ATPase Inhibitory Factor 1—A Novel Marker of Cellular Fitness and Exercise Capacity?
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of Studied Participants
2.2. Association of IF1 with CPET Parameters
3. Discussion
3.1. IF1
3.2. IF1 Origin
3.3. Intracellular ATP, ATPase, and IF1
3.4. Extracellular ATP
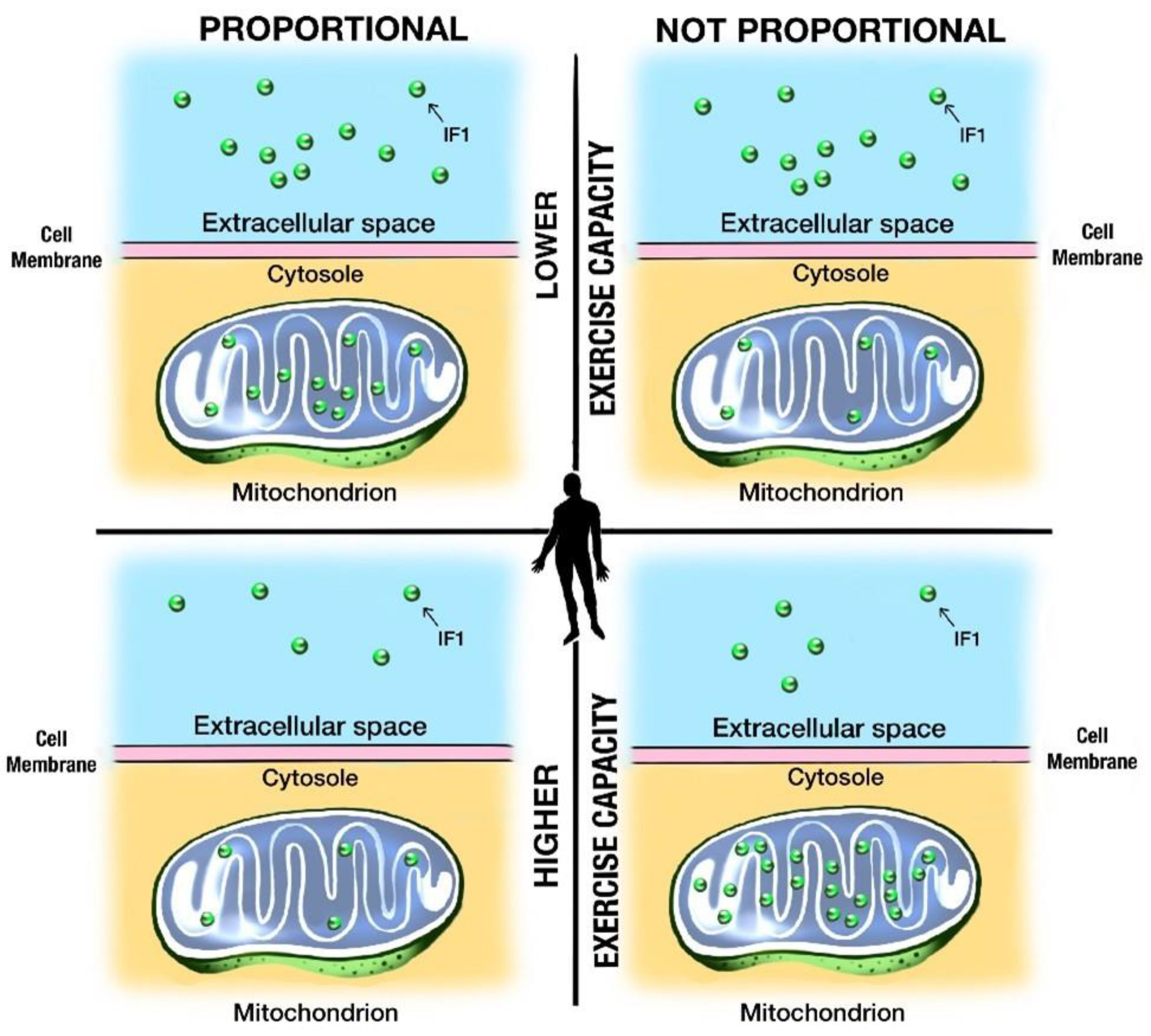
3.5. Exercise Capacity and IF1
3.6. IF1 Is a Potential Marker of Mitochondrial Fitness, Cellular Integrity, and Exercise Capacity
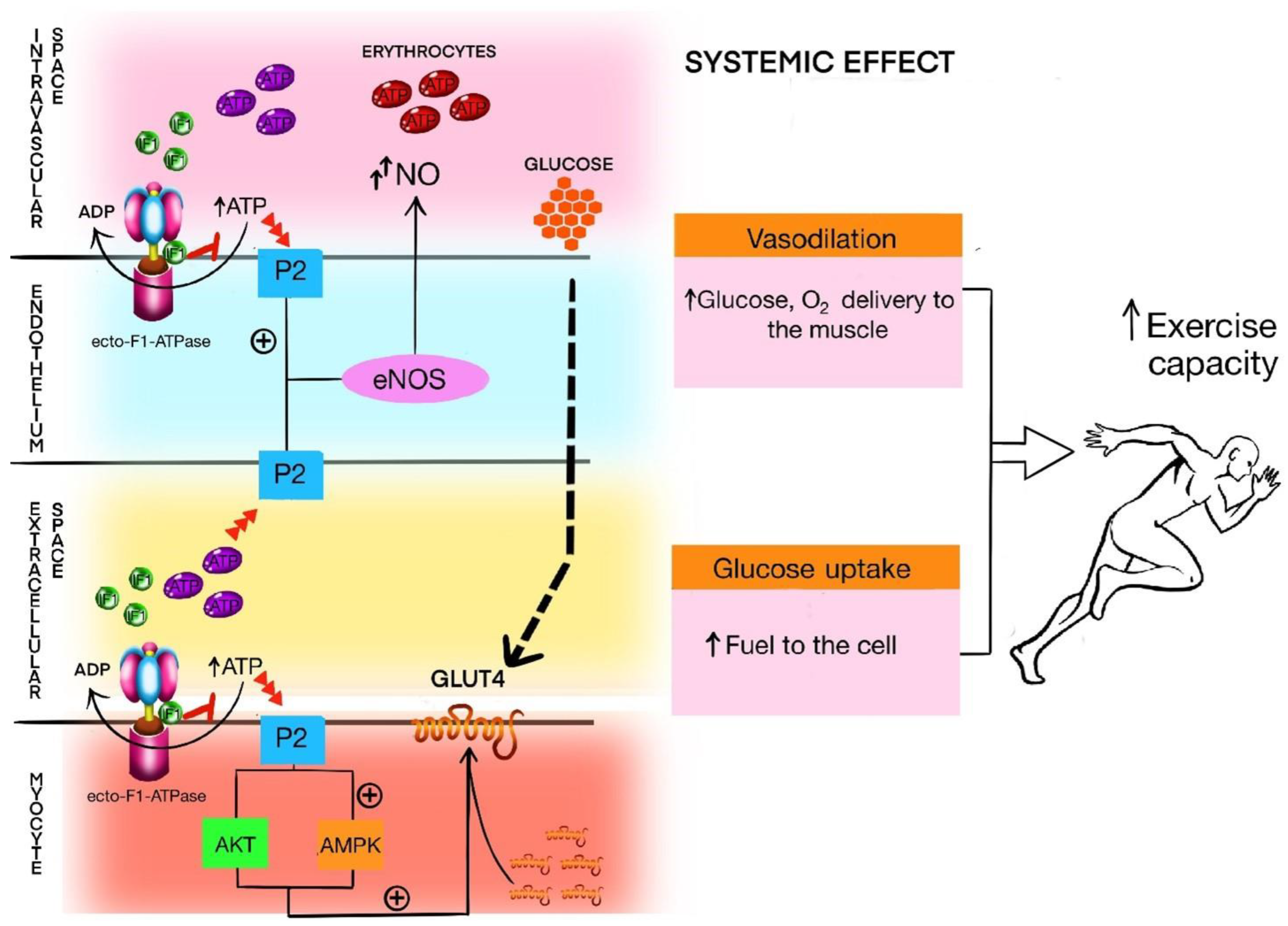
3.7. IF1 in Relation to ecto-F1-ATPase, Purinergic Signaling, and Exercise Capacity
4. Materials and Methods
4.1. Bioethical Issues
4.2. Participants
4.3. Pretest Resting Spirometry
4.4. Cardiopulmonary Exercise Test
- HR—heart rate;
- VO2—the volume of consumed O2 per minute;
- O2pulse—the ratio of VO2 to HR (an indirect measure of stroke volume);
- VCO2—the volume of produced CO2 per minute;
- VE—minute ventilation;
- TV—the tidal volume of a single breath;
- BF—breathing frequency;
- VE/VCO2—the ventilatory equivalent for O2;
- VE/VO2—the ventilatory equivalent for CO2;
- RER—respiratory exchange ratio;
- PETO2—the end-tidal O2 tension in the exhaled air;
- PETCO2—the end-tidal CO2 tension in the exhaled air.
4.5. Blood Sampling, Storage, and Biochemical Analysis
4.6. Statistical Analysis
5. Conclusions
5.1. Limitations
5.2. Perspectives
- ATP is crucial in all features of physical activity and skeletal muscle work, including exercise capacity;
- IF1 regulates the activity of mitochondrial ATPase and ecto-F1-ATPase on the cells surface;
- ATPase and ecto-F1-ATPase regulate the amount of ATP and its metabolites in intra- and extracellular space;
- IF1 serum concentration negatively correlates with indices of exercise capacity;
- How IF1 migrates from mitochondria to extracellular space and further to intravenous space is unknown;
- IF1 might be a novel marker of cellular fitness, cellular integrity, and exercise capacity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- So, B.; Kim, H.J.; Kim, J.; Song, W. Exercise-induced myokines in health and metabolic diseases. Integr. Med. Res. 2014, 3, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Åkerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grannell, A.; Kokkinos, A.; le Roux, C.W. Myokines in Appetite Control and Energy Balance. Muscles 2022, 1, 26–47. [Google Scholar] [CrossRef]
- Zunner, B.E.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Kelly, M.; Gauthier, M.S.; Saha, A.K.; Ruderman, N.B. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: Association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes 2009, 58, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Van Hall, G.; Steensberg, A.; Sacchetti, M.; Fischer, C.; Keller, C.; Schjerling, P.; Hiscock, N.; Møller, K.; Saltin, B.; Febbraio, M.A.; et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 2003, 88, 3005–3010. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; Bellamy, L.; Parise, G.; Baker, S.K.; Phillips, S.M. Muscular and Systemic Correlates of Resistance Training-Induced Muscle Hypertrophy. Alway SE, editor. PLoS ONE 2013, 8, e78636. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Uthman, L.; Somani, Y.; van Royen, N. Short-term exercise-induced protection of cardiovascular function and health: Why and how fast does the heart benefit from exercise? J. Physiol. 2022, 600, 1339–1355. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.C.; Hadj Sassi, A.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Joyner, M.J.; Green, D.J. Mimicking exercise: What matters most and where to next? J. Physiol. 2021, 599, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Wróbel, M.; Gołaś, A.; Rokicka, D.; Pyka, Ł.; Szewczyk, M.; Stołtny, T.; Roczniok, R.; Gąsior, M.; Strojek, K. The influence of resistance training on muscle strength, irisin concentration, and metabolic parameters in type 1 diabetic patients. Endokrynol. Pol. 2022, 73, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Pullman, M.E.; Monroy, G.C. A naturally occurring inhibitor of mitochondrial adenosine triphosphatase. J. Biol. Chem. 1963, 238, 3762–3769. [Google Scholar] [CrossRef] [PubMed]
- Gore, E.; Duparc, T.; Genoux, A.; Perret, B.; Najib, S.; Martinez, L.O. The Multifaceted ATPase Inhibitory Factor 1 (IF1) in Energy Metabolism Reprogramming and Mitochondrial Dysfunction: A New Player in Age-Associated Disorders? Antioxid. Redox Signal. 2022, 37, 370–393. [Google Scholar] [CrossRef]
- Lee, H.J.; Moon, J.; Chung, I.; Chung, J.H.; Park, C.; Lee, J.O.; Han, J.A.; Kang, M.J.; Yoo, E.H.; Kwak, S.Y.; et al. ATP synthase inhibitory factor 1 (IF1), a novel myokine, regulates glucose metabolism by AMPK and Akt dual pathways. FASEB J. 2019, 33, 14825–14840. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Hernández, P.; Domínguez-Ramírez, L.; Estrada-Bernal, A.; Montes-Sánchez, D.G.; Zentella-Dehesa, A.; de Gómez-Puyou, M.T.; Gómez-Puyou, A.; García, J.J. The inhibitor protein of the F1F0-ATP synthase is associated to the external surface of endothelial cells. Biochem. Biophys. Res. Commun. 2005, 330, 844–849. [Google Scholar] [CrossRef]
- Contessi, S.; Comelli, M.; Cmet, S.; Lippe, G.; Mavelli, I. IF(1) distribution in HepG2 cells in relation to ecto-F(0)F (1)ATPsynthase and calmodulin. J. Bioenerg. Biomembr. 2007, 39, 291–300. [Google Scholar] [CrossRef]
- Champagne, E.; Martinez, L.O.; Collet, X.; Barbaras, R. Ecto-F1Fo ATP synthase/F1 ATPase: Metabolic and immunological functions. Curr. Opin. Lipidol. 2006, 17, 279–284. [Google Scholar] [CrossRef]
- Genoux, A.; Pons, V.; Radojkovic, C.; Roux-Dalvai, F.; Combes, G.; Rolland, C.; Malet, N.; Monsarrat, B.; Lopez, F.; Ruidavets, J.B.; et al. Mitochondrial inhibitory factor 1 (IF1) is present in human serum and is positively correlated with HDL-cholesterol. PLoS ONE 2011, 6, e23949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florin, A.; Lambert, C.; Sanchez, C.; Zappia, J.; Durieux, N.; Tieppo, A.M.; Mobasheri, A.; Henrotin, Y. The secretome of skeletal muscle cells: A systematic review. Osteoarthr. Cartil. Open 2020, 2, 100019. [Google Scholar] [CrossRef]
- Senior, A.E.; Nadanaciva, S.; Weber, J. The molecular mechanism of ATP synthesis by F1F0-ATP synthase. Biochim. Biophys. Acta (BBA) Bioenerg. 2002, 1553, 188–211. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Senior, A.E. ATP synthase: What we know about ATP hydrolysis and what we do not know about ATP synthesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2000, 1458, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Caudal, A.; Tang, X.; Chavez, J.D.; McMillen, T.S.; Keller, A.; Villet, O.; Zhao, M.; Liu, Y.; Ritterhoff, J.; et al. Upregulation of mitochondrial ATPase inhibitory factor 1 (ATPIF1) mediates increased glycolysis in mouse hearts. J. Clin. Investig. 2022, 132, e155333. Available online: https://www.jci.org/articles/view/155333 (accessed on 8 July 2022). [CrossRef] [PubMed]
- Campanella, M.; Parker, N.; Tan, C.H.; Hall, A.M.; Duchen, M.R. IF(1): Setting the pace of the F(1)F(o)-ATP synthase. Trends Biochem. Sci. 2009, 34, 343–350. [Google Scholar] [CrossRef]
- Garcia-Bermudez, J.; Cuezva, J.M. The ATPase Inhibitory Factor 1 (IF1): A master regulator of energy metabolism and of cell survival. Biochim. Biophys. Acta 2016, 1857, 1167–1182. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Silvério, M.N.O.; de Oliveira Maciel, S.F.V. Purinergic signaling as a new mechanism underlying physical exercise benefits: A narrative review. Purinergic Signal. 2021, 17, 649–679. [Google Scholar] [CrossRef]
- Marginedas-Freixa, I.; Alvarez, C.L.; Moras, M.; Leal Denis, M.F.; Hattab, C.; Halle, F.; Bihel, F.; Mouro-Chanteloup, I.; Lefevre, S.D.; Le Van Kim, C.; et al. Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. Sci. Rep. 2018, 8, 11384. [Google Scholar] [CrossRef] [Green Version]
- Kirby, B.S.; Crecelius, A.R.; Richards, J.C.; Dinenno, F.A. Sources of intravascular ATP during exercise in humans: Critical role for skeletal muscle perfusion. Exp. Physiol. 2013, 98, 988–998. [Google Scholar] [CrossRef]
- Crecelius, A.R.; Kirby, B.S.; Dinenno, F.A. Intravascular ATP and the Regulation of Blood Flow and Oxygen Delivery in Humans. Exerc. Sport Sci. Rev. 2015, 43, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.P.; Saltin, B. Regulation of the skeletal muscle blood flow in humans. Exp. Physiol. 2014, 99, 1552–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moritz, C.E.J.; Teixeira, B.C.; Rockenbach, L.; Reischak-Oliveira, A.; Casali, E.A.; Battastini, A.M.O. Altered extracellular ATP, ADP, and AMP hydrolysis in blood serum of sedentary individuals after an acute, aerobic, moderate exercise session. Mol. Cell. Biochem. 2017, 426, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zarębska, E.A.; Kusy, K.; Słomińska, E.M.; Kruszyna, Ł.; Zieliński, J. Plasma Nucleotide Dynamics during Exercise and Recovery in Highly Trained Athletes and Recreationally Active Individuals. BioMed Res. Int. 2018, 2018, e4081802. [Google Scholar] [CrossRef]
- Zarębska, E.A.; Kusy, K.; Słomińska, E.M.; Kruszyna, Ł.; Zieliński, J. Alterations in Exercise-Induced Plasma Adenosine Triphosphate Concentration in Highly Trained Athletes in a One-Year Training Cycle. Metabolites 2019, 9, 230. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiari, N.; Hosseinkhani, S.; Larijani, B.; Mohajeri-Tehrani, M.R.; Fallah, A. Red blood cell ATP/ADP & nitric oxide: The best vasodilators in diabetic patients. J. Diabetes Metab. Disord. 2012, 11, 9. [Google Scholar]
- Ulker, P.; Özen, N.; Abdullayeva, G.; Köksoy, S.; Yaraş, N.; Basrali, F. Extracellular ATP activates eNOS and increases intracellular NO generation in Red Blood Cells. Clin. Hemorheol. Microcirc. 2018, 68, 89–101. [Google Scholar] [CrossRef]
- Mcardle William, D.; Katch Frank, I.; Katch Victor, L. Exercise Physiology. Nutrition, Energy, and Human Performance. VIII; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015. [Google Scholar]
- Jones, A.M.; Carter, H. The Effect of Endurance Training on Parameters of Aerobic Fitness. Sports Med. 2000, 29, 373–386. [Google Scholar] [CrossRef]
- Mazaheri, R.; Schmied, C.; Niederseer, D.; Guazzi, M. Cardiopulmonary Exercise Test Parameters in Athletic Population: A Review. J. Clin. Med. 2021, 10, 5073. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; 481p. [Google Scholar]
- Hebisz, P.; Jastrzębska, A.D.; Hebisz, R. Real Assessment of Maximum Oxygen Uptake as a Verification after an Incremental Test Versus without a Test. Front. Physiol. 2021, 12, 739745. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Harber, M.; Wisloff, U.; Stensvold, D.; Kaminsky, L.A. Peak oxygen pulse responses during maximal cardiopulmonary exercise testing: Reference standards from FRIEND (Fitness Registry and the Importance of Exercise: An International Database). Int. J. Cardiol. 2020, 301, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Radak, Z.; Taniguchi, J.; Masaki, T. How Different Respiratory Rate Patterns affect Cardiorespiratory Variables and Performance. Int. J. Exerc. Sci. 2017, 10, 322–329. [Google Scholar]
- Hill, D.W.; Poole, D.C.; Smith, J.C. The relationship between power and the time to achieve VO2max. Med. Sci. Sports Exerc. 2002, 34, 709–714. [Google Scholar] [PubMed]
- MacIntosh, B.R.; Neptune, R.R.; Horton, J.F. Cadence, power, and muscle activation in cycle ergometry. Med. Sci. Sports Exerc. 2000, 32, 1281–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efficiency of Cycling Exercise: Quantification, Mechanisms, and Misunderstandings. Available online: https://onlinelibrary-1wiley-1com-1stm795f10030.han.ump.edu.pl/doi/epdf/10.1111/sms.14149 (accessed on 18 August 2022).
- Majerczak, J.; Korostynski, M.; Nieckarz, Z.; Szkutnik, Z.; Duda, K.; Zoladz, J.A. Endurance training decreases the non-linearity in the oxygen uptake–power output relationship in humans. Exp. Physiol. 2012, 97, 386–399. [Google Scholar] [CrossRef]
- Sietsema, K.; Sue, D.; Stringer, W.; Ward, S. Wasserman & Whipp’s Principles of Exercise Testing and Interpretation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Nilsson, M.I.; Bourgeois, J.M.; Nederveen, J.P.; Leite, M.R.; Hettinga, B.P.; Bujak, A.L.; May, L.; Lin, E.; Crozier, M.; Rusiecki, D.R.; et al. Lifelong aerobic exercise protects against inflammaging and cancer. PLoS ONE 2019, 14, e0210863. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Tarnopolsky, M.A. Mitochondria and Aging—The Role of Exercise as a Countermeasure. Biology 2019, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. Available online: https://www.frontiersin.org/articles/10.3389/fcvm.2019.00069 (accessed on 2 September 2022). [CrossRef] [Green Version]
- Sorriento, D.; Di Vaia, E.; Iaccarino, G. Physical Exercise: A Novel Tool to Protect Mitochondrial Health. Front. Physiol. 2021, 12, 660068. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.J.; Berry, B.J.; Wojtovich, A.P. Exercise and Mitochondrial Dynamics: Keeping in Shape with ROS and AMPK. Antioxidants 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Behnke, B.J.; Musch, T.I. The role of vascular function on exercise capacity in health and disease. J. Physiol. 2021, 599, 889–910. [Google Scholar] [CrossRef] [PubMed]
- Flores-Opazo, M.; McGee, S.L.; Hargreaves, M. Exercise and GLUT4. Exerc. Sport Sci. Rev. 2020, 48, 110–118. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Guzik, P.; Mostowska, A.; Walkowiak, J. Publication ethics of human studies in the light of the Declaration of Helsinki—A mini-review. J. Med. Sci. 2022, 91, e700. [Google Scholar] [CrossRef]
- The Prohibited List [Internet]. World Anti-Doping Agency. Available online: https://www.wada-ama.org/en/prohibited-list (accessed on 17 July 2022).
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Pritchard, A.; Burns, P.; Correia, J.; Jamieson, P.; Moxon, P.; Purvis, J.; Thomas, M.; Tighe, H.; Sylvester, K.P. ARTP statement on cardiopulmonary exercise testing 2021. BMJ Open Respir. Res. 2021, 8, e001121. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J.; Froelicher, V.F. Principles of Exercise Testing and Interpretation. J. Cardiopulm. Rehabil. Prev. 1987, 7, 189. [Google Scholar] [CrossRef]
- Kinnear, W.; Blakey, J. A Practical Guide to the Interpretation of Cardio-Pulmonary Exercise Tests; Oxford University Press: Oxford, UK, 2014; Available online: http://oxfordmedicine.com/view/10.1093/med/9780198702467.001.0001/med-9780198702467 (accessed on 28 October 2020).
- Robergs, R.A.; Dwyer, D.; Astorino, T. Recommendations for Improved Data Processing from Expired Gas Analysis Indirect Calorimetry. Sports Med. 2010, 40, 95–111. [Google Scholar] [CrossRef]
| Median | LQ | UQ | Median | LQ | UQ | Median | LQ | UQ | Median | LQ | UQ | Median | LQ | UQ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STAGE | Rest | Warm-up | VT1 | VT2 | Peak Exercise | ||||||||||
| Age | 29 | 26 | 35 | - | - | - | - | - | - | - | - | - | - | - | - |
| BMI [kg/m2] | 23.67 | 21.63 | 25.93 | - | - | - | - | - | - | - | - | - | - | - | - |
| IF1 serum concentration [ng/L] | 646 | 427 | 1940 | - | - | - | - | - | - | - | - | - | - | - | - |
| BF [breaths/min] | 15.44 | 11.83 | 18.99 | 21.33 | 18.32 | 25.17 | 25.97 | 22.6 | 29.93 | 32.94 | 28.08 | 37.42 | 53.05 | 44.84 | 63.26 |
| VE/VCO2 | 32.68 | 30.32 | 36.09 | 26.69 | 25.24 | 27.81 | 25.26 | 23.61 | 26.9 | 26.35 | 24.39 | 28.08 | 32.48 | 29.98 | 35.67 |
| VE/VO2 | 29.25 | 26.56 | 35.29 | 23.26 | 20.65 | 24.6 | 25.1 | 23.23 | 26.64 | 28.94 | 26.7 | 31.13 | 39.4 | 37.08 | 45.22 |
| HR [beats/min] | 83 | 73 | 95 | 119 | 106 | 128 | 153 | 146 | 162 | 173 | 166 | 182 | 187 | 179 | 192 |
| VE [L/min] | 14.82 | 11.17 | 18.87 | 36.95 | 30.67 | 43 | 64.18 | 52.27 | 76.8 | 95.42 | 78.52 | 107.01 | 149.16 | 122.71 | 173.03 |
| O2pulse [mL/beat] | 4.83 | 3.63 | 6.22 | 12.46 | 10.69 | 14.77 | 15.84 | 13.19 | 18.55 | 18.04 | 15.56 | 20.76 | 18.98 | 16.03 | 21.37 |
| PETCO2 [Pa] | 4221.03 | 3878.64 | 4454.43 | 5248.57 | 5061.57 | 5489.65 | 5539.82 | 5281.43 | 5886.86 | 5367.15 | 5084.8 | 5789.04 | 4469.03 | 4025.27 | 4818.42 |
| PETO2 [Pa] | 15,329.75 | 14,738.12 | 16,115.79 | 13,923.84 | 13,372.9 | 14,350.91 | 14,100.66 | 13,706.29 | 14,523.81 | 14,780.66 | 14,302.99 | 15,108.75 | 16,056.14 | 15,689.28 | 16,482.93 |
| RER | 0.9 | 0.82 | 1.05 | 0.86 | 0.79 | 0.92 | 0.99 | 0.97 | 1 | 1.1 | 1.08 | 1.13 | 1.26 | 1.22 | 1.3 |
| VCO2 [L] | 0.39 | 0.26 | 0.53 | 1.3 | 1.02 | 1.51 | 2.39 | 1.95 | 2.84 | 3.43 | 2.93 | 3.97 | 4.41 | 3.63 | 5.02 |
| VO2/kg | 5.4 | 4.07 | 7.15 | 19.15 | 17.21 | 22.78 | 31.63 | 27.48 | 36.57 | 40.55 | 34.49 | 45.78 | 45.23 | 38.97 | 50.38 |
| VO2 max [L] | 0.42 | 0.3 | 0.53 | 1.49 | 1.23 | 1.74 | 2.42 | 1.96 | 2.91 | 3.09 | 2.6 | 3.65 | 3.43 | 2.97 | 3.98 |
| TV [L] | 0.84 | 0.64 | 1.2 | 1.67 | 1.35 | 2.01 | 2.48 | 2 | 2.95 | 2.93 | 2.45 | 3.36 | 2.83 | 2.44 | 3.16 |
| Load [W] | 70 | 50 | 80 | 183.97 | 139.98 | 224.93 | 254 | 214.99 | 304 | 299 | 260 | 365 | |||
| BR (%) | 90.89 | 87.92 | 92.74 | 76.93 | 72.54 | 80.46 | 59.1 | 52.96 | 66.58 | 41.87 | 30.72 | 48.96 | 8.16 | −6.68 | 21.05 |
| BR [L] | 145.97 | 120.98 | 166.46 | 125.88 | 98.98 | 148.68 | 93.95 | 74.89 | 117.25 | 63.96 | 45.38 | 87.82 | 10.9 | −9.34 | 32.31 |
| Warm-Up | VT1 | VT2 | Peak Exercise | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| VE/VCO2 | 0.07 | 0.5191 | −0.04 | 0.7323 | 0 | 0.9929 | 0.11 | 0.2897 |
| VE/VO2 | 0.01 | 0.888 | −0.04 | 0.7292 | 0 | 0.9626 | 0.09 | 0.4019 |
| HR | 0.03 | 0.7607 | 0.01 | 0.9203 | 0.04 | 0.7297 | 0.14 | 0.1794 |
| VE | −0.13 | 0.1947 | −0.26 | 0.0112 * | −0.23 | 0.0214 * | −0.16 | 0.1193 |
| O2pulse | −0.26 | 0.0111 * | −0.24 | 0.0193 * | −0.22 | 0.0342 * | −0.21 | 0.0373 * |
| PETCO2 | −0.14 | 0.1833 | −0.01 | 0.9276 | −0.04 | 0.7313 | −0.17 | 0.0957 |
| PETO2 | 0.07 | 0.4716 | −0.01 | 0.9228 | 0 | 0.9646 | 0.1 | 0.331 |
| RER | 0.03 | 0.7854 | 0.01 | 0.9114 | −0.05 | 0.6564 | −0.05 | 0.6575 |
| VCO2 | −0.18 | 0.0814 | −0.23 | 0.0211 * | −0.23 | 0.0237 * | −0.21 | 0.0399 * |
| VO2/kg | −0.04 | 0.728 | −0.19 | 0.0692 | −0.15 | 0.14 | −0.12 | 0.2454 |
| VO2 | −0.2 | 0.0473 * | −0.22 | 0.0288 * | −0.21 | 0.0354 * | −0.21 | 0.0431 * |
| TV | −0.15 | 0.1317 | −0.16 | 0.1089 | −0.15 | 0.1321 | −0.18 | 0.076 |
| Load | −0.2 | 0.0526 | −0.27 | 0.0076 * | −0.27 | 0.0067 * | −0.26 | 0.0107 * |
| Breathing reserve (%) | 0.1 | 0.3111 | 0.18 | 0.0811 | 0.15 | 0.1497 | 0.08 | 0.4255 |
| Breathing reserve (L) | −0.04 | 0.7266 | 0 | 0.9983 | 0.03 | 0.7432 | 0.09 | 0.3774 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domin, R.; Pytka, M.; Niziński, J.; Żołyński, M.; Zybek-Kocik, A.; Wrotkowska, E.; Zieliński, J.; Guzik, P.; Ruchała, M. ATPase Inhibitory Factor 1—A Novel Marker of Cellular Fitness and Exercise Capacity? Int. J. Mol. Sci. 2022, 23, 15303. https://doi.org/10.3390/ijms232315303
Domin R, Pytka M, Niziński J, Żołyński M, Zybek-Kocik A, Wrotkowska E, Zieliński J, Guzik P, Ruchała M. ATPase Inhibitory Factor 1—A Novel Marker of Cellular Fitness and Exercise Capacity? International Journal of Molecular Sciences. 2022; 23(23):15303. https://doi.org/10.3390/ijms232315303
Chicago/Turabian StyleDomin, Remigiusz, Michał Pytka, Jan Niziński, Mikołaj Żołyński, Ariadna Zybek-Kocik, Elżbieta Wrotkowska, Jacek Zieliński, Przemysław Guzik, and Marek Ruchała. 2022. "ATPase Inhibitory Factor 1—A Novel Marker of Cellular Fitness and Exercise Capacity?" International Journal of Molecular Sciences 23, no. 23: 15303. https://doi.org/10.3390/ijms232315303
APA StyleDomin, R., Pytka, M., Niziński, J., Żołyński, M., Zybek-Kocik, A., Wrotkowska, E., Zieliński, J., Guzik, P., & Ruchała, M. (2022). ATPase Inhibitory Factor 1—A Novel Marker of Cellular Fitness and Exercise Capacity? International Journal of Molecular Sciences, 23(23), 15303. https://doi.org/10.3390/ijms232315303






