Cross-Regulation between Autophagy and Apoptosis Induced by Vitamin E and Lactobacillus Plantarum through Beclin-1 Network
Abstract
1. Introduction
2. Results
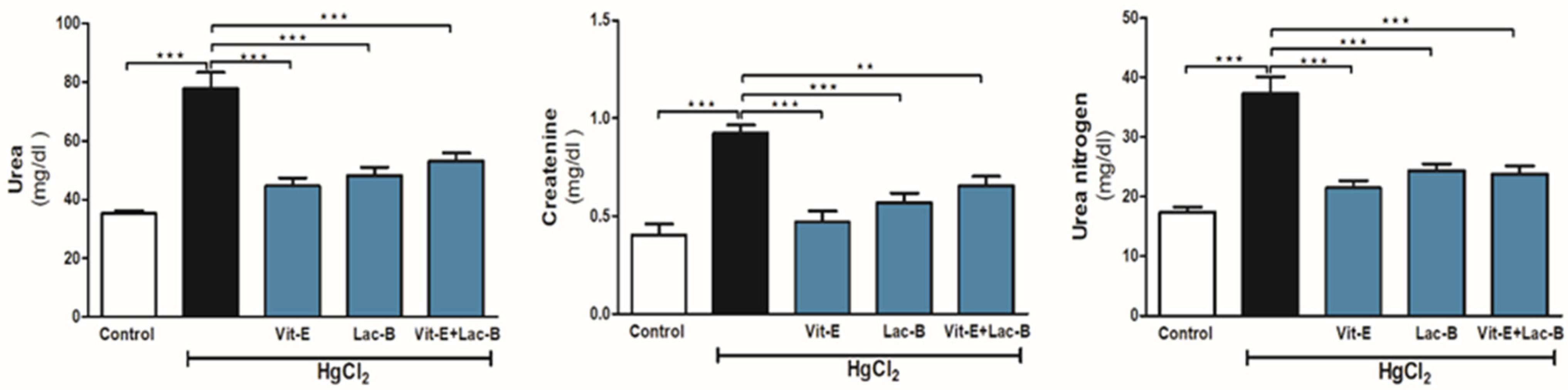
2.1. Vit-E and Lac-B Restored Renal Function after HgCl2 Induced Kidney Injury
2.2. Vit-E and Lac-B Mitigated Oxidative Stress after HgCl2 Induced Kidney Injury
2.3. Vit-E and Lac-B Attenuated the HgCl2 Nephrotoxicity via Increasing Beclin-1 and Bcl-2 Protein Expression
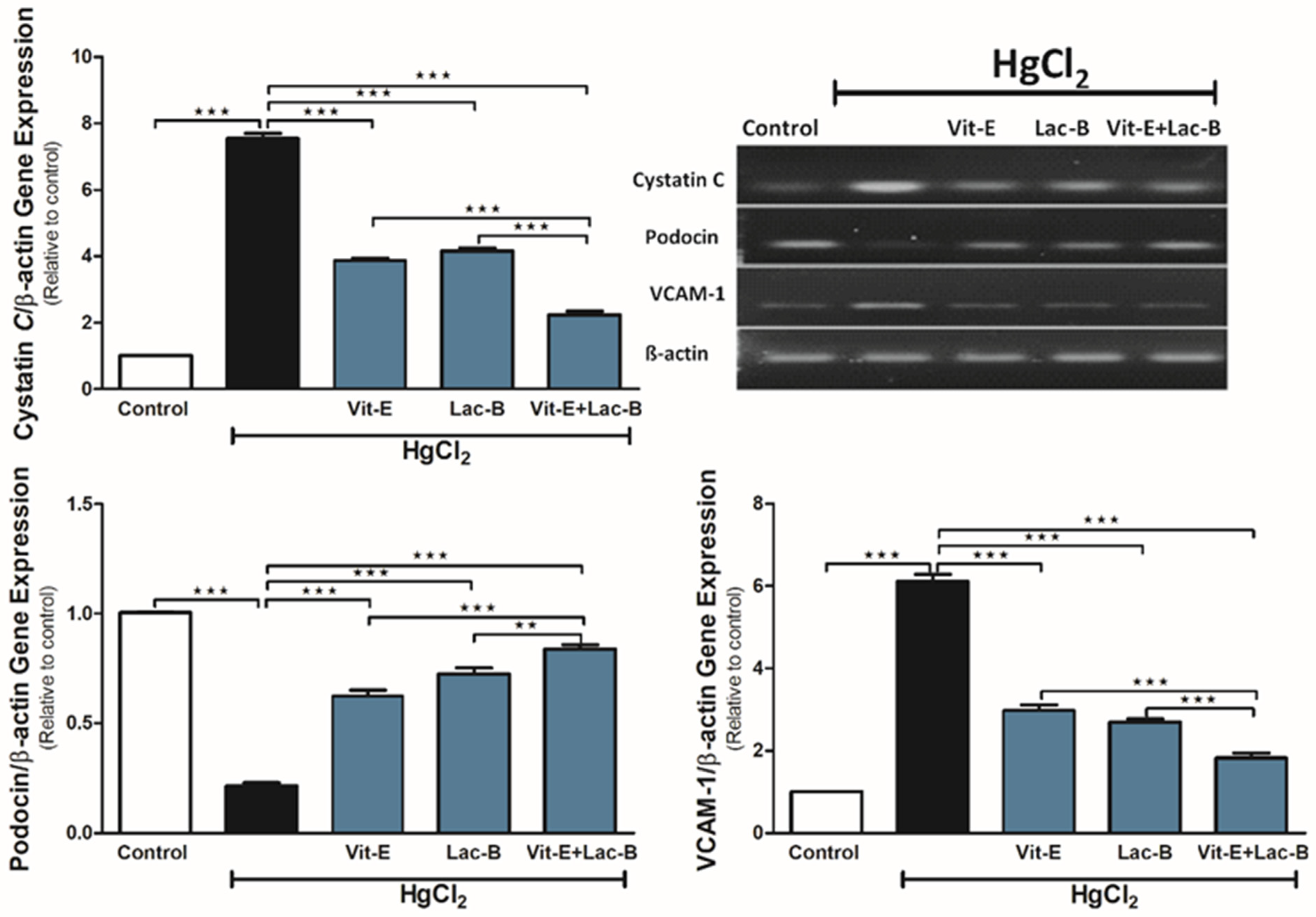
2.4. Vit-E and Lac-B Diminished the HgCl2 Nephrotoxicity via Modulating Cystatin C, VCAM-1 and Podocin Genes
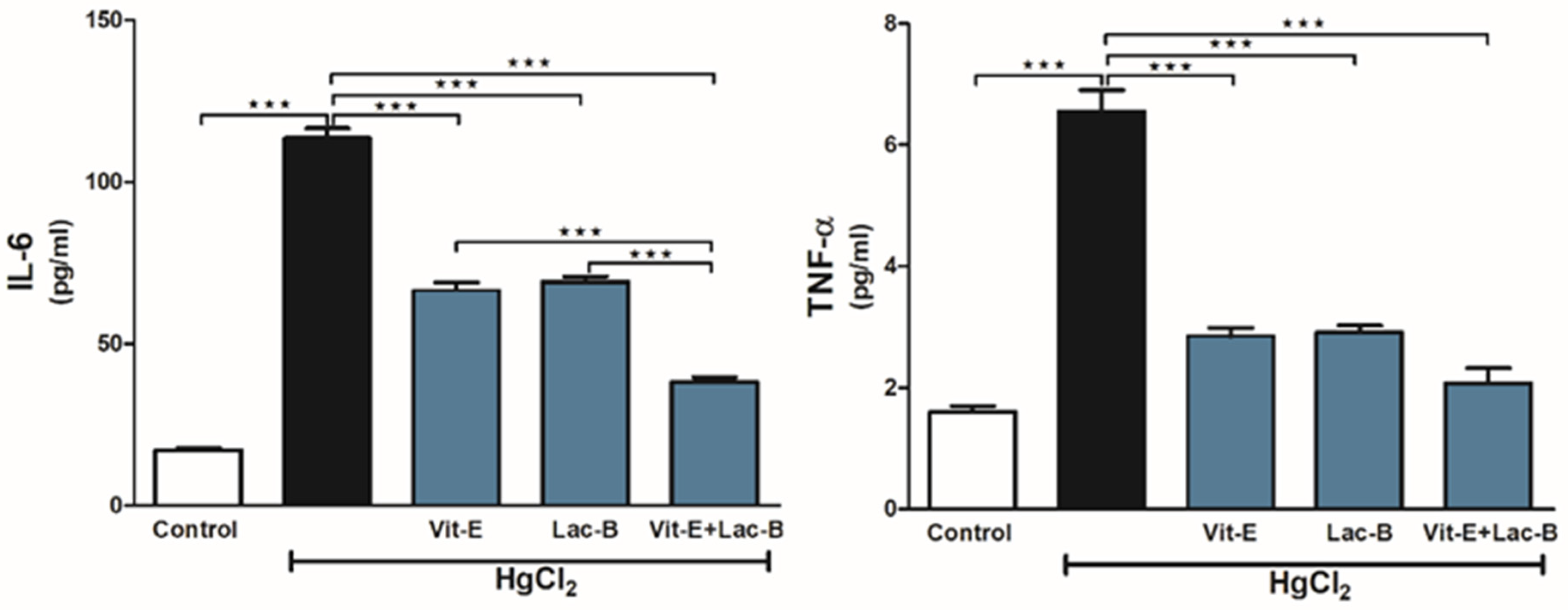
2.5. Vit-E and Lac-B Attenuated Renal Tissue Inflammation after HgCl2 Exposure
2.6. Vit-E and Lac-B Inhibited Renal Apoptotic Cell Damage in Response to HgCl2
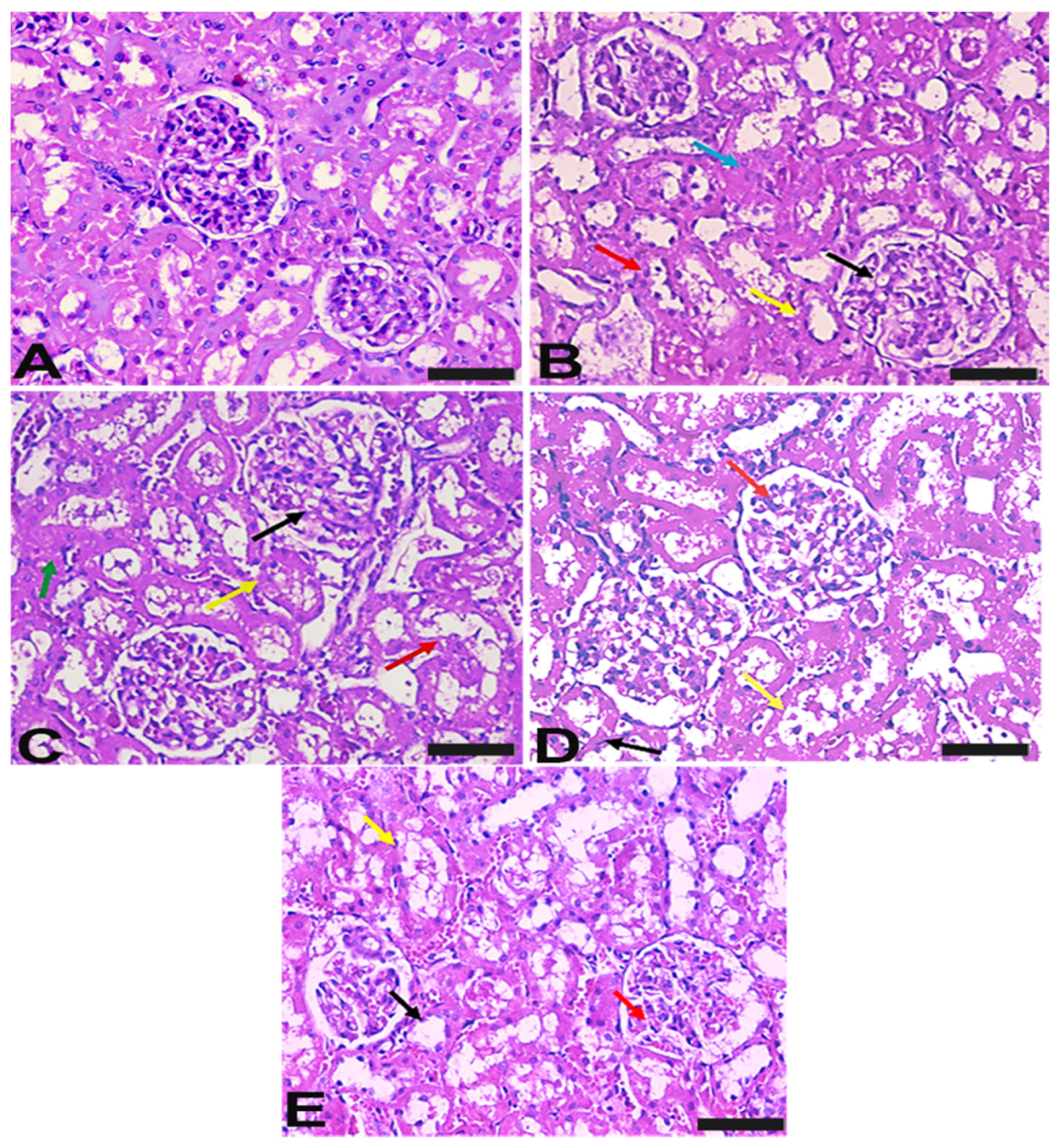
2.7. Vit-E and Lac-B Ameliorated HgCl2 Induced Histopathological Changes in Renal Tissue
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Experimental Design
4.4. Biochemical Analyses
4.4.1. Serum Creatinine, Urea and Urea Nitrogen
4.4.2. Oxidative Stress and Antioxidant Defenses
4.4.3. Inflammatory and Apoptotic Markers
4.4.4. DNA Fragmentation
4.4.5. Protein Expression
4.4.6. Gene Expression
4.4.7. Histological Examination
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Araragi, S.; Kondoh, M.; Kawase, M.; Saito, S.; Higashimoto, M.; Sato, M. Mercuric chloride induces apoptosis via a mitochondrial-dependent pathway in human leukemia cells. Toxicology 2003, 184, 1–9. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Kara, A.; Gedikli, S.; Sengul, E.; Gelen, V.; Ozkanlar, S. Oxidative stress and autophagy. In Free Radicals and Diseases; In Tech Open: London, UK, 2016; pp. 69–86. [Google Scholar]
- Fryer, M.J. Vitamin E as a protective antioxidant in progressive renal failure. Nephrology 2000, 5, 1–7. [Google Scholar] [CrossRef]
- Xing, J.; Wang, G.; Zhang, Q.; Liu, X.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Determining antioxidant activities of lactobacilli cell-free supernatants by cellular antioxidant assay: A comparison with traditional methods. PLoS ONE 2015, 10, e0119058. [Google Scholar] [CrossRef]
- Yu, Z.-Q.; Wang, L.-M.; Yang, W.-X. How vitamin E and its derivatives regulate tumour cells via the MAPK signalling pathway? Gene 2022, 808, 145998. [Google Scholar] [CrossRef]
- Rakowski, M.; Porębski, S.; Grzelak, A. Nutraceuticals as Modulators of Autophagy: Relevance in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 3625. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, Y.; Wang, Y.; Fan, Y.; Zhang, Y.; Xing, X.; Nan, B.; Ai, Z.; Li, X.; Wang, Y. Ameliorative effect of Lactobacillus plantarum Lp2 against cyclophosphamide-induced liver injury in mice. Food Chem. Toxicol. 2022, 169, 113433. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, Q.; Hua, X.; Li, X.; Zhang, W.; Sun, H.; Li, S.; Wang, X.; Li, B. Beclin 1, an autophagy-related gene, augments apoptosis in U87 glioblastoma cells. Oncol. Rep. 2014, 31, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cherubini, E.; Scozzi, D.; Pietrangeli, V.; Tabbì, L.; Raffa, S.; Leone, L.; Visco, V.; Torrisi, M.R.; Bruno, P. Decreased expression of autophagic beclin 1 protein in idiopathic pulmonary fibrosis fibroblasts. J. Cell. Physiol. 2013, 228, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ray, A.; Mukherjee, S.; Agarwal, S.; Kundu, R.; Bhattacharya, S. Low concentration of mercury induces autophagic cell death in rat hepatocytes. Toxicol. Ind. Health 2014, 30, 611–620. [Google Scholar] [CrossRef]
- Kanzawa, T.; Kondo, Y.; Ito, H.; Kondo, S.; Germano, I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003, 63, 2103–2108. [Google Scholar]
- Bolt, A.M.; Byrd, R.M.; Klimecki, W.T. Autophagy is a biological target of arsenic. In Arsenic in Geosphere and Human Diseases; Arsenic 2010; CRC Press: Boca Raton, FL, USA, 2010; pp. 347–348. ISBN 0429206259. [Google Scholar]
- Chargui, A.; Zekri, S.; Jacquillet, G.; Rubera, I.; Ilie, M.; Belaid, A.; Duranton, C.; Tauc, M.; Hofman, P.; Poujeol, P. Cadmium-induced autophagy in rat kidney: An early biomarker of subtoxic exposure. Toxicol. Sci. 2011, 121, 31–42. [Google Scholar] [CrossRef]
- Elsayed, A.; Elkomy, A.; Alkafafy, M.; Elkammar, R.; Fadl, S.E.; Abdelhiee, E.Y.; Abdeen, A.; Youssef, G.; Shaheen, H.; Soliman, A. Ameliorating Effect of Lycopene and N-Acetylcysteine against Cisplatin-Induced Cardiac Injury in Rats. Pak. Vet. J. 2022, 42, 107–111. [Google Scholar]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Forman, H.J.; Sevanian, A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997, 22, 269–285. [Google Scholar] [CrossRef]
- Fadda, L.M.; Alhusaini, A.M.; Al-Qahtani, Q.H.; Ali, H.M.; Hasan, I.H. Role of α-tocopherol and Lactobacillus plantarum in the alleviation of mercuric chloride-induced testicular atrophy in rat’s model: Implication of molecular mechanisms. J. Biochem. Mol. Toxicol. 2020, 34, e22481. [Google Scholar] [CrossRef]
- Alhusaini, A.; Alghilani, S.; Alhuqbani, W.; Hasan, I.H. Vitamin E and Lactobacillus Provide Protective Effects Against Liver Injury Induced by HgCl2: Role of CHOP, GPR87, and mTOR Proteins. Dose-Response 2021, 19, 15593258211011360. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.M.; Daba, M.Y.; Dahab, G.M.; Sharaf el-Din, O.A. Thymoquinone ameliorates renal oxidative damage and proliferative response induced by mercuric chloride in rats. Basic Clin. Pharmacol. Toxicol. 2008, 103, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.M.B.; Albuquerque, P.L.M.M.; Meneses, G.C.; da Silva Junior, G.B.; Martins, A.M.C.; Daher, E.D.F. Role of endothelial biomarkers in predicting acute kidney injury in Bothrops Envenoming. Toxicol. Lett. 2021, 345, 61–66. [Google Scholar] [CrossRef]
- Watanabe, Y.; Fukuda, T.; Hayashi, C.; Nakao, Y.; Toyoda, M.; Kawakami, K.; Shinjo, T.; Iwashita, M.; Yamato, H.; Yotsumoto, K. Extracellular vesicles derived from GMSCs stimulated with TNF-α and IFN-α promote M2 macrophage polarization via enhanced CD73 and CD5L expression. Sci. Rep. 2022, 12, 13344. [Google Scholar] [CrossRef]
- Chu, L.-Y.; Hsueh, Y.-C.; Cheng, H.-L.; Wu, K.K. Cytokine-induced autophagy promotes long-term VCAM-1 but not ICAM-1 expression by degrading late-phase IκBα. Sci. Rep. 2017, 7, 12472. [Google Scholar] [CrossRef]
- Caridi, G.; Perfumo, F.; Ghiggeri, G.M. NPHS2 (Podocin) mutations in nephrotic syndrome. Clinical spectrum and fine mechanisms. Pediatr. Res. 2005, 57, 54–61. [Google Scholar] [CrossRef]
- Garcia-Pliego, E.; Franco-Colin, M.; Rojas-Franco, P.; Blas-Valdivia, V.; Serrano-Contreras, J.I.; Pentón-Rol, G.; Cano-Europa, E. Phycocyanobilin is the molecule responsible for the nephroprotective action of phycocyanin in acute kidney injury caused by mercury. Food Funct. 2021, 12, 2985–2994. [Google Scholar] [CrossRef]
- Salah-Abbès, J.B.; Belgacem, H.; Ezzdini, K.; Abdel-Wahhab, M.A.; Abbès, S. Zearalenone nephrotoxicity: DNA fragmentation, apoptotic gene expression and oxidative stress protected by Lactobacillus plantarum MON03. Toxicon 2020, 175, 28–35. [Google Scholar] [CrossRef]
- Wang, I.; Yen, T.-H.; Hsieh, P.-S.; Ho, H.-H.; Kuo, Y.-W.; Huang, Y.-Y.; Kuo, Y.-L.; Li, C.-Y.; Lin, H.-C.; Wang, J.-Y. Effect of a Probiotic Combination in an Experimental Mouse Model and Clinical Patients With Chronic Kidney Disease: A Pilot Study. Front. Nutr. 2021, 8, 276. [Google Scholar] [CrossRef]
- Bergmann, P.T.T.K.; Simonsen, U.; Lykkesfeldt, J. BCPT policy for experimental and clinical studies. Basic Clin. Pharmacol. Toxicol. 2021, 128, 4–8. [Google Scholar] [CrossRef]
- Peixoto, N.C.; Roza, T.; Flores, É.M.M.; Pereira, M.E. Effects of zinc and cadmium on HgCl2-δ-ALA-D inhibition and Hg levels in tissues of suckling rats. Toxicol. Lett. 2003, 146, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Bakhaat, G.A.; Tammam, H.G.; Mohamed, R.M.; El-Naggar, S.A. Cardioprotective effect of green tea extract and vitamin E on Cisplatin-induced cardiotoxicity in mice: Toxicological, histological and immunohistochemical studies. Biomed. Pharmacother. 2019, 113, 108731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ni, X.; Wang, Q.; Peng, Z.; Niu, L.; Wang, H.; Zhou, Y.; Sun, H.; Pan, K.; Jing, B. Lactobacillus plantarum BSGP201683 isolated from giant panda feces attenuated inflammation and improved gut microflora in mice challenged with Enterotoxigenic Escherichia coli. Front. Microbiol. 2017, 8, 1885. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- MARKLUND, S.; MARKLUND, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Hickey, E.J.; Raje, R.R.; Reid, V.E.; Gross, S.M.; Ray, S.D. Diclofenac induced in vivo nephrotoxicity may involve oxidative stress-mediated massive genomic DNA fragmentation and apoptotic cell death. Free Radic. Biol. Med. 2001, 31, 139–152. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, Z.; Guo, J.; Liu, A.; Lu, Q.; Fatima, Z.; Khaliq, H.; Shabbir, M.A.B.; Maan, M.K.; Wu, Q. Mequindox-induced kidney toxicity is associated with oxidative stress and apoptosis in the mouse. Front. Pharmacol. 2018, 9, 436. [Google Scholar] [CrossRef]



| Gene Name | Primer Code |
|---|---|
| VCAM-1 | Forward: 5′- GAA TTC TCC CAA ATC GAC ATA TTC CC-3′ Reverse: 5′- CTC GAG TTA TTT CTC TTG AAC AGT TAA TT-3′ |
| podocin | Forward: 5′- CCT GTG AGT GGC TTC TTG TCC TC-3′ Reverse: 5′- GGA GAC GCT TCA TAG TGG TTT GCA-3′ |
| cystatin C | Forward: 5′- GCGTACCACAGCCGCGCCAT-3′ Reverse: 5′- TGGGGCTGGTCATGGAAAGGACAGT-3′ |
| β-actin | Forward: 5′- GCA CCA CAC CTT CTA CAA TG-3′ Reverse: 5′- TGC TTG CTG ATC CAC ATC TG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhusaini, A.M.; Alhumaidan, S.A.; Alharbi, G.M.; Alzahrani, E.A.; Sarawi, W.S.; Alomar, H.A.; Alanazi, A.M.; Mattar, D.S.; Hasan, I.H. Cross-Regulation between Autophagy and Apoptosis Induced by Vitamin E and Lactobacillus Plantarum through Beclin-1 Network. Int. J. Mol. Sci. 2022, 23, 15305. https://doi.org/10.3390/ijms232315305
Alhusaini AM, Alhumaidan SA, Alharbi GM, Alzahrani EA, Sarawi WS, Alomar HA, Alanazi AM, Mattar DS, Hasan IH. Cross-Regulation between Autophagy and Apoptosis Induced by Vitamin E and Lactobacillus Plantarum through Beclin-1 Network. International Journal of Molecular Sciences. 2022; 23(23):15305. https://doi.org/10.3390/ijms232315305
Chicago/Turabian StyleAlhusaini, Ahlam M., Sara A. Alhumaidan, Ghaida M. Alharbi, Eman A. Alzahrani, Wedad S. Sarawi, Hatun A. Alomar, Abeer M. Alanazi, Dareen S. Mattar, and Iman H. Hasan. 2022. "Cross-Regulation between Autophagy and Apoptosis Induced by Vitamin E and Lactobacillus Plantarum through Beclin-1 Network" International Journal of Molecular Sciences 23, no. 23: 15305. https://doi.org/10.3390/ijms232315305
APA StyleAlhusaini, A. M., Alhumaidan, S. A., Alharbi, G. M., Alzahrani, E. A., Sarawi, W. S., Alomar, H. A., Alanazi, A. M., Mattar, D. S., & Hasan, I. H. (2022). Cross-Regulation between Autophagy and Apoptosis Induced by Vitamin E and Lactobacillus Plantarum through Beclin-1 Network. International Journal of Molecular Sciences, 23(23), 15305. https://doi.org/10.3390/ijms232315305






