Synthesis of Co3O4 Nanoparticles-Decorated Bi12O17Cl2 Hierarchical Microspheres for Enhanced Photocatalytic Degradation of RhB and BPA
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural, Morphological, and Elemental Analyses
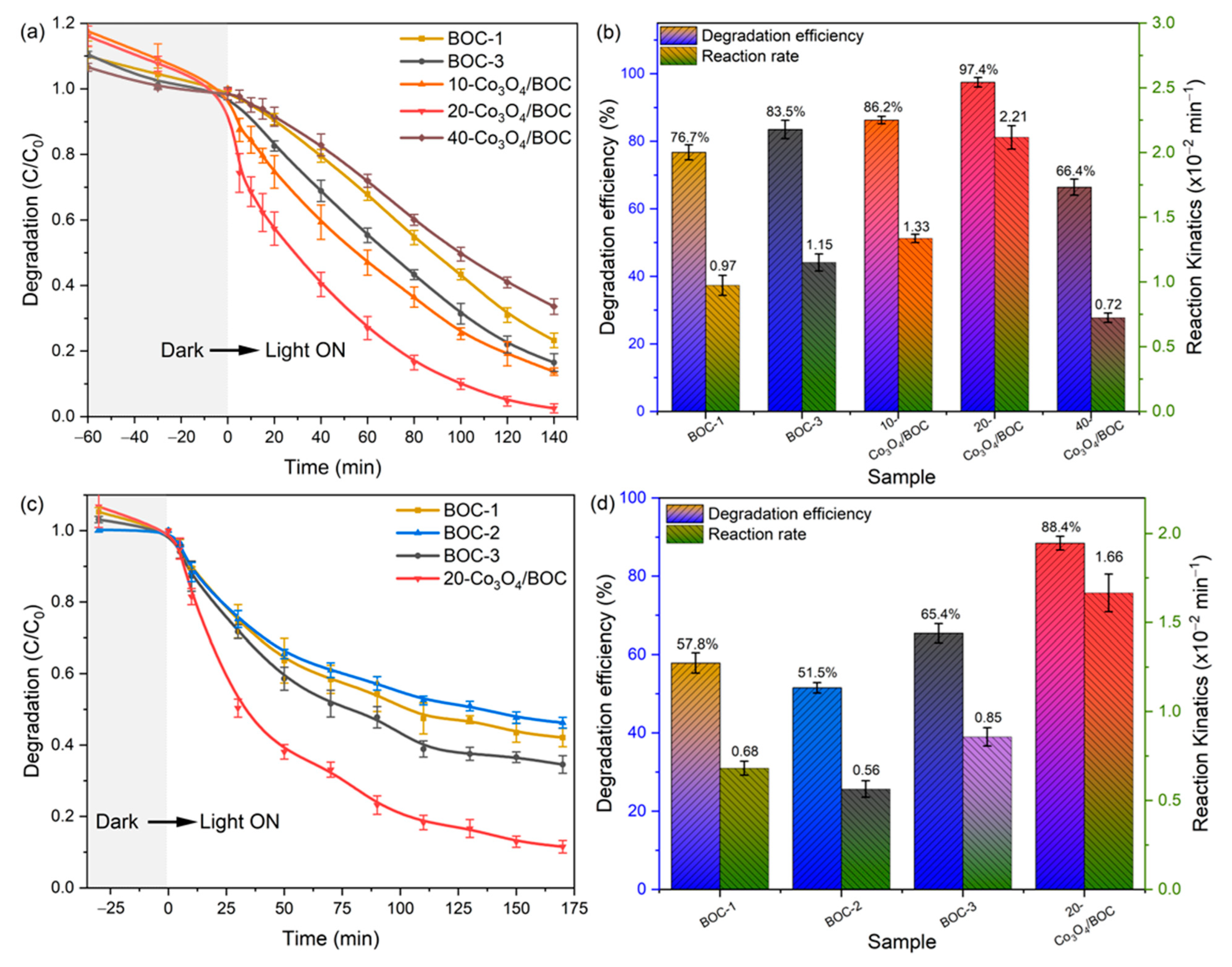
2.2. Photocatalytic Performance
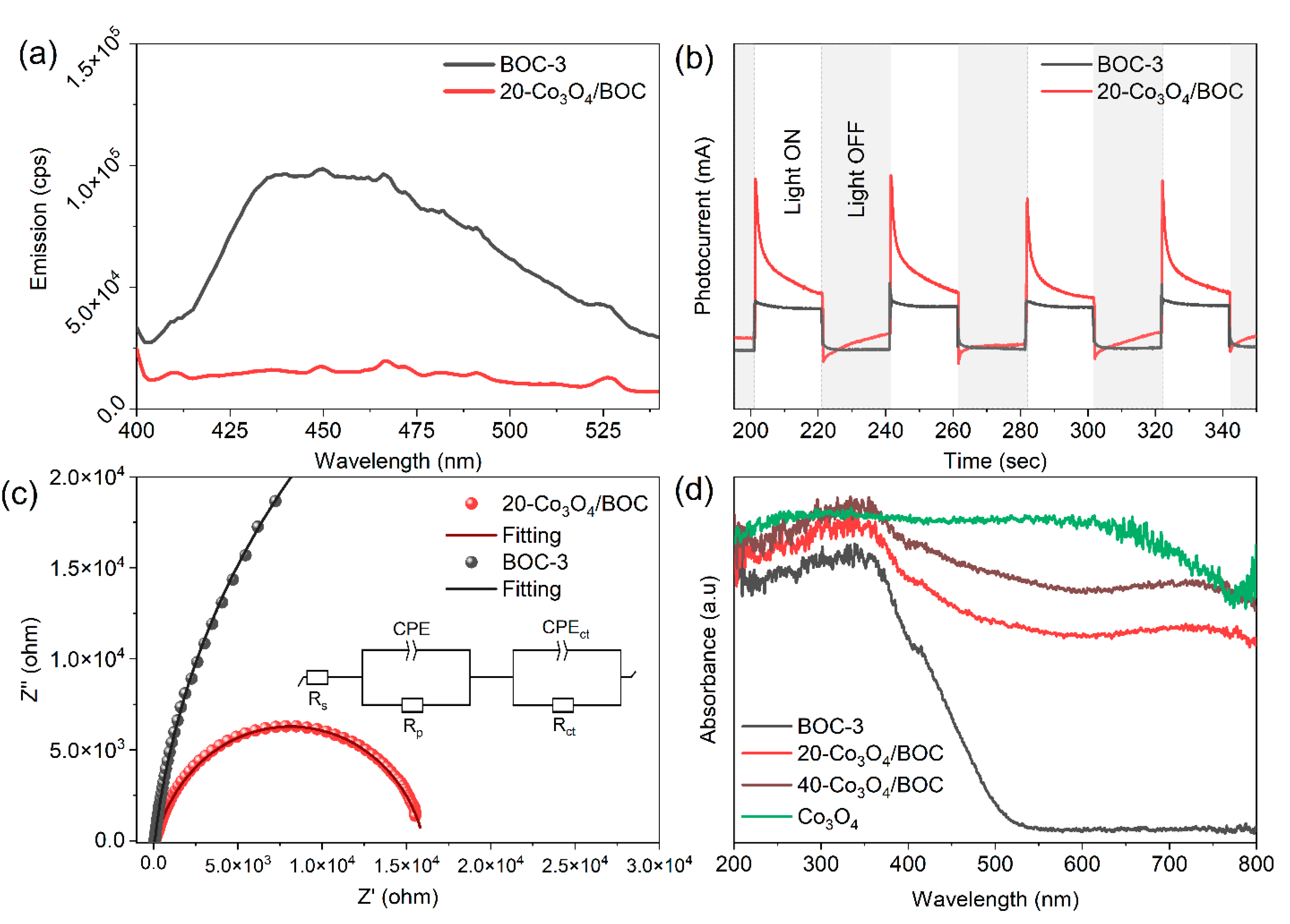
2.3. Analysis of Enhanced Photocatalytic Activity of Co3O4/BOC
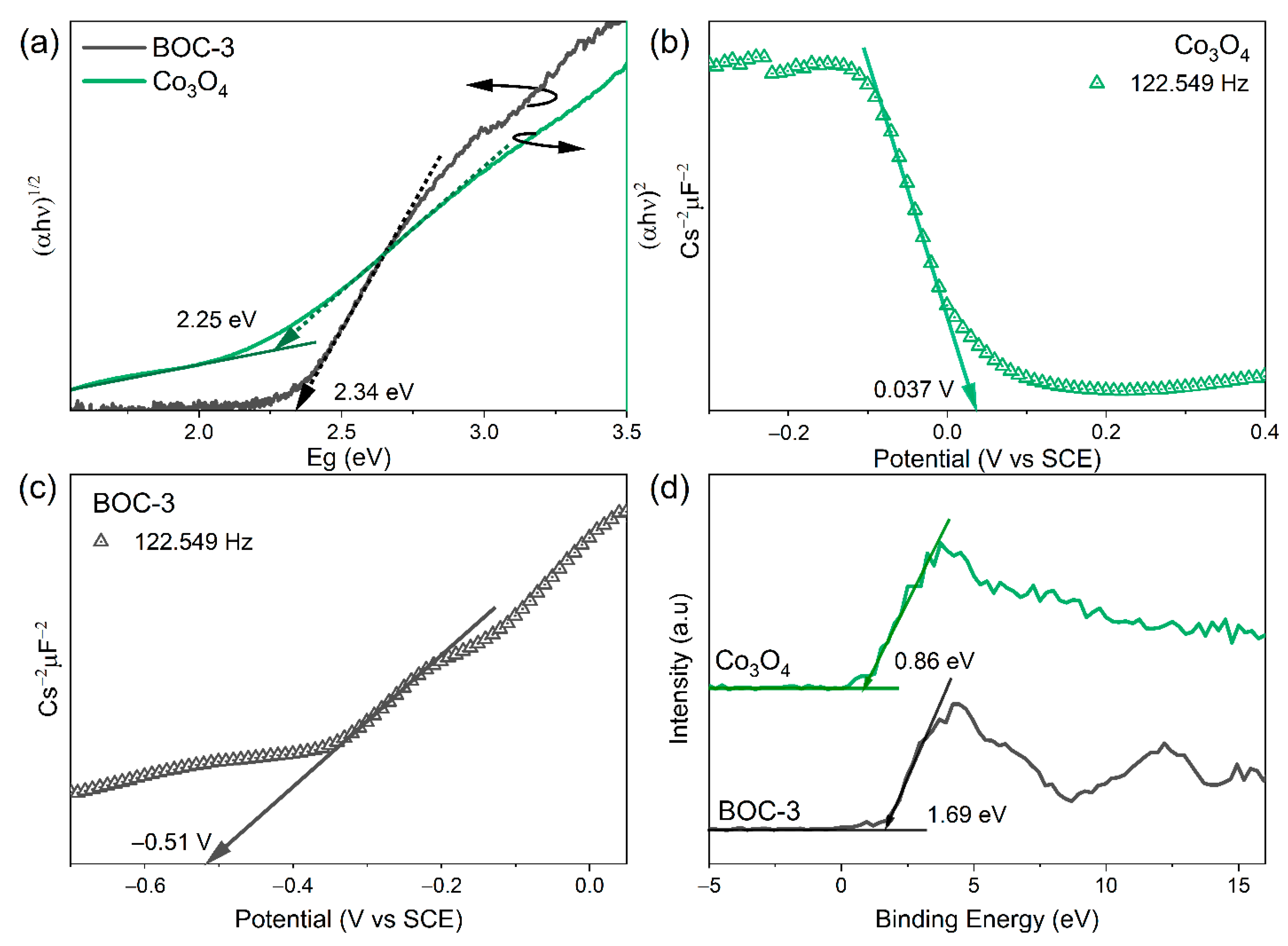
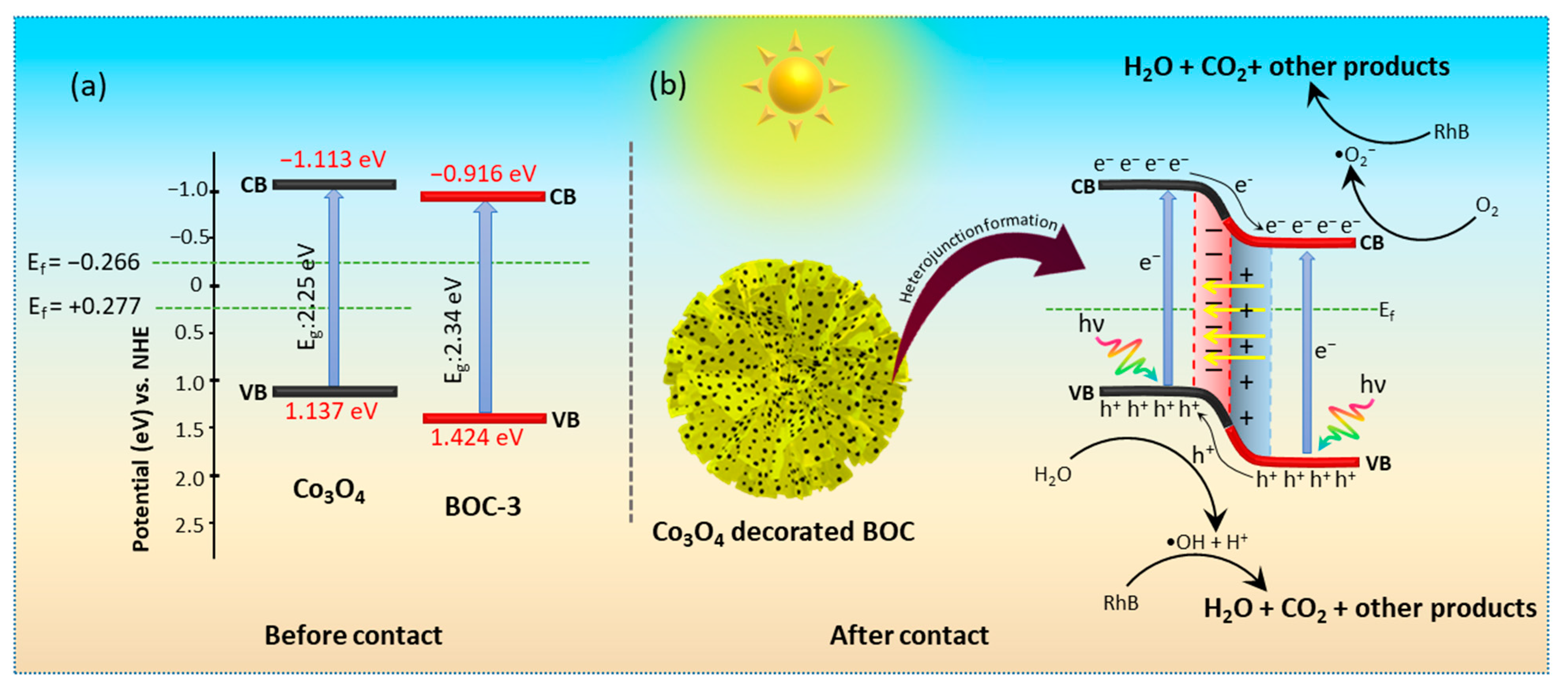
2.4. Interfacial Charge Transfer Behavior and Photocatalytic Reaction Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Co3O4 Nanoparticles
3.3. Preparation of BOC and Co3O4/BOC
3.4. Characterization of Samples
3.5. Photocatalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
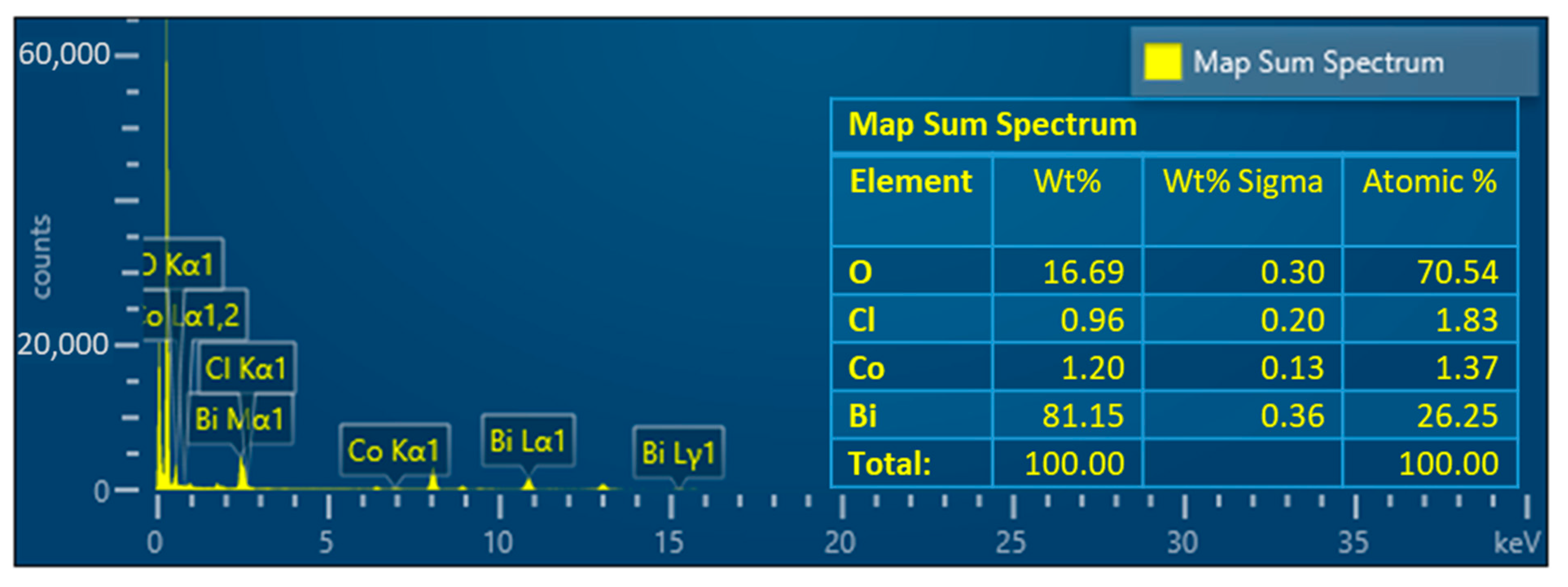
Appendix C
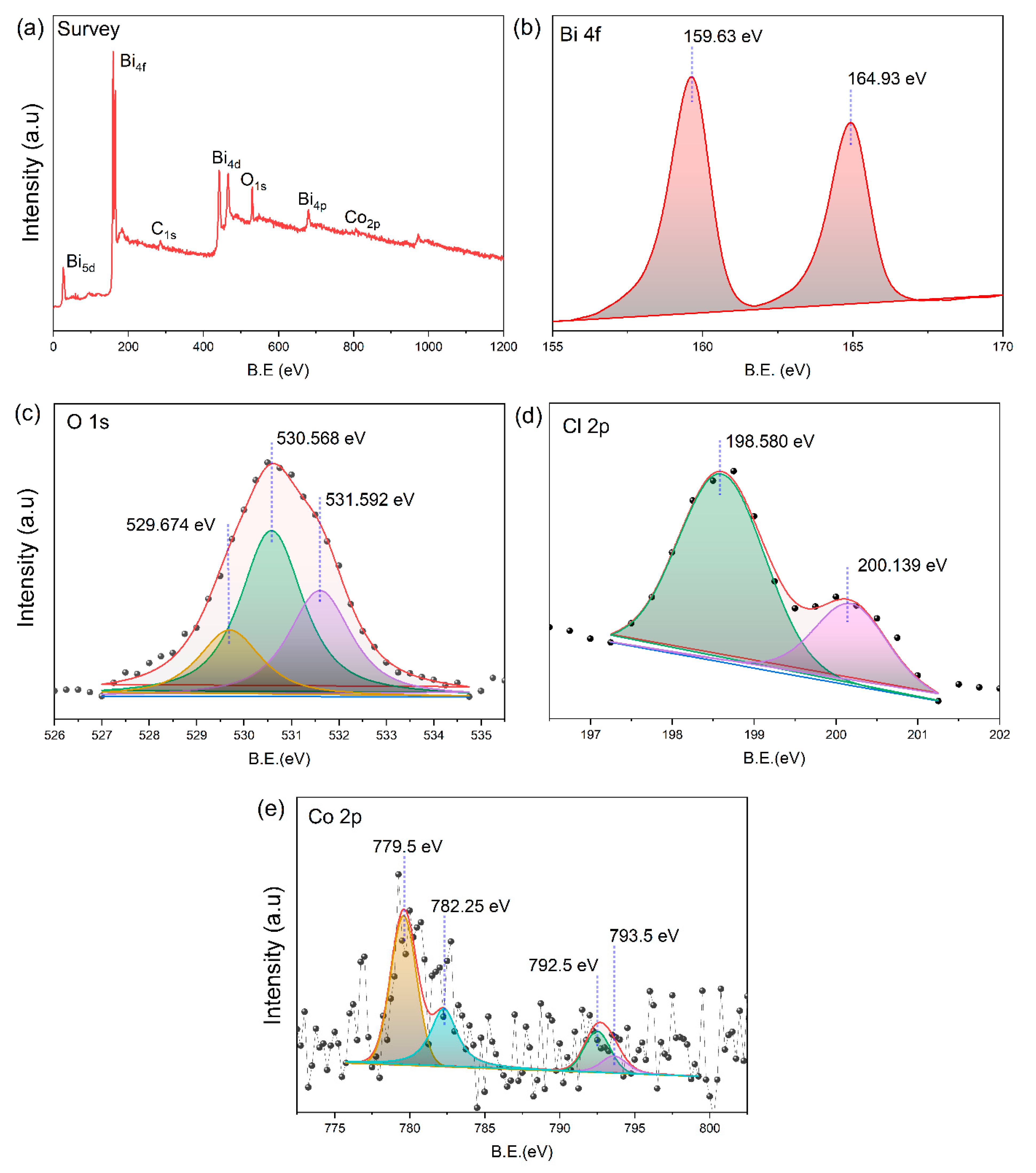
Appendix D
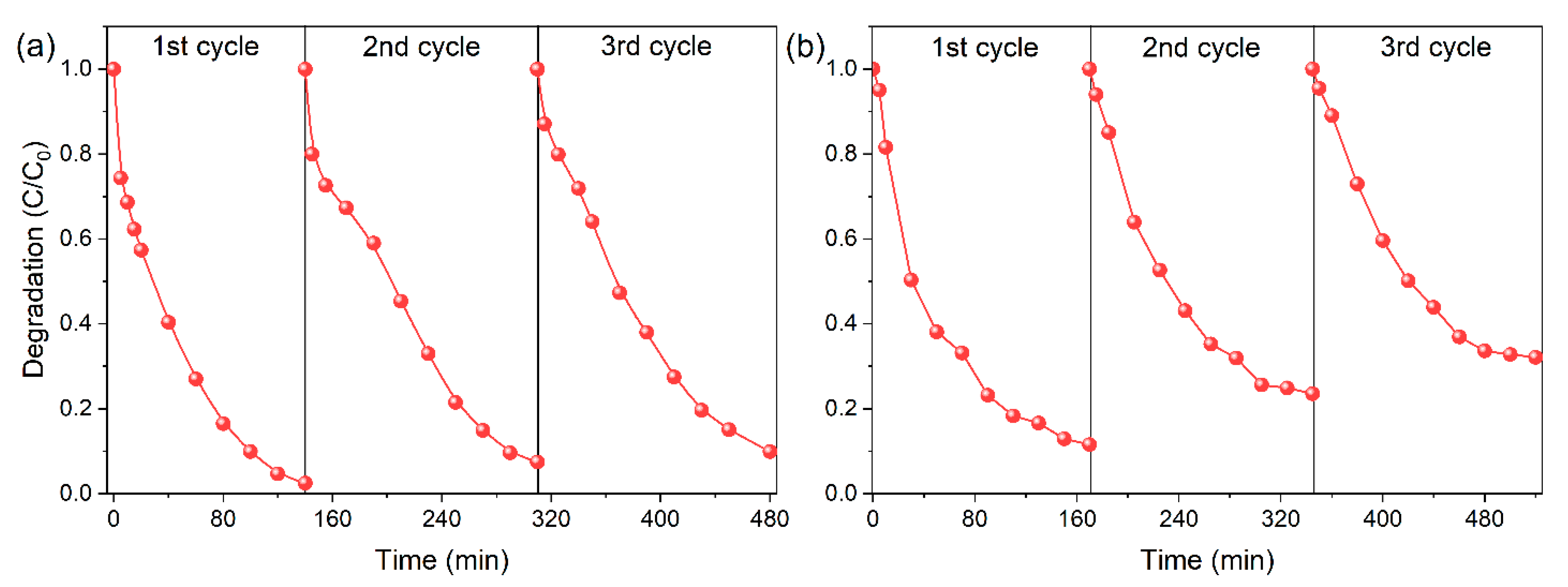
Appendix E
| Photocatalyst | Rate Constant (min−1) | Degradation Efficiency (%) | Degradation Time (min) | Lamp (C) | Amount of Catalyst (mg) | Solution Volume (mL) | Solution Concentration (mg/L) | Ref |
|---|---|---|---|---|---|---|---|---|
| Bi12O17Cl2 | 0.0115 min−1 | 83.5% | 140 | 300 W Xenon lamp, (420–720 nm) | 20 | 40 | 20 | This work |
| Co3O4/Bi12O17Cl2 | 0.021 min−1 | 97.4% | ||||||
| Bi12O17Cl2 | 1.93 × 10−3 min−1 | 40% | 240 | 300 W Xenon lamp, (420–720 nm) | 40 | 80 | 15 | [52] |
| Ag/Bi12O17Cl2 | 10.3 × 10−3 min−1 | 93% | ||||||
| Bi12O17Cl2 | 1.16 × 10−2 min−1 | 83% | 150 | 300 W Xenon lamp, (400–780 nm) | 80 | 80 | 20 | [53] |
| Bi-Bi12O17Cl2 | 2.19 × 10−2 min−1 | 99% | ||||||
| Bi12O17Cl2 | 0.074 min−1 | -- | 20 | 300 W Xenon lamp, (400 nm) | 10 | 50 | 10 | [54] |
| Fe(III)-modified Bi12O17Cl2 | 0.157 min−1 | -- | ||||||
| Bi12O17Cl2 | 0.0613 min−1 | 78% | 20 | 300 W Xenon lamp, (400 nm) | 30 | 50 | 10 | [23] |
| Graphene/Bi12O17Cl2 | 0.160 min−1 | -- | ||||||
| Bi12O17Cl2 | 0.102 min−1 | 83% | 20 | 300 W Xenon lamp, (400 nm) | 30 | 50 | 5 | [55] |
| 2D/2D g-C3N4/Bi12O17Cl2 | 0.353 min−1 | 99% | ||||||
| ZnO/NiO | 0.019 min−1 | -- | 120 | 320 mW/cm2. LED light | 50 | 50 | 10 | [56] |
| g-C3N4 | 1.11 min−1 | 99% | 90 | 150 watt halogen lamp | 50 | 100 | 5 | [57] |
| Carbon dot implanted g-C3N4 | 0.48 min−1 | -- | 80 | 300 W Xenon lamp, (320–780 nm) | 10 | 30 | 15 | [58] |
| BiVO4-Ni/AgVO3 | 0.133 min−1 | -- | 30 | 300 W Xenon lamp, (400 nm) | 30 | 50 | 10 | [59] |
| Fe-BiOBr + H2O2 | 0.0646 min−1 | 98.23% | 60 | 350 W xenon lamp (420 nm) | 30 | 50 | 20 | [60] |
| SrSnO3/g-C3N4 | k1: 0.0083−1 k2: 0.0348−1 | 97.3 | 250 | Sun Light | 100 | 100 | 5 | [61] |
References
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Senthil Kumar, P.; Radin Mohamed, R.M.S.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of Rhodamine B from Textile Wastewater Using Nanoparticle Photocatalysts: A Review for Sustainable Approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [Green Version]
- Wright-Walters, M.; Volz, C.; Talbott, E.; Davis, D. An Updated Weight of Evidence Approach to the Aquatic Hazard Assessment of Bisphenol A and the Derivation a New Predicted No Effect Concentration (Pnec) Using a Non-Parametric Methodology. Sci. Total Environ. 2011, 409, 676–685. [Google Scholar] [CrossRef]
- Sadeghfar, F.; Zalipour, Z.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Chapter 2—Photodegradation Processes. In Interface Science and Technology; Ghaedi, M., Ed.; Photocatalysis: Fundamental Processes and Applications; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 55–124. [Google Scholar]
- Taghizadeh, A.; Taghizadeh, M.; Sabzehmeidani, M.M.; Sadeghfar, F.; Ghaedi, M. Chapter 1—Electronic Structure: From Basic Principles to Photocatalysis. In Interface Science and Technology; Ghaedi, M., Ed.; Photocatalysis: Fundamental Processes and Applications; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 1–53. [Google Scholar]
- Kumar, R.; Raizada, P.; Khan, A.A.P.; Nguyen, V.-H.; Van Le, Q.; Ghotekar, S.; Selvasembian, R.; Gandhi, V.; Singh, A.; Singh, P. Recent Progress in Emerging BiPO4-Based Photocatalysts: Synthesis, Properties, Modification Strategies, and Photocatalytic Applications. J. Mater. Sci. Technol. 2022, 108, 208–225. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Liu, G.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Synthesis of Carnation Flower-like Bi2O2CO3 Photocatalyst and Its Promising Application for Photoreduction of Cr(VI). Adv. Powder Technol. 2022, 33, 103481. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Yi, Z.; Wang, S.; Wu, X.; Li, R.; Yang, H. Comparative Investigation on Synthesis, Morphological Tailoring and Photocatalytic Activities of Bi2O2CO3 Nanostructures. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 644, 128758. [Google Scholar] [CrossRef]
- Li, L.; Sun, X.; Xian, T.; Gao, H.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Template-Free Synthesis of Bi2O2CO3 Hierarchical Nanotubes Self-Assembled from Ordered Nanoplates for Promising Photocatalytic Applications. Phys. Chem. Chem. Phys. 2022, 24, 8279–8295. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Preparation of Core-Shell Heterojunction Photocatalysts by Coating CdS Nanoparticles onto Bi4Ti3O12 Hierarchical Microspheres and Their Photocatalytic Removal of Organic Pollutants and Cr(VI) Ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127918. [Google Scholar] [CrossRef]
- Guo, J.; Shi, L.; Zhao, J.; Wang, Y.; Tang, K.; Zhang, W.; Xie, C.; Yuan, X. Enhanced Visible-Light Photocatalytic Activity of Bi2MoO6 Nanoplates with Heterogeneous Bi2MoO6-X@Bi2MoO6 Core-Shell Structure. Appl. Catal. B Environ. 2018, 224, 692–704. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Li, H.; Mu, Y.; Yang, Z. Synthesis and Application of Bi2WO6 for the Photocatalytic Degradation of Two Typical Fluoroquinolones under Visible Light Irradiation. RSC Adv. 2019, 9, 27768–27779. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, Y.; Sun, D.; Zhang, S.; Tang, T.; Zhang, X.; Cao, S. Bi2O3-Sensitized TiO2 Hollow Photocatalyst Drives the Efficient Removal of Tetracyclines under Visible Light. Inorg. Chem. 2020, 59, 18131–18140. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Liang, J.; Yuan, X.; Jiang, L.; Yu, H.; Sun, H.; Zhu, Z.; Ye, S.; Tang, N.; et al. Fabrication and Regulation of Vacancy-Mediated Bismuth Oxyhalide towards Photocatalytic Application: Development Status and Tendency. Coord. Chem. Rev. 2021, 443, 214033. [Google Scholar] [CrossRef]
- Zhu, G.; Hojamberdiev, M.; Zhang, S.; Din, S.T.U.; Yang, W. Enhancing Visible-Light-Induced Photocatalytic Activity of BiOI Microspheres for NO Removal by Synchronous Coupling with Bi Metal and Graphene. Appl. Surf. Sci. 2019, 467–468, 968–978. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, J.; Zhang, L. Selective Oxidation of Benzyl Alcohol into Benzaldehyde over Semiconductors under Visible Light: The Case of Bi12O17Cl2 Nanobelts. Appl. Catal. B Environ. 2013, 142–143, 487–493. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhang, X.; Qiu, H.-B.; Wang, W.-K.; Huang, G.-X.; Jiang, J.; Yu, H.-Q. Photocatalytic Degradation of Bisphenol A by Oxygen-Rich and Highly Visible-Light Responsive Bi12O17Cl2 Nanobelts. Appl. Catal. B Environ. 2017, 200, 659–665. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Y.; Liu, Z.; Du, C. Enhanced Photocatalytic Activity of Bi12O17Cl2 Preferentially Oriented Growth along [200] with Various Surfactants. J. Mater. Sci. 2018, 53, 14217–14230. [Google Scholar] [CrossRef]
- Fang, K.; Shi, L.; Wang, F.; Yao, L. The Synthesis of 3D Bi12O17Cl2 Hierarchical Structure with Visible-Light Photocatalytic Activity. Mater. Lett. 2020, 277, 128352. [Google Scholar] [CrossRef]
- Zhang, Y.; Di, J.; Zhu, X.; Ji, M.; Chen, C.; Liu, Y.; Li, L.; Wei, T.; Li, H.; Xia, J. Chemical Bonding Interface in Bi2Sn2O7/BiOBr S-Scheme Heterojunction Triggering Efficient N2 Photofixation. Appl. Catal. B Environ. 2023, 323, 122148. [Google Scholar] [CrossRef]
- Guo, M.; He, H.; Cao, J.; Lin, H.; Chen, S. Novel I-Doped Bi12O17Cl2 Photocatalysts with Enhanced Photocatalytic Activity for Contaminants Removal. Mater. Res. Bull. 2019, 112, 205–212. [Google Scholar] [CrossRef]
- Zhang, M.; Bi, C.; Lin, H.; Cao, J.; Chen, S. Construction of Novel Au/Bi12O17Cl2 Composite with Intensive Visible Light Activity Enhancement for Contaminants Removal. Mater. Lett. 2017, 191, 132–135. [Google Scholar] [CrossRef]
- Ma, J.; Shi, L.; Hou, L.; Yao, L.; Lu, C.; Geng, Z. Fabrication of Graphene/Bi12O17Cl2 as an Effective Visible-Light Photocatalyst. Mater. Res. Bull. 2020, 122, 110690. [Google Scholar] [CrossRef]
- He, G.; Xing, C.; Xiao, X.; Hu, R.; Zuo, X.; Nan, J. Facile Synthesis of Flower-like Bi12O17Cl2/β-Bi2O3 Composites with Enhanced Visible Light Photocatalytic Performance for the Degradation of 4-Tert-Butylphenol. Appl. Catal. B Environ. 2015, 170–171, 1–9. [Google Scholar] [CrossRef]
- In Situ Assembly of BiOI@Bi12O17Cl2 P-n Junction: Charge Induced Unique Front-Lateral Surfaces Coupling Heterostructure with High Exposure of BiOI {001} Active Facets for Robust and Nonselective Photocatalysis. Appl. Catal. B Environ. 2016, 199, 75–86. [CrossRef]
- Guo, J.; Sun, H.; Yuan, X.; Jiang, L.; Wu, Z.; Yu, H.; Tang, N.; Yu, M.; Yan, M.; Liang, J. Photocatalytic Degradation of Persistent Organic Pollutants by Co-Cl Bond Reinforced CoAl-LDH/Bi12O17Cl2 Photocatalyst: Mechanism and Application Prospect Evaluation. Water Res. 2022, 219, 118558. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shi, L.; Wang, Z.; Liu, D. Preparation of Ag2O/Bi12O17Cl2 p-n Junction Photocatalyst and Its Photocatalytic Performance under Visible and Infrared Light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127811. [Google Scholar] [CrossRef]
- Kim, K.-H.; Choi, Y.-H. Surface Oxidation of Cobalt Carbonate and Oxide Nanowires by Electrocatalytic Oxygen Evolution Reaction in Alkaline Solution. Mater. Res. Express 2022, 9, 034001. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Zuo, G.; Guo, Y.; Xiao, W.; Dai, Y.; Kong, J.; Xu, X.; Zhou, Y.; Xie, A.; et al. 0D/2D Co3O4/TiO2 Z-Scheme Heterojunction for Boosted Photocatalytic Degradation and Mechanism Investigation. Appl. Catal. B Environ. 2020, 278, 119298. [Google Scholar] [CrossRef]
- Dai, X.; Cui, L.; Yao, L.; Shi, L. Facile Construction of Novel Co3O4/Bi12O17Cl2 Heterojunction Composites with Enhanced Photocatalytic Performance. J. Solid State Chem. 2021, 297, 122066. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, B.; Liu, G.; Li, H.; Xia, J. Carbonized Polymer Dots Modified Ultrathin Bi12O17Cl2 Nanosheets Z-Scheme Heterojunction for Robust CO2 Photoreduction. Chem. Eng. Sci. 2021, 232, 116338. [Google Scholar] [CrossRef]
- Wang, L.; Min, X.; Sui, X.; Chen, J.; Wang, Y. Facile Construction of Novel BiOBr/Bi12O17Cl2 Heterojunction Composites with Enhanced Photocatalytic Performance. J. Colloid Interface Sci. 2020, 560, 21–33. [Google Scholar] [CrossRef]
- Zhu, J.; Fan, J.; Cheng, T.; Cao, M.; Sun, Z.; Zhou, R.; Huang, L.; Wang, D.; Li, Y.; Wu, Y. Bilayer Nanosheets of Unusual Stoichiometric Bismuth Oxychloride for Potassium Ion Storage and CO2 Reduction. Nano Energy 2020, 75, 104939. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Ji, X.; Huang, S.; Xia, J.; Xie, M.; Yan, J.; Xu, H.; Li, H. Conjugated Conducting Polymers PANI Decorated Bi12O17Cl2 Photocatalyst with Extended Light Response Range and Enhanced Photoactivity. Appl. Surf. Sci. 2019, 464, 552–561. [Google Scholar] [CrossRef]
- Superior Visible Light Hydrogen Evolution of Janus Bilayer Junctions via Atomic-Level Charge Flow Steering|Nature Communications. Available online: https://www.nature.com/articles/ncomms11480 (accessed on 15 October 2022).
- Diallo, A.; Beye, A.C.; Doyle, T.B.; Park, E.; Maaza, M. Green Synthesis of Co3O4 Nanoparticles via Aspalathus Linearis: Physical Properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wei, X.; Hu, X.; Zhou, W.; Zhao, Y. Effect of Formic Acid Treatment on the Structure and Catalytic Activity of Co3O4 for N2O Decomposition. Catal. Lett. 2019, 149, 1026–1036. [Google Scholar] [CrossRef]
- Lopes Matias, J.A.; Sabino da Silva, E.B.; Raimundo, R.A.; Ribeiro da Silva, D.; Oliveira, J.B.L.; Morales, M.A. (Bi13Co11)Co2O40–Co3O4 Composites: Synthesis, Structural and Magnetic Properties. J. Alloy. Compd. 2021, 852, 156991. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, B.; Yi, Z.; Wu, X.; Zhang, J.; Yang, H. Synthesis of a Self-Assembled Dual Morphologies Ag-NPs/SrMoO4 Photocatalyst with LSPR Effect for the Degradation of Methylene Blue Dye. ChemistrySelect 2022, 7, e202201274. [Google Scholar] [CrossRef]
- Urgunde, A.B.; Kamboj, V.; Kannattil, H.P.; Gupta, R. Layer-by-Layer Coating of Cobalt-Based Ink for Large-Scale Fabrication of OER Electrocatalyst. Energy Technol. 2019, 7, 1900603. [Google Scholar] [CrossRef]
- Din, S.T.U.; Lee, H.; Yang, W. Z-Scheme Heterojunction of 3-Dimensional Hierarchical Bi3O4Cl/Bi5O7I for a Significant Enhancement in the Photocatalytic Degradation of Organic Pollutants (RhB and BPA). Nanomaterials 2022, 12, 767. [Google Scholar] [CrossRef]
- Li, R.; Hu, B.; Yu, T.; Chen, H.; Wang, Y.; Song, S. Insights into Correlation among Surface-Structure-Activity of Cobalt-Derived Pre-Catalyst for Oxygen Evolution Reaction. Adv. Sci. 2020, 7, 1902830. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Lei, T.; Nan, B.; Kang, J.; Jiang, Y.; He, Y.; Liu, C.T. Mo Doped Porous Ni–Cu Alloy as Cathode for Hydrogen Evolution Reaction in Alkaline Solution. RSC Adv. 2015, 5, 82078–82086. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Bi, F.; Chen, J.; Wang, Y.; Cui, L.; Xu, J.; Zhang, X. Highly Efficient Photothermal Catalysis of Toluene over Co3O4/TiO2 p-n Heterojunction: The Crucial Roles of Interface Defects and Band Structure. Appl. Catal. B Environ. 2022, 315, 121550. [Google Scholar] [CrossRef]
- Rational Design of Carbon-Doped Carbon Nitride/Bi12O17Cl2 Composites: A Promising Candidate Photocatalyst for Boosting Visible-Light-Driven Photocatalytic Degradation of Tetracycline|ACS Sustainable Chemistry & Engineering. Available online: https://pubs.acs.org/doi/full/10.1021/acssuschemeng.8b00782 (accessed on 2 November 2022).
- Guerrero-Araque, D.; Acevedo-Peña, P.; Ramírez-Ortega, D.; Lartundo-Rojas, L.; Gómez, R. SnO2–TiO2 Structures and the Effect of CuO, CoO Metal Oxide on Photocatalytic Hydrogen Production. J. Chem. Technol. Biotechnol. 2017, 92, 1531–1539. [Google Scholar] [CrossRef]
- Patel, M.; Park, W.-H.; Ray, A.; Kim, J.; Lee, J.-H. Photoelectrocatalytic Sea Water Splitting Using Kirkendall Diffusion Grown Functional Co3O4 Film. Sol. Energy Mater. Sol. Cells 2017, 171, 267–274. [Google Scholar] [CrossRef]
- Zhu, G.; Hojamberdiev, M.; Zhang, W.; Taj Ud Din, S.; Joong Kim, Y.; Lee, J.; Yang, W. Enhanced Photocatalytic Activity of Fe-Doped Bi4O5Br2 Nanosheets Decorated with Au Nanoparticles for Pollutants Removal. Appl. Surf. Sci. 2020, 526, 146760. [Google Scholar] [CrossRef]
- Xiong, J.; Cheng, G.; Li, G.; Qin, F.; Chen, R. Well-Crystallized Square-like 2D BiOCl Nanoplates: Mannitol-Assisted Hydrothermal Synthesis and Improved Visible-Light-Driven Photocatalytic Performance. RSC Adv. 2011, 1, 1542–1553. [Google Scholar] [CrossRef]
- Kato, D.; Tomita, O.; Nelson, R.; Kirsanova, M.A.; Dronskowski, R.; Suzuki, H.; Zhong, C.; Tassel, C.; Ishida, K.; Matsuzaki, Y.; et al. Bi12O17Cl2 with a Sextuple Bi—O Layer Composed of Rock-Salt and Fluorite Units and Its Structural Conversion through Fluorination to Enhance Photocatalytic Activity. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adfm.202204112 (accessed on 6 October 2022).
- Chen, Y.; Liu, G.; Dong, L.; Liu, X.; Liu, M.; Wang, X.; Gao, C.; Wang, G.; Teng, Z.; Yang, W.; et al. A Microwave-Assisted Solvothermal Method to Synthesize BiOCl Microflowers with Oxygen Vacancies and Their Enhanced Photocatalytic Performance. J. Alloy. Compd. 2023, 930, 167331. [Google Scholar] [CrossRef]
- Chang, F.; Wang, X.; Luo, J.; Wang, J.; Xie, Y.; Deng, B.; Hu, X. Ag/Bi12O17Cl2 Composite: A Case Study of Visible-Light-Driven Plasmonic Photocatalyst. Mol. Catal. 2017, 427, 45–53. [Google Scholar] [CrossRef]
- Chang, F.; Lei, B.; Zhang, X.; Xu, Q.; Chen, H.; Deng, B.; Hu, X. The Reinforced Photocatalytic Performance of Binary-Phased Composites Bi-Bi12O17Cl2 Fabricated by a Facile Chemical Reduction Protocol. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 290–298. [Google Scholar] [CrossRef]
- Meng, X.; Shi, L.; Yao, L.; Zhang, Y.; Cui, L. Fe (III) Clusters Modified Bi12O17Cl2 Nanosheets Photocatalyst for Boosting Photocatalytic Performance through Interfacial Charge Transfer Effect. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124658. [Google Scholar] [CrossRef]
- Shi, L.; Si, W.; Wang, F.; Qi, W. Construction of 2D/2D Layered g-C3N4/Bi12O17Cl2 Hybrid Material with Matched Energy Band Structure and Its Improved Photocatalytic Performance. RSC Adv. 2018, 8, 24500–24508. [Google Scholar] [CrossRef]
- Ma, L.; Ai, X.; Chen, Y.; Liu, P.; Lin, C.; Lu, K.; Jiang, W.; Wu, J.; Song, X. Improved Photocatalytic Activity via N-Type ZnO/p-Type NiO Heterojunctions. Nanomaterials 2022, 12, 3665. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, D.; Kędzierski, T.; Aleksandrzak, M.; Mijowska, E.; Zielińska, B. Influence of Hydrogenation on Morphology, Chemical Structure and Photocatalytic Efficiency of Graphitic Carbon Nitride. Int. J. Mol. Sci. 2021, 22, 13096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Xia, Y.; Xun, M.; Chen, H.; Liu, X.; Yin, X. Metal-Free Carbon Quantum Dots Implant Graphitic Carbon Nitride: Enhanced Photocatalytic Dye Wastewater Purification with Simultaneous Hydrogen Production. Int. J. Mol. Sci. 2020, 21, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Liu, F.; Chi, X.; Tian, Y.; Zhu, Z.; Guan, R.; Song, J. A Mesoporous Nanofibrous BiVO4-Ni/AgVO3 Z-Scheme Heterojunction Photocatalyst with Enhanced Photocatalytic Reduction of Cr6+ and Degradation of RhB under Visible Light. Appl. Surf. Sci. 2022, 603, 154416. [Google Scholar] [CrossRef]
- An, W.; Wang, H.; Yang, T.; Xu, J.; Wang, Y.; Liu, D.; Hu, J.; Cui, W.; Liang, Y. Enriched Photocatalysis-Fenton Synergistic Degradation of Organic Pollutants and Coking Wastewater via Surface Oxygen Vacancies over Fe-BiOBr Composites. Chem. Eng. J. 2023, 451, 138653. [Google Scholar] [CrossRef]
- de Sousa Filho, I.A.; Arana, L.R.; Doungmo, G.; Grisolia, C.K.; Terrashke, H.; Weber, I.T. SrSnO3/g-C3N4 and Sunlight: Photocatalytic Activity and Toxicity of Degradation Byproducts. J. Environ. Chem. Eng. 2020, 8, 103633. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Din, S.T.U.; Xie, W.-F.; Yang, W. Synthesis of Co3O4 Nanoparticles-Decorated Bi12O17Cl2 Hierarchical Microspheres for Enhanced Photocatalytic Degradation of RhB and BPA. Int. J. Mol. Sci. 2022, 23, 15028. https://doi.org/10.3390/ijms232315028
Din STU, Xie W-F, Yang W. Synthesis of Co3O4 Nanoparticles-Decorated Bi12O17Cl2 Hierarchical Microspheres for Enhanced Photocatalytic Degradation of RhB and BPA. International Journal of Molecular Sciences. 2022; 23(23):15028. https://doi.org/10.3390/ijms232315028
Chicago/Turabian StyleDin, Syed Taj Ud, Wan-Feng Xie, and Woochul Yang. 2022. "Synthesis of Co3O4 Nanoparticles-Decorated Bi12O17Cl2 Hierarchical Microspheres for Enhanced Photocatalytic Degradation of RhB and BPA" International Journal of Molecular Sciences 23, no. 23: 15028. https://doi.org/10.3390/ijms232315028
APA StyleDin, S. T. U., Xie, W.-F., & Yang, W. (2022). Synthesis of Co3O4 Nanoparticles-Decorated Bi12O17Cl2 Hierarchical Microspheres for Enhanced Photocatalytic Degradation of RhB and BPA. International Journal of Molecular Sciences, 23(23), 15028. https://doi.org/10.3390/ijms232315028







