Insights into the Relationship between Pentraxin-3 and Cancer
Abstract
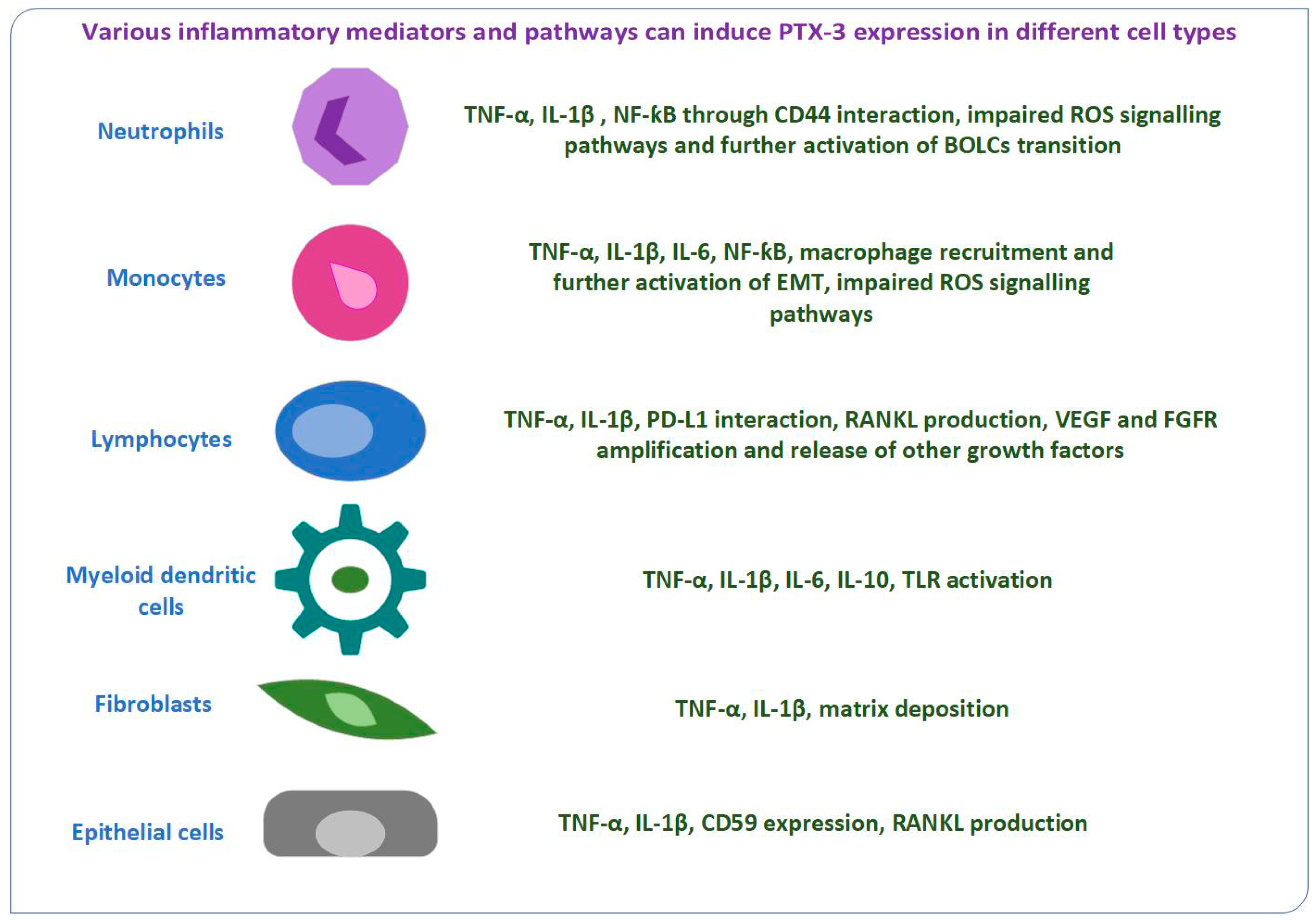
1. Introduction
2. Prostate Cancer
3. Breast Cancer
4. Glioblastoma and Meningioma
5. Gynecological Cancers
6. Colorectal Cancer
7. Gastric Cancer
8. Pancreatic Cancer
9. Lung Cancer
10. Hepatic Cancer
11. Renal Cancer
12. Hematologic Malignancies
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4E-BP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| Akt | Serine/threonine protein kinase (protein-kinase B) |
| ALCAM | Activated leukocyte cell adhesion molecule |
| ATRA | All-trans-retinoic acid |
| AXL | Member of receptor tyrosine kinases family |
| BDNF | Brain-derived neurotrophic factor |
| BOLCs | Osteoblast-like cells |
| CD44 | Type I glycoprotein |
| CD59 | Protectin |
| CRP | C-reactive protein |
| EMT | Epithelial–mesenchymal transition |
| ESR | Erythrocyte sedimentation rate |
| FGF | Fibroblast growth factors |
| FGFR | Fibroblast growth factor receptor |
| HIF-1α | Hypoxia-inducible factor 1α |
| HPV | Human papillomavirus |
| IL | Interleukin |
| miR-224 | Precursor of microRNAs |
| mTOR | Mammalian target of rapamycin |
| NF-ƙkB | Nuclear factor-kappa B |
| Nrf2 | Nuclear factor-like 2 |
| p53 | Tumor supressor protein |
| PCT | Procalcitonin |
| PD-1 | Programmed death 1 receptor |
| PD-L1 | Programmed death ligand 1 |
| PSA | Prostate-SpecificAntigen |
| PTX-3 | Pentraxin 3 |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| RELB | Protein coding gene (RELB Proto-Oncogene, NF-ƙKB Subunit) |
| ROS | Reactive oxygen species |
| SIR2 | Member 2 of sirtuin family |
| SRC | Proto-oncogene tyrosine kinase |
| STAT3 | Signal transducer and activator of transcription 3 |
| sTRAIL | Soluble TNF-related apoptosis-inducing ligand |
| suPAR | Soluble urokinase plasminogen activator receptor |
| TGF-β1 | Transforming growth factor β-1 |
| TLR | Toll-like receptors |
| TNF-α | Tumor necrosis factor α |
| TrkB | Tropomyosin receptor kinase B |
| VEGF | Vascular endothelial growth factor |
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- WHO. Cancer Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 2 August 2022).
- Lobato-Delgado, B.; Priego-Torres, B.; Sanchez-Morillo, D. Combining Molecular, Imaging, and Clinical Data Analysis for Predicting Cancer Prognosis. Cancers 2022, 14, 3215. [Google Scholar] [CrossRef]
- Aghamajidi, A.; MalekiVareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Bhatia, K.; Coban, S. Inflammation and Gastric Cancer. Diseases 2022, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer Overview. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 2 August 2022).
- Choi, H.; Hwang, W. Perioperative Inflammatory Response and Cancer Recurrence in Lung Cancer Surgery: A Narrative Review. Front. Surg. 2022, 9, 888630. [Google Scholar] [CrossRef]
- Bleve, S.; Cursano, M.C.; Casadei, C.; Schepisi, G.; Menna, C.; Urbini, M.; Gianni, C.; De Padova, S.; Filograna, A.; Gallà, V.; et al. Inflammatory Biomarkers for Outcome Prediction in Patients With Metastatic Testicular Cancer. Front. Oncol. 2022, 12, 910087. [Google Scholar] [CrossRef]
- Chatterjee, D.; Das, P.; Chakrabarti, O. Mitochondrial Epigenetics Regulating Inflammation in Cancer and Aging. Front. Cell Dev. Biol. 2022, 10, 929708. [Google Scholar] [CrossRef] [PubMed]
- Liotti, F.; Marotta, M.; Melillo, R.M.; Prevete, N. The Impact of Resolution of Inflammation on Tumor Microenvironment: Exploring New Ways to Control Cancer Progression. Cancers 2022, 14, 3333. [Google Scholar] [CrossRef]
- Chela, H.K.; Gangu, K.; Ertugrul, H.; Juboori, A.A.; Daglilar, E.; Tahan, V. The 8th Wonder of the Cancer World: Esophageal Cancer and Inflammation. Diseases 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Lee, H.J.; Chang, J.E. Inflammatory Cytokine: An Attractive Target for Cancer Treatment. Biomedicines 2022, 10, 2116. [Google Scholar] [CrossRef]
- Macovei, D.G.; Irimes, M.B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2022. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Liu, S.; Yang, M. Crosstalk between gut microbiota and COVID-19 impacts pancreatic cancer progression. World J. Gastrointest. Oncol. 2022, 14, 1456–1468. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, R.; Zhang, Y.; Zhu, T.; Li, Q.; Zhang, W. Interleukin-17 as a potential therapeutic target for chronic pain. Front. Immunol. 2022, 13, 999407. [Google Scholar] [CrossRef] [PubMed]
- Jarocki, M.; Karska, J.; Kowalski, S.; Kiełb, P.; Nowak, Ł.; Krajewski, W.; Saczko, J.; Kulbacka, J.; Szydełko, T.; Małkiewicz, B. Interleukin 17 and Its Involvement in Renal Cell Carcinoma. J. Clin. Med. 2022, 11, 4973. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J. IL-6: The Link Between Inflammation, Immunity and Breast Cancer. Front. Oncol. 2022, 12, 903800. [Google Scholar] [CrossRef] [PubMed]
- Janiczek-Polewska, M.; Szylberg, Ł.; Malicki, J.; Marszałek, A. Role of Interleukins and New Perspectives in Mechanisms of Resistance to Chemotherapy in Gastric Cancer. Biomedicines 2022, 10, 1600. [Google Scholar] [CrossRef]
- Leung, J.H.; Ng, B.; Lim, W.W. Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer. Cells 2022, 11, 2257. [Google Scholar] [CrossRef]
- Margiana, R.; Sharma, S.K.; Khan, B.I.; Alameri, A.A.; Opulencia, M.J.C.; Hammid, A.T.; Hamza, T.A.; Babakulov, S.K.; Abdelbasset, W.K.; Jawhar, Z.H. The pathogenicity of COVID-19 and the role of pentraxin-3: An updated review study. Pathol. Res. Pract. 2022, 238, 154128. [Google Scholar] [CrossRef] [PubMed]
- Parente, R.; Sobacchi, C.; Bottazzi, B.; Mantovani, A.; Grčevic, D.; Inforzato, A. The Long Pentraxin PTX3 in Bone Homeostasis and Pathology. Front. Immunol. 2019, 10, 2628. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zou, H.; Dai, Z.; Feng, S.; Zhang, M.; Xiao, G.; Liu, Z.; Cheng, Q. The Basic Characteristics of the Pentraxin Family and Their Functions in Tumor Progression. Front. Immunol. 2020, 11, 1757. [Google Scholar] [CrossRef]
- Brilland, B.; Vinatier, E.; Subra, J.F.; Jeannin, P.; Augusto, J.F.; Delneste, Y. Anti-Pentraxin Antibodies in Autoimmune Diseases: Bystanders or Pathophysiological Actors? Front. Immunol. 2021, 11, 626343. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yu, Y.; Lu, L. The Role of Pentraxin 3 in Aspergillosis: Reality and Prospects. Mycobiology 2020, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stravalaci, M.; Ferrara, M.; Pathak, V.; Davi, F.; Bottazzi, B.; Mantovani, A.; Medina, R.J.; Romano, M.R.; Inforzato, A. The Long Pentraxin PTX3 as a New Biomarker and Pharmacological Target in Age-Related Macular Degeneration and Diabetic Retinopathy. Front. Pharmacol. 2022, 12, 811344. [Google Scholar] [CrossRef] [PubMed]
- Doni, A.; Mantovani, A.; Bottazzi, B.; Russo, R.C. PTX3 Regulation of Inflammation, Hemostatic Response, Tissue Repair, and Resolution of Fibrosis Favors a Role in Limiting Idiopathic Pulmonary Fibrosis. Front. Immunol. 2021, 12, 676702. [Google Scholar] [CrossRef]
- Camaioni, A.; Klinger, F.G.; Campagnolo, L.; Salustri, A. The Influence of Pentraxin 3 on the Ovarian Function and Its Impact on Fertility. Front. Immunol. 2018, 9, 2808. [Google Scholar] [CrossRef]
- Rauten, A.M.; Silosi, I.; Stratul, S.I.; Foia, L.; Camen, A.; Toma, V.; Cioloca, D.; Surlin, V.; Surlin, P.; Bogdan, M. Expression of Pentraxin 3 and Thrombospondin 1 in Gingival Crevicular Fluid during Wound Healing after Gingivectomy in Postorthodontic Patients. J. Immunol. Res. 2016, 2016, 4072543. [Google Scholar] [CrossRef]
- Ma, Y.J.; Garred, P. Pentraxins in Complement Activation and Regulation. Front. Immunol. 2018, 9, 3046. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, Z.; Wu, W.; Zhang, N.; Zhang, L.; Hu, J.; Luo, P.; Zhang, J.; Liu, Z.; et al. Molecular insight into pentraxin-3: Update advances in innate immunity, inflammation, tissue remodeling, diseases, and drug role. Biomed. Pharmacother. 2022, 156, 113783. [Google Scholar] [CrossRef]
- Stallone, G.; Netti, G.S.; Cormio, L.; Castellano, G.; Infante, B.; Pontrelli, P.; Divella, C.; Selvaggio, O.; Spadaccino, F.; Ranieri, E.; et al. Modulation of complement activation by pentraxin-3 in prostate cancer. Sci. Rep. 2020, 10, 18400. [Google Scholar] [CrossRef]
- Urbano, N.; Scimeca, M.; Crocco, A.; Mauriello, A.; Bonanno, E.; Schillaci, O. 18F-Choline PET/CT Identifies High-Grade Prostate Cancer Lesions Expressing Bone Biomarkers. J. Clin. Med. 2019, 8, 1657. [Google Scholar] [CrossRef] [PubMed]
- Falagario, U.G.; Busetto, G.M.; Netti, G.S.; Sanguedolce, F.; Selvaggio, O.; Infante, B.; Ranieri, E.; Stallone, G.; Carrieri, G.; Cormio, L. Prospective Validation of Pentraxin-3 as a Novel Serum Biomarker to Predict the Risk of Prostate Cancer in Patients Scheduled for Prostate Biopsy. Cancers 2021, 13, 1611. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Urbano, N.; Cerroni, C.; Anemona, L.; Montanaro, M.; Fazi, S.; Schillaci, O.; Mauriello, A.; Bonanno, E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, 297.e19–297.e31. [Google Scholar] [CrossRef] [PubMed]
- Stallone, G.; Cormio, L.; Netti, G.S.; Infante, B.; Selvaggio, O.; Fino, G.D.; Ranieri, E.; Bruno, F.; Prattichizzo, C.; Sanguedolce, F.; et al. Pentraxin 3: A novel biomarker for predicting progression from prostatic inflammation to prostate cancer. Cancer Res. 2014, 74, 4230–4238. [Google Scholar] [CrossRef]
- Güzel, Ö.; Kösem, A.; Aslan, Y.; Asfuroglu, A.; Balci, M.; Senel, C.; Tuncel, A. The Role of Pentraxin-3, Fetuin-A and Sirtuin-7 in the Diagnosis of Prostate Cancer. Urol. J. 2021, 19, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Bedini, N.; Cicchetti, A.; Palorini, F.; Magnani, T.; Zuco, V.; Pennati, M.; Campi, E.; Allavena, P.; Pesce, S.; Villa, S.; et al. Evaluation of Mediators Associated with the Inflammatory Response in Prostate Cancer Patients Undergoing Radiotherapy. Dis. Markers 2018, 2018, 9128128. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Schultz, M.; Gaardsting, A.; Ladelund, S.; Garred, P.; Iversen, K.; Eugen-Olsen, J.; Helms, M.; David, K.P.; Kjaer, A.; et al. Inflammatory biomarkers and cancer: CRP and suPAR as markers of incident cancer in patients with serious nonspecific symptoms and signs of cancer. Int. J. Cancer 2017, 141, 191–199. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Varone, F.; Ciuffa, S.; Mauriello, A.; Bonanno, E. Calcifications in prostate cancer: An active phenomenon mediated by epithelial cells with osteoblast-phenotype. Microsc. Res. Tech. 2018, 81, 745–748. [Google Scholar] [CrossRef]
- Chorny, A.; Casas-Recasens, S.; Sintes, J.; Shan, M.; Polentarutti, N.; Garcia-Escudero, R.; Walland, A.C.; Yeiser, J.R.; Cassis, L.; Carrillo, J.; et al. The soluble pattern recognition receptor PTX3links humoral innate and adaptive immune responses by helping marginalzone B cells. J. Exp. Med. 2016, 213, 2167–2185. [Google Scholar] [CrossRef]
- Chang, W.C.; Wu, S.L.; Huang, W.C.; Hsu, J.Y.; Chan, S.H.; Wang, J.M.; Tsai, J.-P.; Chen, B.-K. PTX3 gene activation in EGF-induced head and neck cancer cell metastasis. Oncotarget 2015, 6, 7741–7757. [Google Scholar] [CrossRef]
- Choi, B.; Lee, E.J.; Shin, M.K.; Park, Y.S.; Ryu, M.H.; Kim, S.M.; Kim, E.Y.; Lee, H.K.; Chang, E.J. Upregulation of brain-derived neurotrophic factor in advanced gastric cancer contributes to bone metastatic osteolysis by inducing long pentraxin 3. Oncotarget 2016, 7, 55506–55517. [Google Scholar] [CrossRef]
- Choi, B.; Lee, E.J.; Song, D.H.; Yoon, S.C.; Chung, Y.H.; Jang, Y.; Kim, S.M.; Song, Y.; Kang, S.W.; Yoon, S.Y.; et al. Elevated Pentraxin 3 in bone metastatic breast cancer is correlated with osteolytic function. Oncotarget 2014, 5, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ferrero, S.; Martinelli Boneschi, F.; Boiocchi, L.; Zavanone, M.; Maria Gaini, S.; Bello, L.; Valentino, S.; Barbati, E.; Nebuloni, M.; et al. The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. J. Neuroimmunol. 2013, 260, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Bonavita, E.; Gentile, S.; Rubino, M.; Maina, V.; Papait, R.; Kunderfranco, P.; Greco, C.; Feruglio, F.; Molgora, M.; Laface, I.; et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell 2015, 160, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Ronca, R.; Alessi, P.; Coltrini, D.; Di Salle, E.; Giacomini, A.; Leali, D.; Corsini, M.; Belleri, M.; Tobia, C.; Garlanda, C.; et al. Long pentraxin-3 as an epithelial-stromal fibroblast growth factor-targeting inhibitor in prostate cancer. J. Pathol. 2013, 230, 228–238. [Google Scholar] [CrossRef]
- Hsiao, Y.W.; Chi, J.Y.; Li, C.F.; Chen, L.Y.; Chen, Y.T.; Liang, H.Y.; Lo, Y.C.; Hong, J.Y.; Chuu, C.P.; Hung, L.Y.; et al. Disruption of the pentraxin 3/CD44 interaction as an efficient therapy for triple-negative breast cancers. Clin. Transl. Med. 2022, 12, e724. [Google Scholar] [CrossRef]
- Bonfiglio, R.; Scimeca, M.; Toschi, N.; Pistolese, C.A.; Giannini, E.; Antonacci, C.; Ciuffa, S.; Tancredi, V.; Tarantino, U.; Albonici, L.; et al. Radiological, Histological and Chemical Analysis of Breast Microcalcifications: Diagnostic Value and Biological Significance. J. Mammary Gland. Biol. Neoplasia 2018, 23, 89–99. [Google Scholar] [CrossRef]
- Lee, J.; Hong, B.S.; Ryu, H.S.; Lee, H.B.; Lee, M.; Park, I.A.; Kim, J.; Han, W.; Noh, D.Y.; Moon, H.G. Transition into inflammatory cancer-associated adipocytes in breast cancer microenvironment requires microRNA regulatory mechanism. PLoS ONE 2017, 12, e0174126. [Google Scholar] [CrossRef]
- Scimeca, M.; Antonacci, C.; Colombo, D.; Bonfiglio, R.; Buonomo, O.C.; Bonanno, E. Emerging prognostic markers related to mesenchymal characteristics of poorly differentiated breast cancers. Tumor Biol. 2016, 37, 5427–5435. [Google Scholar] [CrossRef] [PubMed]
- Nirgude, S.; Desai, S.; Mahadeva, R.; Ravindran, F.; Choudhary, B. ST08 Altered NF-κB Pathway in Breast Cancer Cells In Vitro as Revealed by miRNA-mRNA Analysis and Enhanced the Effect of Cisplatin on Tumour Reduction in EAC Mouse Model. Front. Oncol. 2022, 12, 835027. [Google Scholar] [CrossRef]
- Thomas, C.; Henry, W.; Cuiffo, B.G.; Collmann, A.Y.; Marangoni, E.; Benhamo, V.; Bhasin, M.K.; Fan, C.; Fuhrmann, L.; Baldwin, A.S.; et al. Pentraxin-3 is a PI3K signaling target that promotes stem cell-like traits in basal-like breast cancers. Sci. Signal. 2017, 10, eaah4674. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Antonacci, C.; Toschi, N.; Giannini, E.; Bonfiglio, R.; Buonomo, C.O.; Pistolese, C.A.; Tarantino, U.; Bonanno, E. Breast Osteoblast-like Cells: A Reliable Early Marker for Bone Metastases From Breast Cancer. Clin. Breast Cancer 2018, 18, e659–e669. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Ueno, H.; Hosoi, H.; Hashimoto, J.; Morizane, C.; Koizumi, F.; Tamura, K.; Okusaka, T. Clinical impact ofpentraxin family expression on prognosis of pancreatic carcinoma. Br. J. Cancer 2013, 109, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Camozzi, M.; Moroni, E.; Bottazzi, B.; Peri, G.; Indraccolo, S.; Amadori, A.; Mantovani, A.; Presta, M. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood 2004, 104, 92–99. [Google Scholar] [CrossRef]
- Leali, D.; Alessi, P.; Coltrini, D.; Ronca, R.; Corsini, M.; Nardo, G.; Indraccolo, S.; Presta, M. Long pentraxin-3 inhibitsFGF8b-dependent angiogenesis and growth of steroid hormone regulated tumors. Mol. Cancer Ther. 2011, 10, 1600–1610. [Google Scholar] [CrossRef]
- Nome, M.E.; Euceda, L.R.; Jabeen, S.; Debik, J.; Bathen, T.F.; Giskeødegård, G.F.; Taskén, K.A.; Maelandsmo, G.M.; Halvorsen, B.; Yndestad, A.; et al. Serum levels of inflammation-related markers and metabolites predict response to neoadjuvant chemotherapy with and without bevacizumab in breast cancers. Int. J. Cancer 2020, 146, 223–235. [Google Scholar] [CrossRef]
- Ronca, R.; Tamma, R.; Coltrini, D.; Ruggieri, S.; Presta, M.; Ribatti, D. Fibroblast growth factor modulates mast cell recruitment in a murine model of prostate cancer. Oncotarget 2017, 8, 82583–82592. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wu, F.; You, Z.Y.; Zhang, Z.M.; Guo, Y.L.; Zhong, L.X. Analyzing the differentially expressed genes and pathway cross-talk in aggressive breast cancer. J. Obstet. Gynaecol. Res. 2015, 41, 132–140. [Google Scholar] [CrossRef]
- Player, A.; Abraham, N.; Burrell, K.; Bengone, I.O.; Harris, A.; Nunez, L.; Willaims, T.; Kwende, S.; Walls, W. Identification of candidate genes associated with triple negative breast cancer. Genes Cancer 2017, 8, 659–672. [Google Scholar] [CrossRef]
- Pavlou, M.P.; Dimitromanolakis, A.; Diamandis, E.P. Coupling proteomics and transcriptomics in the quest of subtype-specific proteins in breast cancer. Proteomics 2013, 13, 1083–1095. [Google Scholar] [CrossRef]
- Ke, H.H.; Hueng, D.Y.; Tsai, W.C. Low expression of pentraxin 3 and nuclear factor-like 2 implying a relatively longer overall survival time in gliomas. Chin. J. Physiol. 2019, 62, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Zhao, Y.; Liu, T.; Wang, Z.; Zhang, N.; Dai, Z.; Wu, W.; Cao, H.; Feng, S.; et al. PTX3 mediates the infiltration, migration, and inflammation-resolving-polarization of macrophages in glioblastoma. CNS Neurosci. Ther. 2022, 28, 1748–1766. [Google Scholar] [CrossRef] [PubMed]
- Petterson, S.A.; Sørensen, M.D.; Kristensen, B.W. Expression Profiling of Primary and Recurrent Glioblastomas Reveals a Reduced Level of Pentraxin 3 in Recurrent Glioblastomas. J. Neuropathol. Exp. Neurol. 2020, 79, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, C.H.; Sun, G.; Zhang, Z.W.; Qian, B.M.; Zhu, Y.F.; Cai, M.-Y.; Pandey, S.; Zhao, D.; Wang, Y.W.; et al. IL-17induces the proliferation andmigration of glioma cells through the activation of PI3K/Akt1/NF-kappaB-p65. Cancer Lett. 2019, 447, 93–104. [Google Scholar] [CrossRef]
- Zhou, W.; Wahl, D.R. Metabolic abnormalities in glioblastoma and metabolic strategies to overcome treatment resistance. Cancers 2019, 11, 1231. [Google Scholar] [CrossRef]
- Chandrika, G.; Natesh, K.; Ranade, D.; Chugh, A.; Shastry, P. Suppression of the invasive potential of Glioblastoma cells by mTOR inhibitors involves modulation of NFκB and PKC-α signaling. Sci. Rep. 2016, 6, 22455. [Google Scholar] [CrossRef]
- Cherry, E.M.; Lee, D.W.; Jung, J.U.; Sitcheran, R. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) promotes glioma cell invasion through induction of NF-kappa B-inducing kinase (NIK) and non canonical NF-kappa B signaling. Mol. Cancer 2015, 14, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, S.; Du, Y.; Xu, F.; Sun, W.; Xu, Z.; Wang, X.; Qian, P.; Zhang, Q.; Feng, J.; et al. RelB upregulates PD-L1and exacerbates prostate cancer immune evasion. J. Exp. Clin. Cancer Res. 2022, 41, 66. [Google Scholar] [CrossRef]
- Li, K.; Zhong, C.; Wang, B.; He, J.; Bi, J. Nrf2 expression participates ingrowth and differentiation of endometrial carcinoma cells in vitro and in vivo. J. Mol. Histol. 2014, 45, 161–167. [Google Scholar] [CrossRef]
- Soini, Y.; Eskelinen, M.; Juvonen, P.; Kärjä, V.; Haapasaari, K.M.; Saarela, A.; Karihtala, P. Nuclear Nrf2 expression is related to a poor survival in pancreatic adenocarcinoma. Pathol. Res. Pract. 2014, 210, 35–39. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, H.; Dai, Z.; Zhang, Y.; Xia, Z.; Cao, H.; Yang, K.; Hu, S.; Guo, Y.; Ding, F.; et al. A comprehensive prognostic signature for glioblastoma patients based on transcriptomics and single cell sequencing. Cell. Oncol. 2021, 44, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Ishida, C.; Iwase, A.; Osuka, S.; Goto, M.; Takikawa, S.; Nakamura, T.; Kotani, T.; Kikkawa, F. Serum pentraxin 3 as a possible marker for mature cystic teratomas. Gynecol. Endocrinol. 2016, 32, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Aboulouard, S.; Wisztorski, M.; Duhamel, M.; Saudemont, P.; Cardon, T.; Narducci, F.; Lemaire, A.S.; Kobeissy, F.; Leblanc, E.; Fournier, I.; et al. In-depth proteomics analysis of sentinel lymph nodes from individuals with endometrial cancer. Cell Rep. Med. 2021, 2, 100318. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Bonavita, E.; Gentile, S.; Rubino, M.; Laface, I.; Garlanda, C.; Mantovani, A. The long pentraxin PTX3 as a key component of humoral innate immunity and a candidate diagnostic for inflammatory diseases. Int. Arch. Allergy Immunol. 2014, 165, 165–178. [Google Scholar] [CrossRef]
- Uusitalo-Seppälä, R.; Huttunen, R.; Aittoniemi, J.; Koskinen, P.; Leino, A.; Vahlberg, T.; Rintala, E.M. Pentraxin 3(PTX3) is associated with severe sepsis and fatal disease inemergency room patients with suspected infection: A prospectivecohort study. PLoS ONE 2013, 8, e53661. [Google Scholar] [CrossRef]
- Berggrund, M.; Enroth, S.; Lundberg, M.; Assarsson, E.; Stålberg, K.; Lindquist, D.; Hallmans, G.; Grankvist, K.; Olovsson, M.; Gyllensten, U. Identification of Candidate Plasma Protein Biomarkers for Cervical Cancer Using the Multiplex Proximity Extension Assay. Mol. Cell. Proteom. 2019, 18, 735–743. [Google Scholar] [CrossRef]
- Giacomini, A.; Ghedini, G.C.; Presta, M.; Ronca, R. Long pentraxin 3: A novel multifaceted player in cancer. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 53–63. [Google Scholar] [CrossRef]
- Ying, T.H.; Lee, C.H.; Chiou, H.L.; Yang, S.F.; Lin, C.L.; Hung, C.H.; Tsai, J.P.; Hsieh, Y.H. Knockdown of Pentraxin 3 suppresses tumorigenicity and metastasis of human cervical cancer cells. Sci. Rep. 2016, 6, 29385. [Google Scholar] [CrossRef]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-kappa B: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Yu, L.M.; Wang, W.W.; Qi, R.; Leng, T.G.; Zhang, X.L. MicroRNA-224 inhibition prevents progression of cervical carcinoma by targeting PTX3. J. Cell. Biochem. 2018, 119, 10278–10290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Chou, Y.H.; Wang, C.H.; Hsiao, Y.H.; Lee, C.Y.; Yang, S.F.; Wang, P.H. Impact of pentraxin 3 genetic variants on uterine cervical cancer clinicopathologic characteristics. Int. J. Med. Sci. 2021, 18, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, X.; Guo, X.; Tian, Z.; Su, M.; Long, Y.; Huang, C.; Zhou, F.; Liu, M.; Wu, X.; et al. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol. Med. Rep. 2012, 5, 753–760. [Google Scholar] [PubMed]
- Wang, Y.; Lee, C.G. Role of miR-224 in hepatocellular carcinoma: A tool for possible therapeutic intervention? Epigenomics 2011, 3, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.; Kunder, R.; Goel, A.; Sarin, R.; Moiyadi, A.; Shenoy, A.; Mamidipally, C.; Noronha, S.; Kannan, S.; Shirsat, N.V. Distinctive micro RNA signature of medulloblastomas associated with the WNT signaling pathway. J. Cancer Res. Ther. 2010, 6, 521–529. [Google Scholar]
- Fu, J.; Tang, W.; Du, P.; Wang, G.; Chen, W.; Li, J.; Zhu, Y.; Gao, J.; Cui, L. Identifying microRNA-mRNA regulatory network in colorectal cancer by a combination of expression profile and bioinformatics analysis. BMC Syst. Biol. 2012, 6, 68. [Google Scholar] [CrossRef]
- Pai, R.; Nehru, G.A.; Samuel, P.; Selvan, B.; Kumar, R.; Jacob, P.M.; Nair, A. Discriminating thyroid cancers from benign lesions based on differential expression of a limited set of miRNA using paraffin embedded tissues. Indian J. Pathol. Microbiol. 2012, 55, 158–162. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Wang, F.F.; Lv, W.; Xie, X.; Cheng, X. Overexpressed miR-224 promotes the progression of cervical cancer via targeting RASSF8. PLoS ONE 2016, 11, e0162378. [Google Scholar]
- Rudnicki, A.; Shivatzki, S.; Beyer, L.A.; Takada, Y.; Raphael, Y.; Avraham, K.B. microRNA-224 regulates pentraxin 3, a component of the humoral arm of innate immunity, in inner ear inflammation. Hum. Mol. Genet. 2014, 23, 3138–3146. [Google Scholar] [CrossRef]
- Mouková, L.; Nenutil, R.; Fabián, P.; Chovanec, J. Prognostic factors for cervical cancer. Klin. Onkol. 2013, 26, 83–90. [Google Scholar] [CrossRef]
- Chang, X.; Li, D.; Liu, C.; Zhang, Z.; Wang, T. Pentraxin 3 is a diagnostic and prognostic marker for ovarian epithelial cancer patients based on comprehensive bioinformatics and experiments. Cancer Cell Int. 2021, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, Y.; Guo, L. Increased serum pentraxin-3 level predicts poor prognosis in patients with colorectal cancer after curative surgery, a cohort study. Medicine 2018, 97, e11780. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.B.; Maggard, M.A.; Ko, C.Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J. Natl. Cancer Inst. 2004, 96, 1420–1425. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.Y.; Niu, X.C. Increased Plasma Levels of Pentraxin 3 Are Associated with Poor Prognosis of Colorectal Carcinoma Patients. Tohoku J. Exp. Med. 2016, 240, 39–46. [Google Scholar] [CrossRef]
- Papila, K.B.; Sozer, V.; Cigdem, K.P.; Durmus, S.; Kurtulus, D.; Papila, C.; Gelisgen, R.; Uzun, H. Circulating nuclear factor-kappa B mediates cancer-associated inflammation in human breast and colon cancer. J. Med. Biochem. 2021, 40, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Rubino, M.; Kunderfranco, P.; Basso, G.; Greco, C.M.; Pasqualini, F.; Serio, S.; Roncalli, M.; Laghi, L.; Mantovani, A.; Papait, R.; et al. Epigenetic regulation of the extrinsic oncosuppressor PTX3 gene in inflammation and cancer. Oncoimmunology 2017, 6, e1333215. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, K.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. A prospective study of plasma inflammatory markers and risk of colorectal cancer in men. Br. J. Cancer 2013, 108, 1891–1898. [Google Scholar] [CrossRef]
- Sasaki, Y.; Takeda, H.; Sato, T.; Orii, T.; Nishise, S.; Nagino, K.; Iwano, D.; Yaoita, T.; Yoshizawa, K.; Saito, H.; et al. Serum Interleukin-6, insulin, and HOMAIR in male individuals with colorectal adenoma. Clin. Cancer Res. Off. J. Am. Cancer Res. 2012, 18, 392–399. [Google Scholar] [CrossRef]
- Di Caro, G.; Carvello, M.; Pesce, S.; Erreni, M.; Marchesi, F.; Todoric, J.; Sacchi, M.; Montorsi, M.; Allavena, P.; Spinelli, A. Circulating Inflammatory Mediators as Potential Prognostic Markers of Human Colorectal Cancer. PLoS ONE 2016, 11, e0148186. [Google Scholar] [CrossRef]
- Okugawa, Y.; Tanaka, K.; Inoue, Y.; Kawamura, M.; Kawamoto, A.; Hiro, J.; Saigusa, S.; Toiyama, Y.; Ohi, M.; Uchida, K.; et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br. J. Cancer 2013, 108, 121–130. [Google Scholar] [CrossRef]
- Tanaka, K.; Mohri, Y.; Nishioka, J.; Kobayashi, M.; Ohi, M.; Miki, C.; Tonouchi, H.; Nobori, T.; Kusunoki, M. Neurotrophic receptor, tropomyosin-related kinase B as an independent prognostic marker in gastric cancer patients. J. Surg. Oncol. 2009, 99, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.S.; Sun, C.Y.; Zhang, L.; Zhou, S.C.; Chu, Z.B.; Qin, Y.; Wang, Y.D.; Zeng, W.; Yan, H.; Guo, T.; et al. Inhibition of BDNF in multiple myeloma blocks osteoclastogenesis via down-regulated stroma-derived RANKL expression both in vitro and in vivo. PLoS ONE 2012, 7, e46287. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.S.; Sun, C.Y.; Wang, Y.D.; Zhang, L.; Chu, Z.B.; Qin, Y.; Gao, F.; Yan, H.; Guo, T.; Chen, L.; et al. Gene silencing of the BDNF/TrkB axis in multiple myeloma blocks bone destruction and tumor burden in vitro and in vivo. Int. J. Cancer 2013, 133, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Ueda, N.; Kurihara, M.; Matsushima, S.; Ohmori, H.; Fujii, K.; Bhawal, U.K.; Yamamoto, K.; Kirita, T.; Kuniyasu, H. Tropomyosin receptor kinases B and C are tumor progressive and metastatic marker in colorectal carcinoma. Hum. Pathol. 2013, 44, 1098–1106. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef]
- Choi, B.; Lee, E.J.; Park, Y.S.; Kim, S.M.; Kim, E.Y.; Song, Y.; Kang, S.W.; Rhu, M.H.; Chang, E.J. Pentraxin-3 Silencing Suppresses Gastric Cancer-related Inflammation by Inhibiting Chemotactic Migration of Macrophages. Anticancer Res. 2015, 35, 2663–2668. [Google Scholar]
- Cui, X.; Zhang, H.; Cao, A.; Cao, L.; Hu, X. Cytokine TNF-α promotes invasion and metastasis of gastric cancer by down-regulating Pentraxin3. J. Cancer 2020, 11, 1800–1807. [Google Scholar] [CrossRef]
- Cui, X.; Qin, T.; Zhao, Z.; Yang, G.; Sanches, J.G.P.; Zhang, Q.; Fan, S.; Cao, L.; Hu, X. Pentraxin-3 inhibits milky spots metastasis of gastric cancer by inhibiting M2 macrophage polarization. J. Cancer 2021, 12, 4686–4697. [Google Scholar] [CrossRef]
- Yashiro, M.; Matsuoka, T. Fibroblast growth factor receptor signaling as therapeutic targets in gastric cancer. World J Gastroenterol. 2016, 22, 2415–2423. [Google Scholar] [CrossRef]
- Hosoda, K.; Yamashita, K.; Ushiku, H.; Ema, A.; Moriya, H.; Mieno, H.; Washio, M.; Watanabe, M. Prognostic relevance of FGFR2 expression in stage II/III. gastric cancer with curative resection and S-1 chemotherapy. Oncol. Lett. 2018, 15, 1853–1860. [Google Scholar] [CrossRef]
- Nagatsuma, A.K.; Aizawa, M.; Kuwata, T.; Doi, T.; Ohtsu, A.; Fujii, H.; Ochiai, A. Expression profiles of HER2, EGFR, MET and FGFR2in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 2015, 18, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Betts, G.; Valentine, H.; Pritchard, S.; Swindell, R.; Williams, V.; Morgan, S.; Griffiths, E.A.; Welch, I.; West, C.; Womack, C. FGFR2, HER2 and cMetin gastric adenocarcinoma: Detection, prognostic significance and assessment of downstream pathway activation. Virchows Arch. 2014, 464, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Lieto, E.; Ferraraccio, F.; Orditura, M.; Castellano, P.; Mura, A.L.; Pinto, M.; Zamboli, A.; De Vita, F.; Galizia, G. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann. Surg. Oncol. 2008, 15, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yeni, M.; Korkut, E.; Aksungur, N.; Kara, S.; Askin, S.; Kartal, M. Determination of Pentraxin-3, Interleukin-8 and Vascular Endothelial Growth Factor Levels in Patients with Gastric Adenocarcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.S.; Xiao, H.S. Micro RNAs as potential biomarkers for gastric cancer. World J. Gastroenterol. 2014, 20, 12007–12017. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef]
- Wu, H.H.; Lin, W.C.; Tsai, K.W. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Expert Rev. Mol. Med. 2014, 16, e1. [Google Scholar] [CrossRef]
- Kamal, M.A.; Siddiqui, I.; Belgiovine, C.; Barbagallo, M.; Paleari, V.; Pistillo, D.; Chiabrando, C.; Schiarea, S.; Bottazzi, B.; Leone, R.; et al. Oncogenic KRAS-Induced Protein Signature in the Tumor Secretome Identifies Laminin-C2 and Pentraxin-3 as Useful Biomarkers for the Early Diagnosis of Pancreatic Cancer. Cancers 2022, 14, 2653. [Google Scholar] [CrossRef]
- Resovi, A.; Bani, M.R.; Porcu, L.; Anastasia, A.; Minoli, L.; Allavena, P.; Cappello, P.; Novelli, F.; Scarpa, A.; Morandi, E.; et al. Soluble stroma-related biomarkers of pancreatic cancer. EMBO Mol. Med. 2018, 10, e8741. [Google Scholar] [CrossRef]
- Goulart, M.R.; Watt, J.; Siddiqui, I.; Lawlor, R.T.; Imrali, A.; Hughes, C.; Saad, A.; ChinAleong, J.; Hurt, C.; Cox, C.; et al. Pentraxin 3 is a stromally-derived biomarker for detection of pancreatic ductal adenocarcinoma. NPJ Precis. Oncol. 2021, 5, 61. [Google Scholar] [CrossRef]
- Ene-Obong, A.; Clear, A.J.; Watt, J.; Wang, J.; Fatah, R.; Riches, J.C.; Marshall, J.F.; Chin-Aleong, J.; Chelala, C.; Gribben, J.G.; et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013, 145, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Bottazzi, B.; Bastone, A.; Mantovani, A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 2005, 23, 337–366. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.H.; Gundewar, C.; Said Hilmersson, K.; Ni, L.; Saleem, M.A.; Andersson, R. Conditionally immortalized human pancreatic stellate cell lines demonstrate enhanced proliferation and migration in response to IGF-I. Exp. Cell Res. 2015, 330, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef]
- Hwang, R.F.; Moore, T.; Arumugam, T.; Ramachandran, V.; Amos, K.D.; Rivera, A.; Ji, B.; Evans, D.B.; Logsdon, C.D. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008, 68, 918–926. [Google Scholar] [CrossRef]
- Kocher, H.M.; Basu, B.; Froeling, F.E.M.; Sarker, D.; Slater, S.; Carlin, D.; deSouza, N.M.; De Paepe, K.N.; Goulart, M.R.; Hughes, C.; et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020, 11, 4841. [Google Scholar] [CrossRef]
- Froeling, F.E.; Feig, C.; Chelala, C.; Dobson, R.; Mein, C.E.; Tuveson, D.A.; Clevers, H.; Hart, I.R.; Kocher, H.M. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 2011, 141, 1486–1497. [Google Scholar] [CrossRef]
- Peng, X.; Xue, H.; Lü, L.; Shi, P.; Wang, J.; Wang, J. Accumulated promoter methylation as a potential biomarker for esophageal cancer. Oncotarget 2017, 8, 679–691. [Google Scholar] [CrossRef]
- Watt, J.; Siddique, I.; Dowe, T.; Crnogorac-Jurcevic, T.; Allavena, P.; Kocher, H. Role of PTX3 in pancreatic cancer. Lancet 2014, 383, 57. [Google Scholar] [CrossRef]
- Hu, T.; Qiao, L.; Li, H.; Ren, H.; Ning, Q.; Zhou, H.; Chen, X.; Sun, Z.; Shen, L. Pentraxin 3 (PTX-3) Levels in Bronchoalveolar Lavage Fluid as a Lung Cancer Biomarker. Dis. Markers 2020, 2020, 4652483. [Google Scholar] [CrossRef]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, W.H.; Gao, Y.; Wang, N.Y.; Wu, W.J. Clinical significance and prognostic value of pentraxin-3 as serologic biomarker for lung cancer. Asian Pac. J. Cancer Prev. 2013, 14, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Halvorsen, A.R.; Bengtson, M.B.; Taskén, K.A.; Mælandsmo, G.M.; Yndestad, A.; Halvorsen, B.; Brustugun, O.T.; Aukrust, P.; Ueland, T.; et al. Levels and prognostic impact of circulating markers of inflammation, endothelial activation and extracellular matrix remodelling in patients with lung cancer and chronic obstructive pulmonary disease. BMC Cancer 2018, 18, 739. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Allavena, P.; Garlanda, C.; Nebuloni, M.; Morenghi, E.; Rahal, D.; Roncalli, M.; Cavuto, S.; Pesce, S.; Monari, M.; et al. Prognostic and diagnostic potential of local and circulating levels of pentraxin 3 in lung cancer patients. Int. J. Cancer 2016, 138, 983–991. [Google Scholar] [CrossRef]
- Takeda, N.; Asai, K.; Ijiri, N.; Yamamoto, N.; Watanabe, T.; Kamoi, H.; Kanazawa, H.; Hirata, K. Pentraxin-3 as a Biomarker for Febrile Neutropenia in Patients with Lung Cancer. Osaka City Med. J. 2015, 61, 43–52. [Google Scholar]
- Dongel, I.; Gokmen, A.A.; Camas, H.E.; Gonen, I.; Kaya, S. Diagnostic significance of biochemical markers and pentraxin-3 in the differential diagnosis of malign, benign pleural effusion and empyema. J. Pak. Med. Assoc. 2020, 70, 860–864. [Google Scholar] [CrossRef]
- Asiedu, M.K.; Beauchamp-Perez, F.D.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 2014, 33, 1316–1324. [Google Scholar] [CrossRef]
- Liu, C.; Yao, Y.; Wang, W. Pentraxin-3 as a prognostic marker in patients with small-cell lung cancer. Med. Oncol. 2014, 31, 207. [Google Scholar] [CrossRef]
- Ahmmed, B.; Kampo, S.; Khan, M.; Faqeer, A.; Kumar, S.P.; Yulin, L.; Liu, J.W.; Yan, Q. Rg3 inhibits gemcitabine-induced lung cancer cell invasiveness through ROS-dependent, NF-κB- and HIF-1α-mediated downregulation of PTX3. J. Cell. Physiol. 2019, 234, 10680–10697. [Google Scholar] [CrossRef]
- Ahmmed, B.; Khan, M.N.; Nisar, M.A.; Kampo, S.; Zheng, Q.; Li, Y.; Yan, Q. Tunicamycin enhances the suppressive effects of cisplatin on lung cancer growth through PTX3 glycosylation via AKT/NF-κB signaling pathway. Int. J. Oncol. 2019, 54, 431–442. [Google Scholar] [CrossRef]
- Gu, X.; Chu, L.; Kang, Y. Angiogenic Factor-Based Signature Predicts Prognosis and Immunotherapy Response in Non-Small-Cell Lung Cancer. Front. Genet. 2022, 13, 894024. [Google Scholar] [CrossRef] [PubMed]
- Cabiati, M.; Gaggini, M.; De Simone, P.; Del Ry, S. Do pentraxin 3 and neural pentraxin 2 have different facet function in hepatocellular carcinoma? Clin. Exp. Med. 2021, 21, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Wang, C.; Guo, C.; Liu, Q.; Zheng, X. Pentraxin 3 overexpression accelerated tumor metastasis and indicated poor prognosis in hepatocellular carcinoma via driving epithelial-mesenchymal transition. J. Cancer 2018, 9, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Cabiati, M.; Gaggini, M.; De Simone, P.; Del Ry, S. Data mining of key genes expression in hepatocellular carcinoma: Novel potential biomarkers of diagnosis prognosis or progression. Clin. Exp. Metastasis 2022, 39, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Fan, X.; Wang, X.; Zeng, L.; Zhang, K.; Zhang, X.; Li, N.; Han, Q.; Lv, Y.; Liu, Z. Serum pentraxin 3 as a biomarker of hepatocellular carcinoma in chronic hepatitis B virus infection. Sci. Rep. 2020, 10, 20276. [Google Scholar] [CrossRef] [PubMed]
- Carmo, R.F.; Aroucha, D.; Vasconcelos, L.R.; Pereira, L.M.; Moura, P.; Cavalcanti, M.S. Genetic variation in PTX3 and plasma levels associated with hepatocellular carcinoma in patients with HCV. J. Viral Hepat. 2016, 23, 116–122. [Google Scholar] [CrossRef]
- Dinney, C.M.; Zhao, L.D.; Conrad, C.D.; Duker, J.M.; Karas, R.O.; Hu, Z.; Hamilton, M.A.; Gillis, T.R.; Parker, T.M.; Fan, B.; et al. Regulation of HBV-specific CD8+ T cell-mediated inflammation is diversified in different clinical presentations of HBV infection. J. Microbiol. 2015, 53, 718–724. [Google Scholar] [CrossRef]
- Feder, S.; Haberl, E.M.; Spirk, M.; Weiss, T.S.; Wiest, R.; Buechler, C. Pentraxin-3 is not related to disease severity in cirrhosis and hepatocellular carcinoma patients. Clin. Exp. Med. 2020, 20, 289–297. [Google Scholar] [CrossRef]
- Netti, G.S.; Lucarelli, G.; Spadaccino, F.; Castellano, G.; Gigante, M.; Divella, C.; Rocchetti, M.T.; Rascio, F.; Mancini, V.; Stallone, G.; et al. PTX3 modulates the immunoflogosis in tumor microenvironment and is a prognostic factor for patients with clear cell renal cell carcinoma. Aging 2020, 12, 7585–7602. [Google Scholar] [CrossRef]
- Urquidi, V.; Goodison, S.; Ross, S.; Chang, M.; Dai, Y.; Rosser, C.J. Diagnostic potential of urinary α1-antitrypsin and apolipoprotein E in the detection of bladder cancer. J. Urol. 2012, 188, 2377–2383. [Google Scholar] [CrossRef]
- Matarazzo, S.; Melocchi, L.; Rezzola, S.; Grillo, E.; Maccarinelli, F.; Giacomini, A.; Turati, M.; Taranto, S.; Zammataro, L.; Cerasuolo, M.; et al. Long Pentraxin-3 Follows and Modulates Bladder Cancer Progression. Cancers 2019, 11, 1277. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, E.; Tomlinson, D.C.; Knowles, M.A. A Decade of FGF Receptor Research in Bladder Cancer: Past, Present, and Future Challenges. Adv. Urol. 2012, 2012, 429213. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.F.; Matarazzo, S.; Maccarinelli, F.; Foglio, E.; Giacomini, A.; Silva Nunes, J.P.; Presta, M.; Dias, A.A.M.; Ronca, R. Long Pentraxin 3-Mediated Fibroblast Growth Factor Trapping Impairs Fibrosarcoma Growth. Front. Oncol. 2018, 8, 472. [Google Scholar] [CrossRef]
- Doni, A.; Stravalaci, M.; Inforzato, A.; Magrini, E.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Link Between Innate Immunity, Tissue Remodeling, and Cancer. Front. Immunol. 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Goodison, S.; Chang, M.; Dai, Y.; Urquidi, V.; Rosser, C.J. A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS ONE 2012, 7, e47469. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Veletic, I.; Harris, D.M.; Li, P.; Liu, Z.; Jain, P.; Manshouri, T.; Ferrajoli, A.; Burger, J.A.; Bose, P.; et al. STAT3 Activates the Pentraxin 3 Gene in Chronic Lymphocytic Leukemia Cells. J. Immunol. 2022, 208, 2847–2855. [Google Scholar] [CrossRef] [PubMed]
- Brunel, A.S.; Wójtowicz, A.; Lamoth, F.; Spertini, O.; Neofytos, D.; Calandra, T.; Marchetti, O.; Bochud, P.Y. Pentraxin-3 polymorphisms and invasive mold infections in acute leukemia patients receiving intensive chemotherapy. Haematologica 2018, 103, e527–e530. [Google Scholar] [CrossRef]
- Chen, W.; Pilling, D.; Gomer, R.H. C-reactive protein (CRP) but not the related pentraxins serum amyloid P and PTX3 inhibits the proliferation and induces apoptosis of the leukemia cell line Mono Mac 6. BMC Immunol. 2017, 18, 47. [Google Scholar] [CrossRef]
- Herrero-Sánchez, M.C.; Angomás, E.B.; de Ramón, C.; Tellería, J.J.; Corchete, L.A.; Alonso, S.; Ramos, M.D.C.; Peñarrubia, M.J.; Márquez, S.; Fernández, N.; et al. Polymorphisms in Receptors Involved in Opsonic and Nonopsonic Phagocytosis, and Correlation with Risk of Infection in Oncohematology Patients. Infect. Immun. 2018, 86, e00709-18. [Google Scholar] [CrossRef]
- Ma, Y.J.; Doni, A.; Skjoedt, M.O.; Honore, C.; Arendrup, M.; Mantovani, A.; Garred, P. Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system. J. Biol. Chem. 2011, 286, 3405–3417. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2022, 33, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Lawler, M.; Davies, L.; Oberst, S.; Oliver, K.; Eggermont, A.; Schmutz, A.; La Vecchia, C.; Allemani, C.; Lievens, Y.; Naredi, P.; et al. European Groundshot-addressing Europe’s cancer research challenges: A Lancet Oncology Commission. Lancet Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
| Type of Cancer | Mechanismof Action and Involvement of PTX-3 in Cancer | Cut-Off Value |
|---|---|---|
| Prostate cancer |
| 3.25 ng/mL (88.5% specificity and 89.3% sensitivity), proving utility as discriminating factor of benign prostatic hyperplasia from prostate cancer [34] |
| Breast cancer |
| - |
| Glioblastoma and meningioma | ||
| Gynecological cancer |
| PTX-3 could be useful as both diagnostic and differentiating tool (>3.5 ng/mL) for mature cystic teratomas from endometriomas [74] |
| Colorectal cancer | At 12.6 ng/mL (68% sensitivity and 71.7% specificity) PTX-3 can be used to identify colorectal cancer in patients with poorer 5 years overall survival rate [94] | |
| Gastric cancer |
| - |
| Pancreatic cancer | 4.34 ng/mL (86% sensitivity and 86% specificity) for neoplasia diagnose [122] | |
| Lung cancer |
| 8.03 ng/mL (72.8% sensitivity and 77.3% specificity) [134] |
| Hepatic cancer |
| 9.231 ng/mL (79.4% sensitivity and 89.9% specificity)—useful to differentiate between chronic hepatitis B infections and early cancer [147] |
| Renal cancer | - | |
| Hematologic malignancies |
| - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, M.; Meca, A.-D.; Turcu-Stiolica, A.; Oancea, C.N.; Kostici, R.; Surlin, M.V.; Florescu, C. Insights into the Relationship between Pentraxin-3 and Cancer. Int. J. Mol. Sci. 2022, 23, 15302. https://doi.org/10.3390/ijms232315302
Bogdan M, Meca A-D, Turcu-Stiolica A, Oancea CN, Kostici R, Surlin MV, Florescu C. Insights into the Relationship between Pentraxin-3 and Cancer. International Journal of Molecular Sciences. 2022; 23(23):15302. https://doi.org/10.3390/ijms232315302
Chicago/Turabian StyleBogdan, Maria, Andreea-Daniela Meca, Adina Turcu-Stiolica, Carmen Nicoleta Oancea, Roxana Kostici, Marin Valeriu Surlin, and Cristina Florescu. 2022. "Insights into the Relationship between Pentraxin-3 and Cancer" International Journal of Molecular Sciences 23, no. 23: 15302. https://doi.org/10.3390/ijms232315302
APA StyleBogdan, M., Meca, A.-D., Turcu-Stiolica, A., Oancea, C. N., Kostici, R., Surlin, M. V., & Florescu, C. (2022). Insights into the Relationship between Pentraxin-3 and Cancer. International Journal of Molecular Sciences, 23(23), 15302. https://doi.org/10.3390/ijms232315302








