IL-1b in the Secretomes of MSCs Seeded on Human Decellularized Allogeneic Bone Promotes Angiogenesis
Abstract
:1. Introduction
2. Results
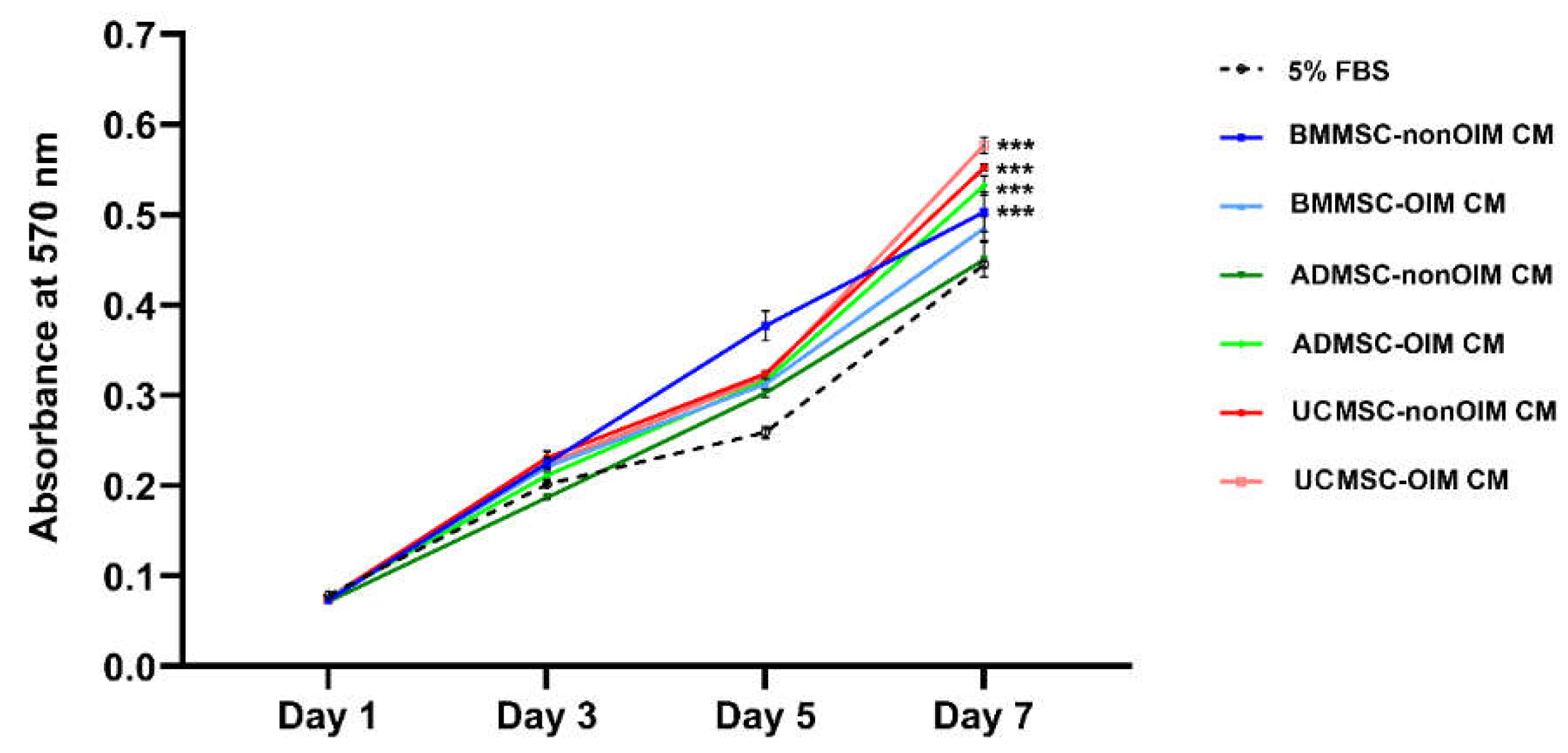
2.1. MSC-CMs Promote Cell Growth
2.2. The Effect of MSC-CMs on Cell Migration
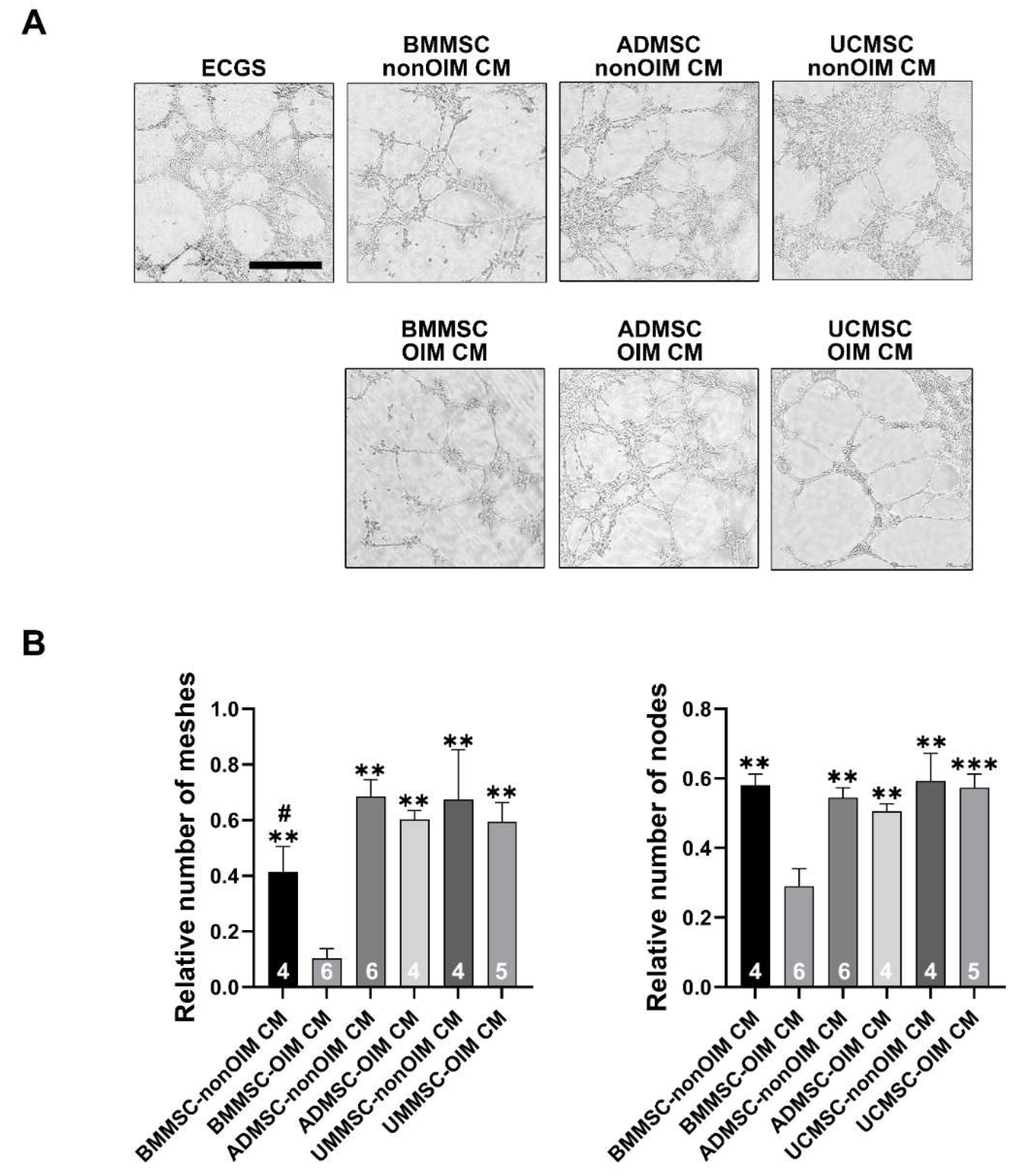
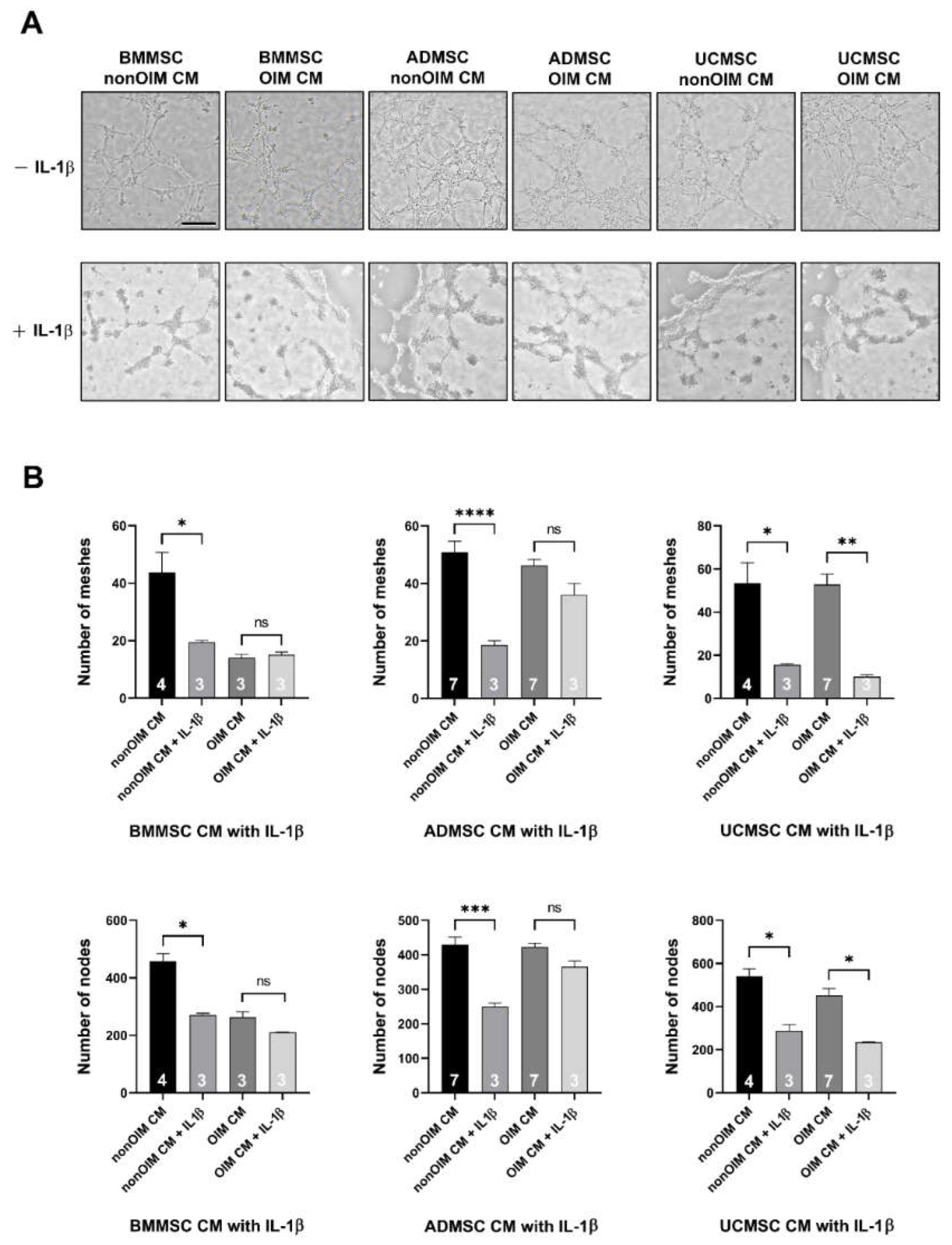
2.3. Analysis of Angiogenic Potential of MSC Secretomes by Tube Formation Assay
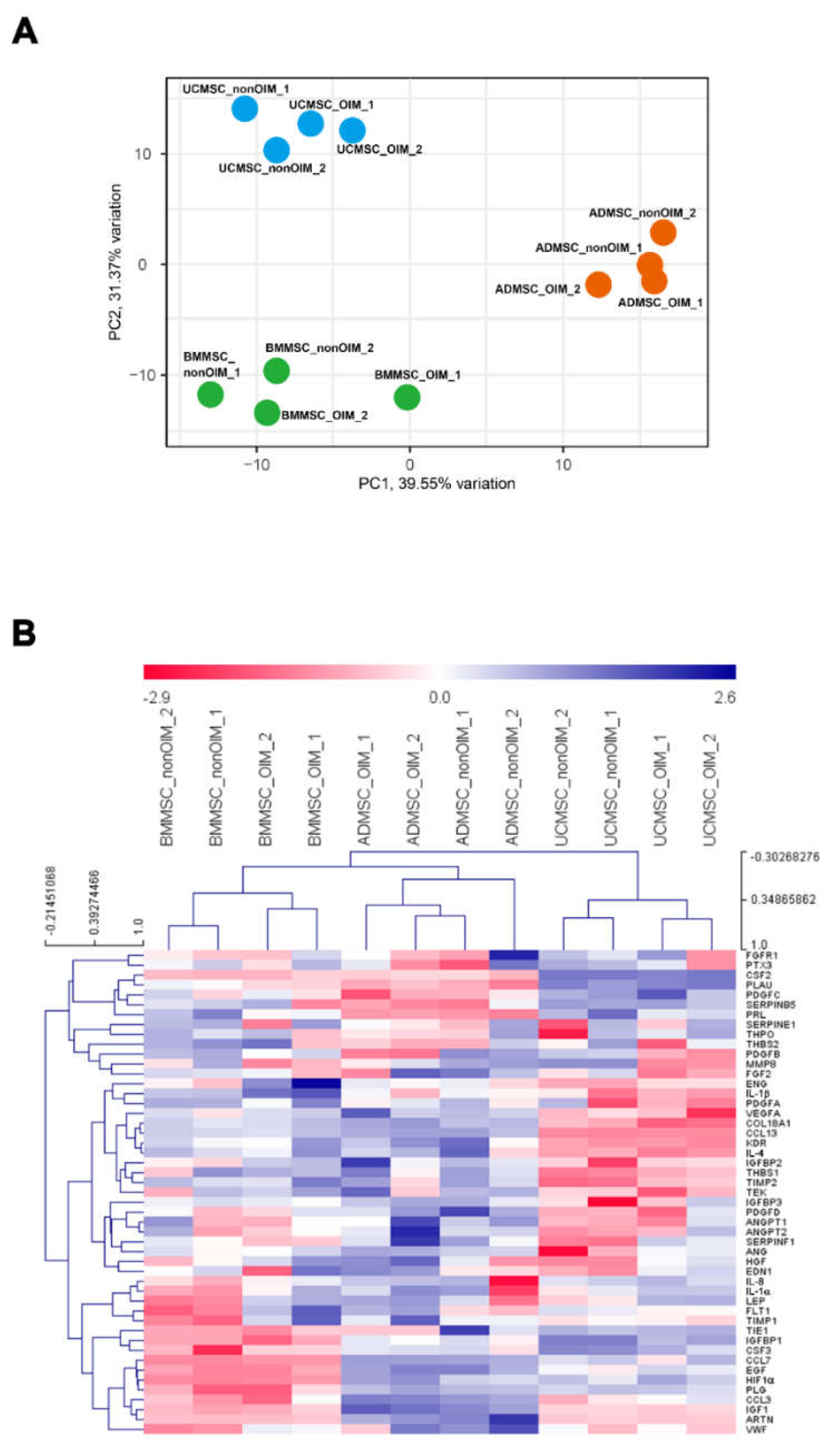
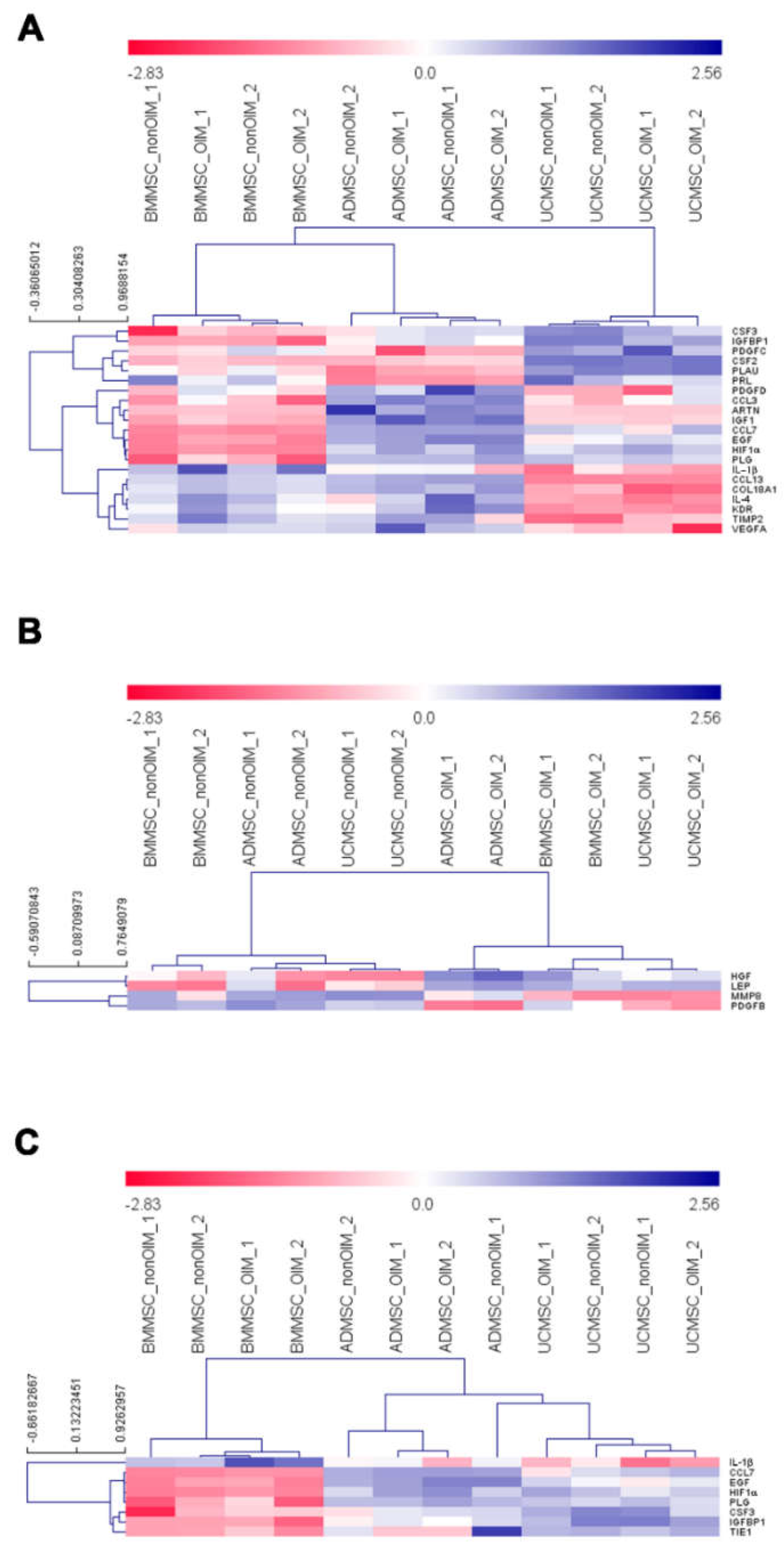
2.4. qPCR Profiling of Angiogenic Genes
2.5. Validation of Analyzed Results by IL-1b Neutralization
3. Discussion
4. Materials and Methods
4.1. MSC Culture
4.2. Processing of Allogeneic Bone
4.3. HUVEC Culture
4.4. Preparation of Conditioned Media
4.5. MTT Viability Assay
4.6. Wound Healing Assay
4.7. Tube Formation Assay
4.8. Real-Time Quantitative Polymerase Chain Reaction Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beamer, B.; Hettrich, C.; Lane, J. Vascular endothelial growth factor: An essential component of angiogenesis and fracture healing. HSS J. 2010, 6, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef]
- Camussi, G.; Albano, E.; Tetta, C.; Bussolino, F. The molecular action of tumor necrosis factor-alpha. Eur. J. Biochem. 1991, 202, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef] [Green Version]
- Vacca, A.; Ribatti, D. Bone marrow angiogenesis in multiple myeloma. Leukemia 2006, 20, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol. Ther. 2019, 27, 25–33. [Google Scholar] [CrossRef]
- Merimi, M.; El-Majzoub, R.; Lagneaux, L.; Moussa Agha, D.; Bouhtit, F.; Meuleman, N.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Najar, M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021, 9, 661532. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollini, S.; Gentili, C.; Tasso, R.; Cancedda, R. The Regenerative Role of the Fetal and Adult Stem Cell Secretome. J. Clin. Med. 2013, 2, 302–327. [Google Scholar] [CrossRef] [Green Version]
- Todeschi, M.R.; El Backly, R.; Capelli, C.; Daga, A.; Patrone, E.; Introna, M.; Cancedda, R.; Mastrogiacomo, M. Transplanted Umbilical Cord Mesenchymal Stem Cells Modify the In Vivo Microenvironment Enhancing Angiogenesis and Leading to Bone Regeneration. Stem Cells Dev. 2015, 24, 1570–1581. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhang, B.; Wan, T.; Zhou, C.; Fan, Y.; Tian, W.; Jing, W. A 3D-printed biphasic calcium phosphate scaffold loaded with platelet lysate/gelatin methacrylate to promote vascularization. J. Mater. Chem. B 2022, 10, 3138–3151. [Google Scholar] [CrossRef]
- Supphaprasitt, W.; Charoenmuang, L.; Thuaksuban, N.; Sangsuwan, P.; Leepong, N.; Supakanjanakanti, D.; Vongvatcharanon, S.; Suwanrat, T.; Srimanok, W. A Three-Dimensional Printed Polycaprolactone-Biphasic-Calcium-Phosphate Scaffold Combined with Adipose-Derived Stem Cells Cultured in Xenogeneic Serum-Free Media for the Treatment of Bone Defects. J. Funct. Biomater. 2022, 13, 93. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Badylak, S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef]
- Harper, C. Permacol: Clinical experience with a new biomaterial. Hosp. Med. 2001, 62, 90–95. [Google Scholar] [CrossRef]
- Chen, C.F.; Chen, Y.C.; Fu, Y.S.; Tsai, S.W.; Wu, P.K.; Chen, C.M.; Chang, M.C.; Chen, W.M. Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 8987. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.; Lee, H.J.; Na, K.S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Layrolle, P.; Mooney, D.J. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909125. [Google Scholar] [CrossRef]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Liu, X.; Dou, H.; Hou, Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment—Specific factors involved in the regulation of MSC plasticity. Genes Dis. 2022, 9, 296–309. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef]
- Kachgal, S.; Putnam, A.J. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 2011, 14, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.Z.; Moreno-Luna, R.; Zhou, B.; Pu, W.T.; Melero-Martin, J.M. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 2012, 15, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Hoch, A.I.; Binder, B.Y.; Genetos, D.C.; Leach, J.K. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS ONE 2012, 7, e35579. [Google Scholar] [CrossRef] [Green Version]
- Cun, Y.; Jin, Y.; Wu, D.; Zhou, L.; Zhang, C.; Zhang, S.; Yang, X.; Zuhong, W.; Zhang, P. Exosome in Crosstalk between Inflammation and Angiogenesis: A Potential Therapeutic Strategy for Stroke. Mediat. Inflamm. 2022, 2022, 7006281. [Google Scholar] [CrossRef]
- Saijo, Y.; Tanaka, M.; Miki, M.; Usui, K.; Suzuki, T.; Maemondo, M.; Hong, X.; Tazawa, R.; Kikuchi, T.; Matsushima, K.; et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: In vivo analysis of tumor-stromal interaction. J. Immunol. 2002, 169, 469–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viveiros, M.M.H.; Viveiros, M.E.M.; Silva, M.G.; Kaneno, R.; Avelino, N.P.; Rainho, C.A.; Schellini, S.A. Expression of inflammatory cytokines in mesenchymal stem cells derived from proximal humerus fractures. Stem Cell Investig. 2022, 9, 3. [Google Scholar] [CrossRef]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Osaki, M.; Kawamata, M.; Kato, T.; Okochi, H.; Ochiya, T. IFATS collection: In vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 2008, 26, 2705–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bou-Ghannam, S.; Kim, K.; Grainger, D.W.; Okano, T. 3D cell sheet structure augments mesenchymal stem cell cytokine production. Sci. Rep. 2021, 11, 8170. [Google Scholar] [CrossRef]
- Pescarmona, R.; Belot, A.; Villard, M.; Besson, L.; Lopez, J.; Mosnier, I.; Mathieu, A.L.; Lombard, C.; Garnier, L.; Frachette, C.; et al. Comparison of RT-qPCR and Nanostring in the measurement of blood interferon response for the diagnosis of type I interferonopathies. Cytokine 2019, 113, 446–452. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Gentile, M.T.; Pastorino, O.; Bifulco, M.; Colucci-D’Amato, L. HUVEC Tube-formation Assay to Evaluate the Impact of Natural Products on Angiogenesis. J. Vis. Exp. 2019, 148, e58591. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Accession No. | Product Size (bp) |
|---|---|---|---|---|
| ANG | ACTGGAACCCATCTCCAGGAACA | CAACACAGGCTCCCAGGAGGAA | NM_001097577 | 100 |
| ANGPT1 | TCAGTGGCTGCAAAAACTTGAGA | CAGCATGGTAGCCGTGTGGT | NM_001146 | 103 |
| ANGPT2 | GCTAAGGACCCCACTGTTGCTA | TCCATGTCACAGTAGGCCTTGAT | NM_001147 | 146 |
| ARTN | GCTGTTTGAGCTTCGGGGGA | TGTTCCCCCACCCTCCTGTT | NM_057091 | 104 |
| CCL13 | ACAGCAGCTTTCAACCCCCA | TGAAGCAGCAAGTAGATGGGACG | NM_005408 | 70 |
| CCL3 | GGTGTCATCTTCCTAACCAAGCGAA | CTCAGGCACTCAGCTCCAGGTC | NM_002983 | 100 |
| CCL7 | AGGCTGGAGAGCTACAGAAGGAC | CTGTGTGGGGTCAGCACAGAT | NM_006273 | 102 |
| COL18A1 | CGACTTCCAGCCGGTGCTC | GACAGGAAGGCGCGGAAGG | NM_001379500 | 141 |
| CSF2 | CGGAAACTTCCTGTGCAACCC | CCTCATCTGGCCGGTCTCACT | NM_000758 | 126 |
| CSF3 | CTGGACAGTGCAGGAAGCCAC | TAGGTGGCACACAGCTTCTCCT | NM_172219 | 135 |
| EDN1 | GCCAAAAAGACAAGAAGTGCTGG | TCCATAATGTCTTCAGCCCTGAGTT | NM_001955 | 70 |
| EGF | AGGAGAACATCTCTCAACCACGAG | AGGCCTTGGAGGGAAGAACTTT | NM_001963 | 112 |
| ENG | AGCCCCACAAGTCTTGCAGAA | CACGCAGCCCTTCGAGACCT | NM_001114753 | 105 |
| FGF2 | CGGGTGCAGTGGCTCATGCCTATA | CGGGGTTTCACCAGGTTGGTCTTG | NM_002006 | 100 |
| FGFR1 | GCATGGTGGGGTCGGTCATC | CTGGAGTCAGCAGACACTGTTACC | NM_023110 | 136 |
| FLT1 | TGACCCACATTGGCCACCATC | GTGTAGTGCTGCATCCTTGTTGA | NM_002019 | 167 |
| HGF | CAGCATCATCGAGGGAAGGTGACTC | CCCCTCACATGGTCCTGATCCAA | NM_000601 | 84 |
| HIF1A | GACATCGCGGGGACCGATTC | CGCCGAGATCTGGCTGCAT | NM_001530 | 112 |
| IGF1 | AGCTGGTGGATGCTCTTCAGTTCG | CACTCATCCACGATGCCTGTCTGA | NM_001111283 | 115 |
| IGFBP1 | CAACCTCTGCACGCCCTCAC | TCTCCGTGCTCTCTGGGCTT | NM_000596 | 112 |
| IGFBP2 | CTCCCTGCACATCCCCAACTG | CTTCCCGGTGTTGGGGTTCA | NM_000597 | 115 |
| IGFBP3 | ACCACCAAGGGGAAGGAGGA | AGCTGCTGGTCATGTCCTTGG | NM_000598 | 130 |
| IL-1A | TGTGACTGCCCAAGATGAAGACCA | TGCCGTGAGTTTCCCAGAAGAAGAG | NM_000575 | 113 |
| IL-1B | GAAGTACCTGAGCTCGCCAGT | GCCTGAAGCCCTTGCTGTAGT | NM_000576 | 181 |
| IL-4 | GAGAAGGACACTCGCTGCCTG | GAGGTTCCTGTCGAGCCGTT | NM_000589 | 93 |
| IL-8 | GACCACACTGCGCCAACACAGAA | CCACAACCCTCTGCACCCAGTTT | NM_000584 | 96 |
| KDR | CAAGTGGCTAAGGGCATGGA | ATTTCAAAGGGAGGCGAGCA | NM_002253 | 181 |
| LEP | TAGGAATCGCAGCGCCAGC | TGTGTGAAATGTCATTGATCCTGGT | NM_000230 | 198 |
| MMP8 | CCAGCAACTACTCACTCCCTCAAG | CAGGGTTTGGGTGTGCTTGGT | NM_002424 | 106 |
| PDGFA | ATCGGGAAGAGGACACGGGAA | ATCTGGTTGGCTGCTTTAGGTGG | NM_002607 | 89 |
| PDGFB | ACCACCTGGCATGCAAGTGTGAGAC | TCCGAATGGTCACCCGAGTTTGG | NM_002608 | 114 |
| PDGFC | CTGAACCAGGGTTCTGCATCCACT | AGGGGGTAGCACTGAAGGACTCACA | NM_016205 | 80 |
| PDGFD | GGAAGATTTCCAACCCGCAGCA | GTCCAGAGCATCCGCAATCAGAGT | NM_025208 | 124 |
| PLAU | GGACCCCTCGTCTGTTCCCT | GGTGTGACTGCGGATCCAGG | NM_002658 | 138 |
| PLG | GCTGACCGGACCGAATGTTT | GAGTTCGGTGGATTGGACTCTTCC | NM_000301 | 153 |
| PRL | GGCTTCTAGAGGGCATGGAGC | CGTAGGCAGTGGAGCAGGTT | NM_000948 | 154 |
| PTX3 | GGCAGACGCGAGCCGA | AAGCCTCATTGGTCTCACTGGATG | NM_002852 | 143 |
| SERPINB5 | TGGAGGCCACGTTCTGTATGG | GGGTAGTAGGATGAACATGCTGAG | NM_002639 | 101 |
| SERPINE1 | GCCCATGATGGCTCAGACCAA | CTGAGGGTGTCCCCGTGGTA | NM_000602 | 105 |
| SERPINF1 | AGGCGAAGTCACCAAGTCCC | TCAAAGCCAGCCCGGTGTTC | NM_002615 | 123 |
| TEK | TGCCACCATCACTCAGTATCAGCTC | TCCGCTGGTTGCTTGAGATTCTG | NM_000459 | 147 |
| THBS1 | TGCTGGTGGTAGACTAGGGTTGTT | ATCCTGGGGGTTTTCTCAAGCC | NM_003246 | 173 |
| THBS2 | GGGCGGCTGGGTCTATTTGT | GCACAGGGCATTGCCGGA | NM_003247 | 113 |
| THPO | TGGGTCCTGGAGCCCTTCTC | GGAGGCGGCTTAGGCTCTTG | NM_000460 | 101 |
| TIE1 | GTACGAGCTGATGCGTCAGTGCT | GCCCGCGTAAGTGAAGTTCTCA | NM_005424 | 145 |
| VEGFA | CCATGCCAAGTGGTCCCAGG | GATGGCAGTAGCTGCGCTGA | NM_001025366 | 104 |
| VWF | CAATGAGTTCCAACTGCAGCTCAGC | TGCCATCCCTCAGCATGAAGTCA | NM_000552 | 110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Fu, Y.-S.; Tsai, S.-W.; Wu, P.-K.; Chen, C.-M.; Chen, W.-M.; Chen, C.-F. IL-1b in the Secretomes of MSCs Seeded on Human Decellularized Allogeneic Bone Promotes Angiogenesis. Int. J. Mol. Sci. 2022, 23, 15301. https://doi.org/10.3390/ijms232315301
Chen Y-C, Fu Y-S, Tsai S-W, Wu P-K, Chen C-M, Chen W-M, Chen C-F. IL-1b in the Secretomes of MSCs Seeded on Human Decellularized Allogeneic Bone Promotes Angiogenesis. International Journal of Molecular Sciences. 2022; 23(23):15301. https://doi.org/10.3390/ijms232315301
Chicago/Turabian StyleChen, Yi-Chun, Yu-Show Fu, Shang-Wen Tsai, Po-Kuei Wu, Chao-Ming Chen, Wei-Ming Chen, and Cheng-Fong Chen. 2022. "IL-1b in the Secretomes of MSCs Seeded on Human Decellularized Allogeneic Bone Promotes Angiogenesis" International Journal of Molecular Sciences 23, no. 23: 15301. https://doi.org/10.3390/ijms232315301
APA StyleChen, Y.-C., Fu, Y.-S., Tsai, S.-W., Wu, P.-K., Chen, C.-M., Chen, W.-M., & Chen, C.-F. (2022). IL-1b in the Secretomes of MSCs Seeded on Human Decellularized Allogeneic Bone Promotes Angiogenesis. International Journal of Molecular Sciences, 23(23), 15301. https://doi.org/10.3390/ijms232315301





