Stem Cells in Burn Wound Management. International Journal of Molecular Sciences. 2022; 23(23):15339. https://doi.org/10.3390/ijms232315339
Chicago/Turabian StyleSurowiecka, Agnieszka, Anna Chrapusta, Maria Klimeczek-Chrapusta, Tomasz Korzeniowski, Justyna Drukała, and Jerzy Strużyna. 2022. "Mesenchymal Stem Cells in Burn Wound Management" International Journal of Molecular Sciences 23, no. 23: 15339. https://doi.org/10.3390/ijms232315339
APA StyleSurowiecka, A., Chrapusta, A., Klimeczek-Chrapusta, M., Korzeniowski, T., Drukała, J., & Strużyna, J. (2022). Mesenchymal Stem Cells in Burn Wound Management. International Journal of Molecular Sciences, 23(23), 15339. https://doi.org/10.3390/ijms232315339






