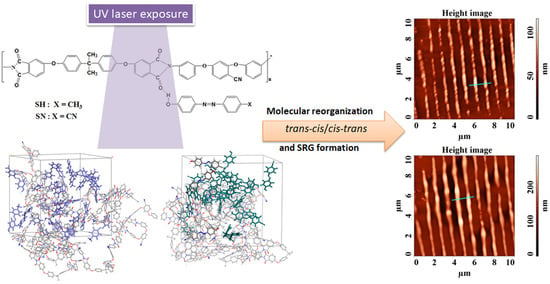
The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics
Abstract
:1. Introduction
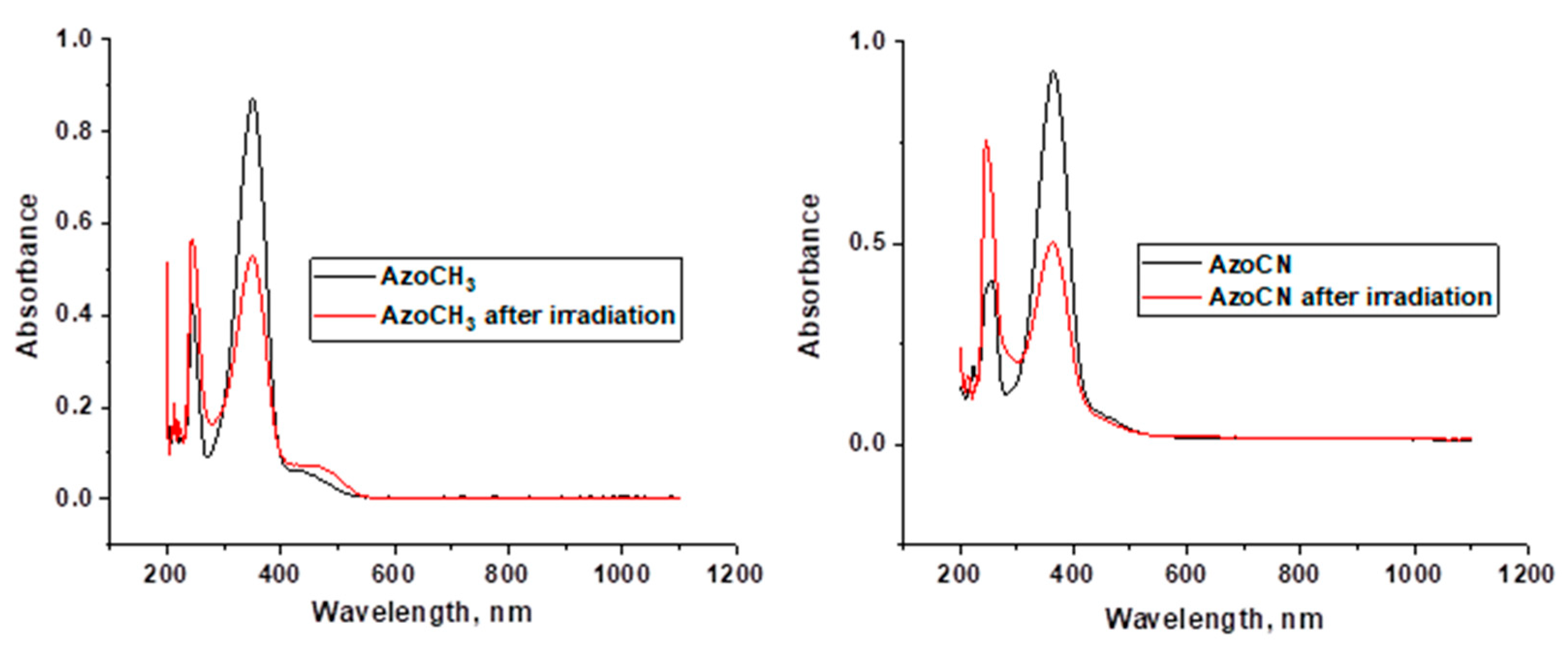
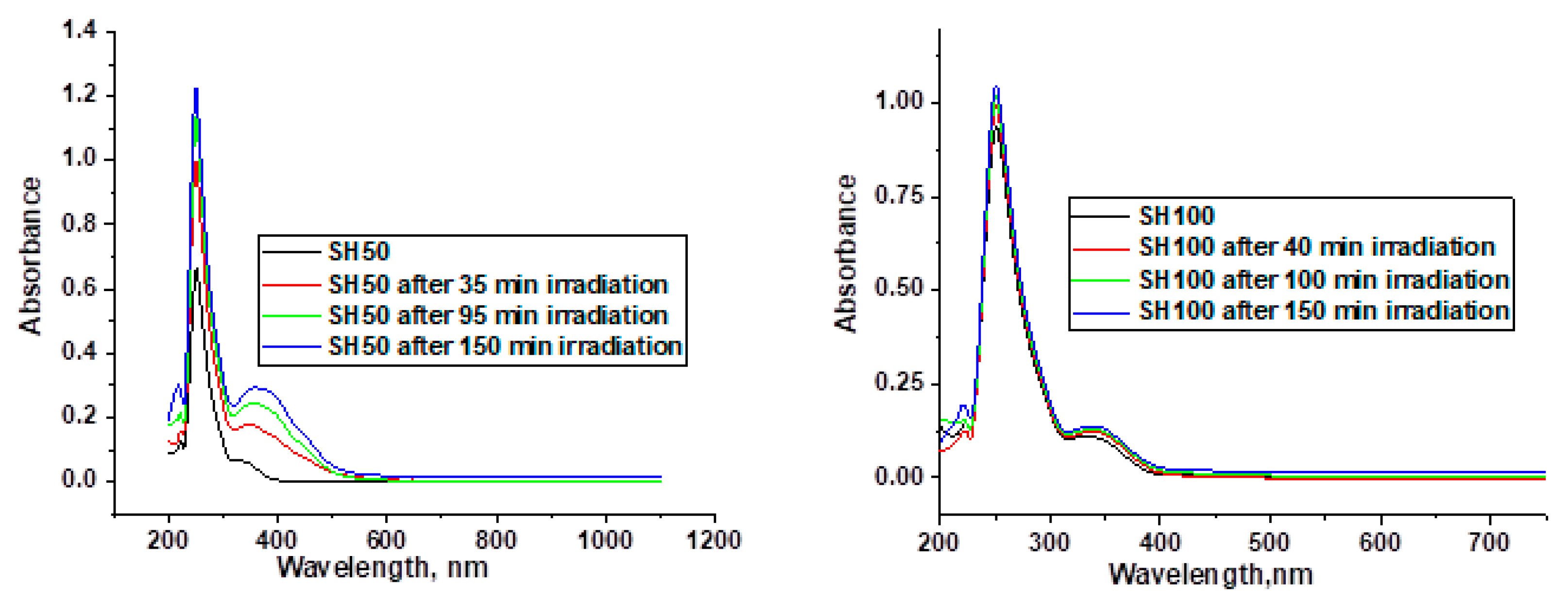
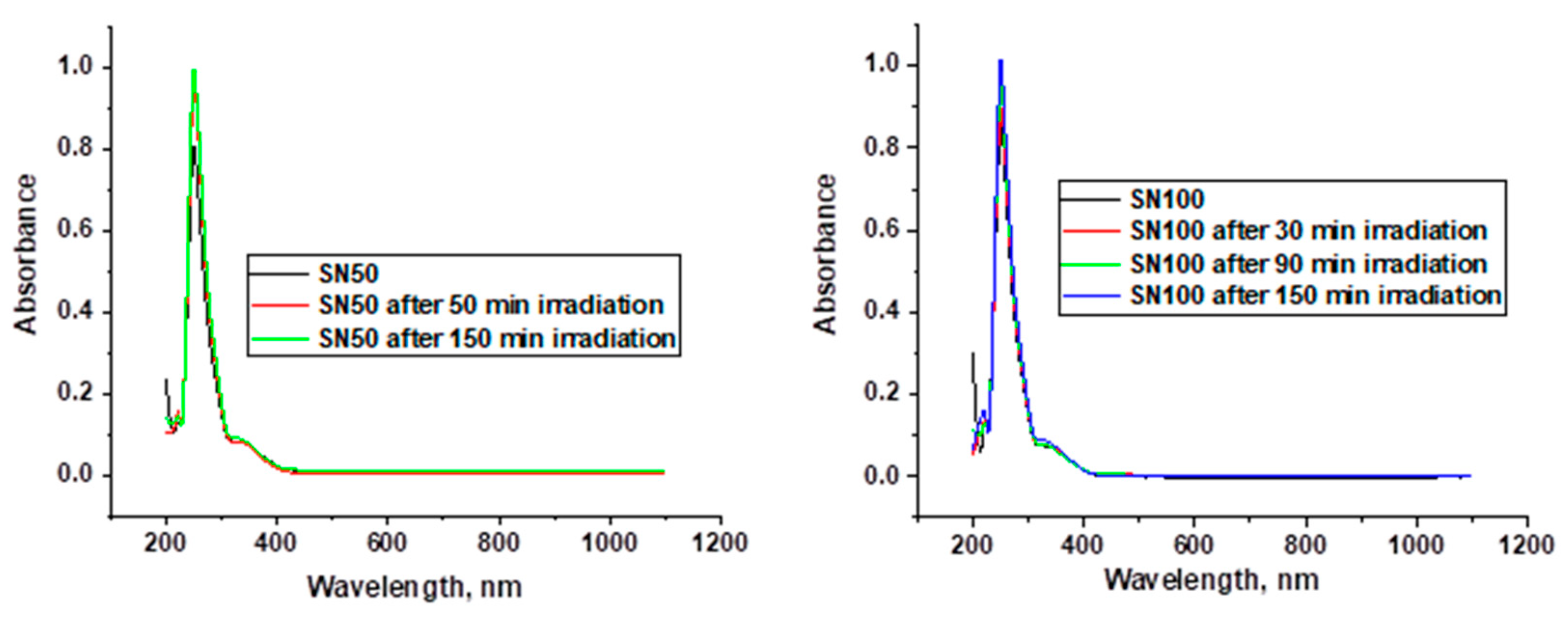
2. Results and Discussion
2.1. Colorimetry Study
2.2. Thermal Stability
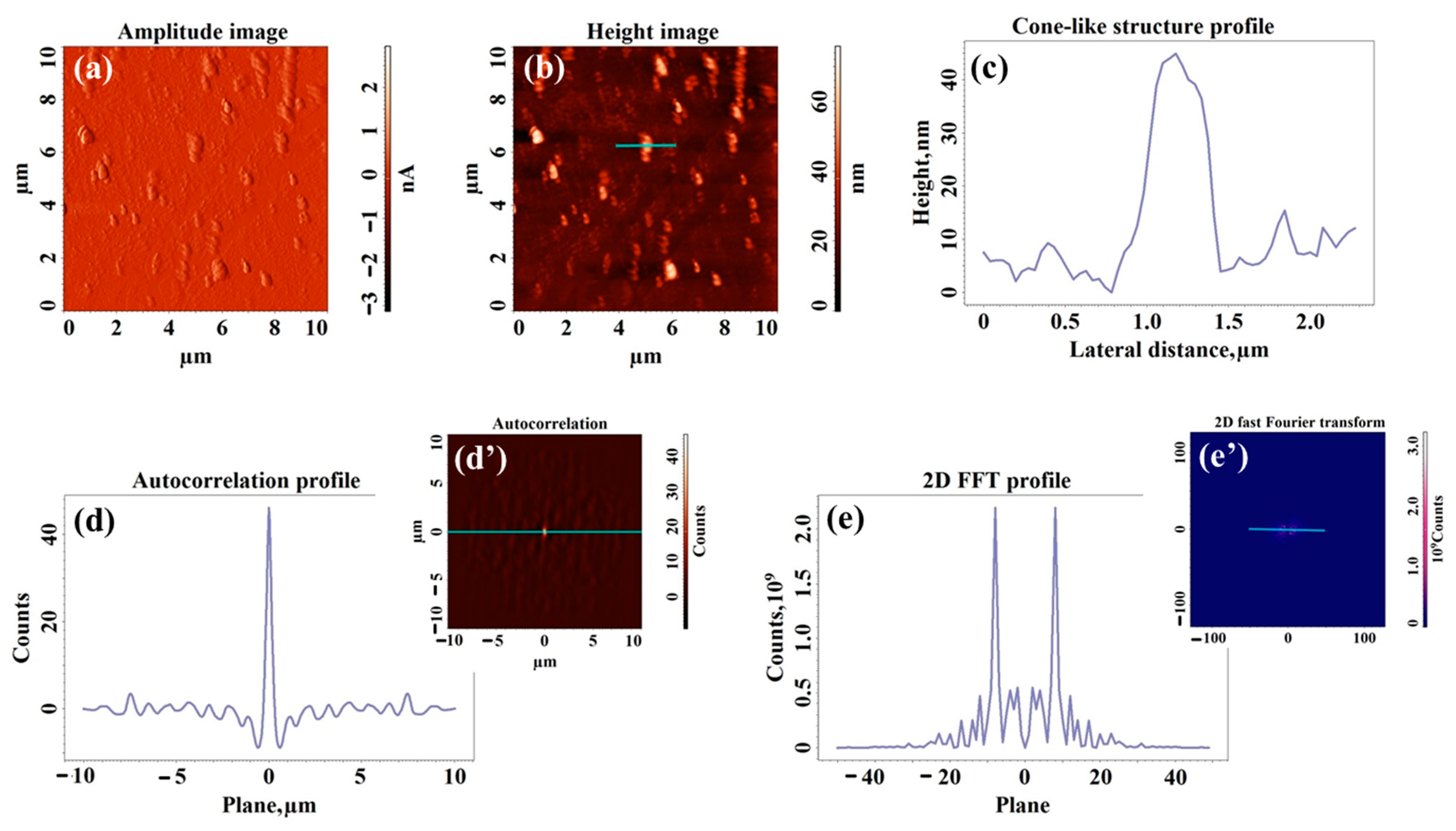
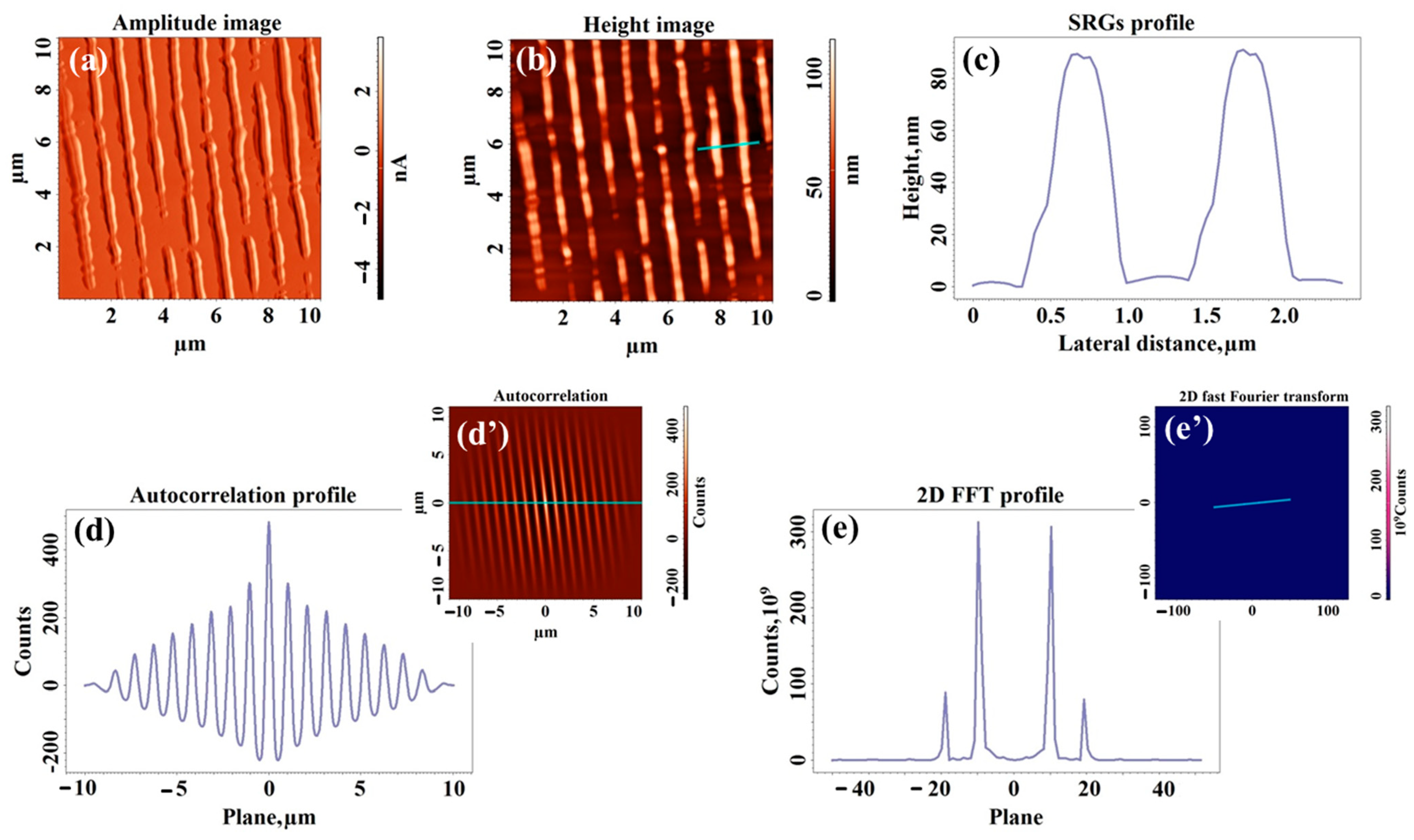
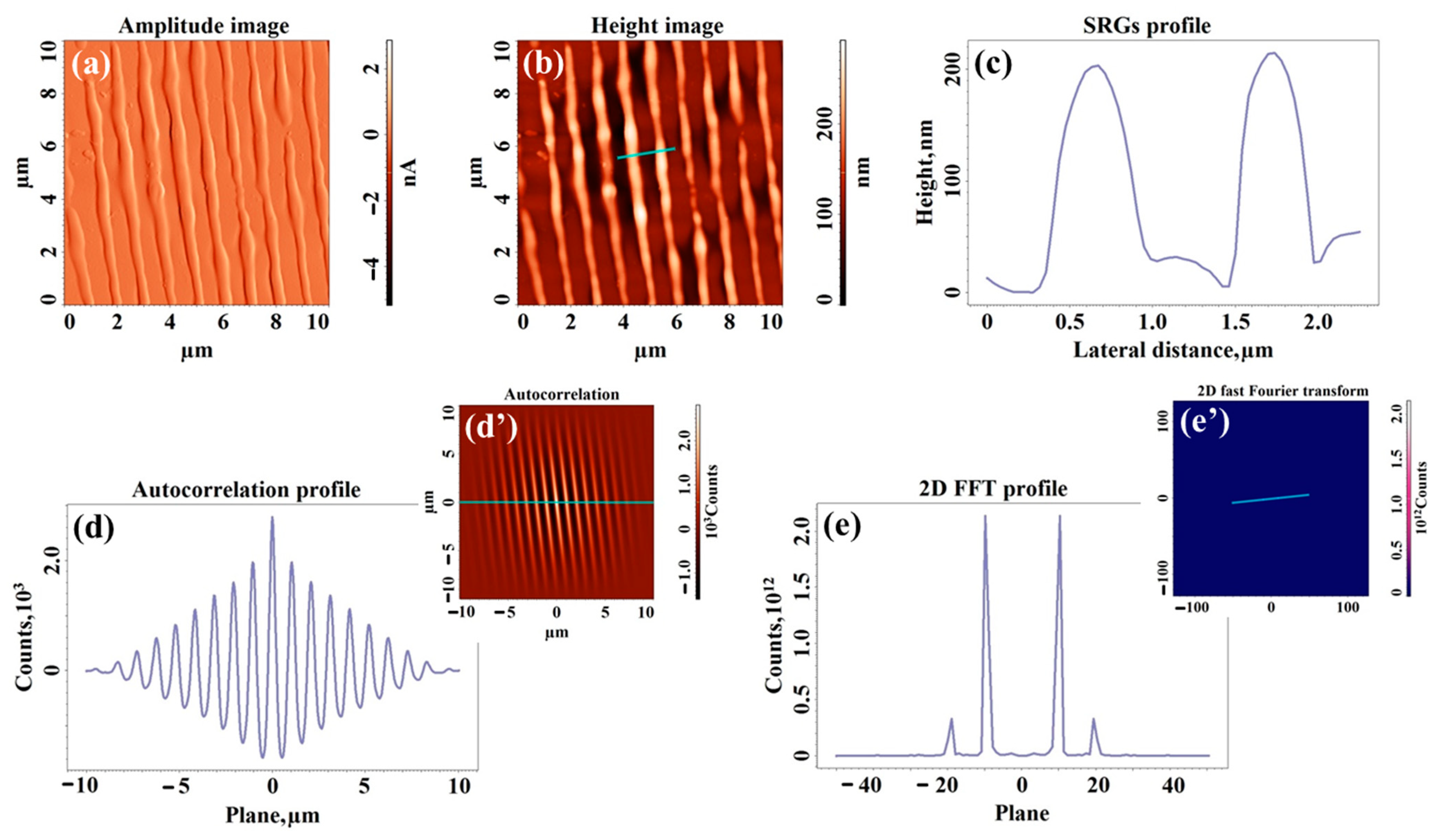
2.3. Morphological Study of UV-Laser Induced Modulations
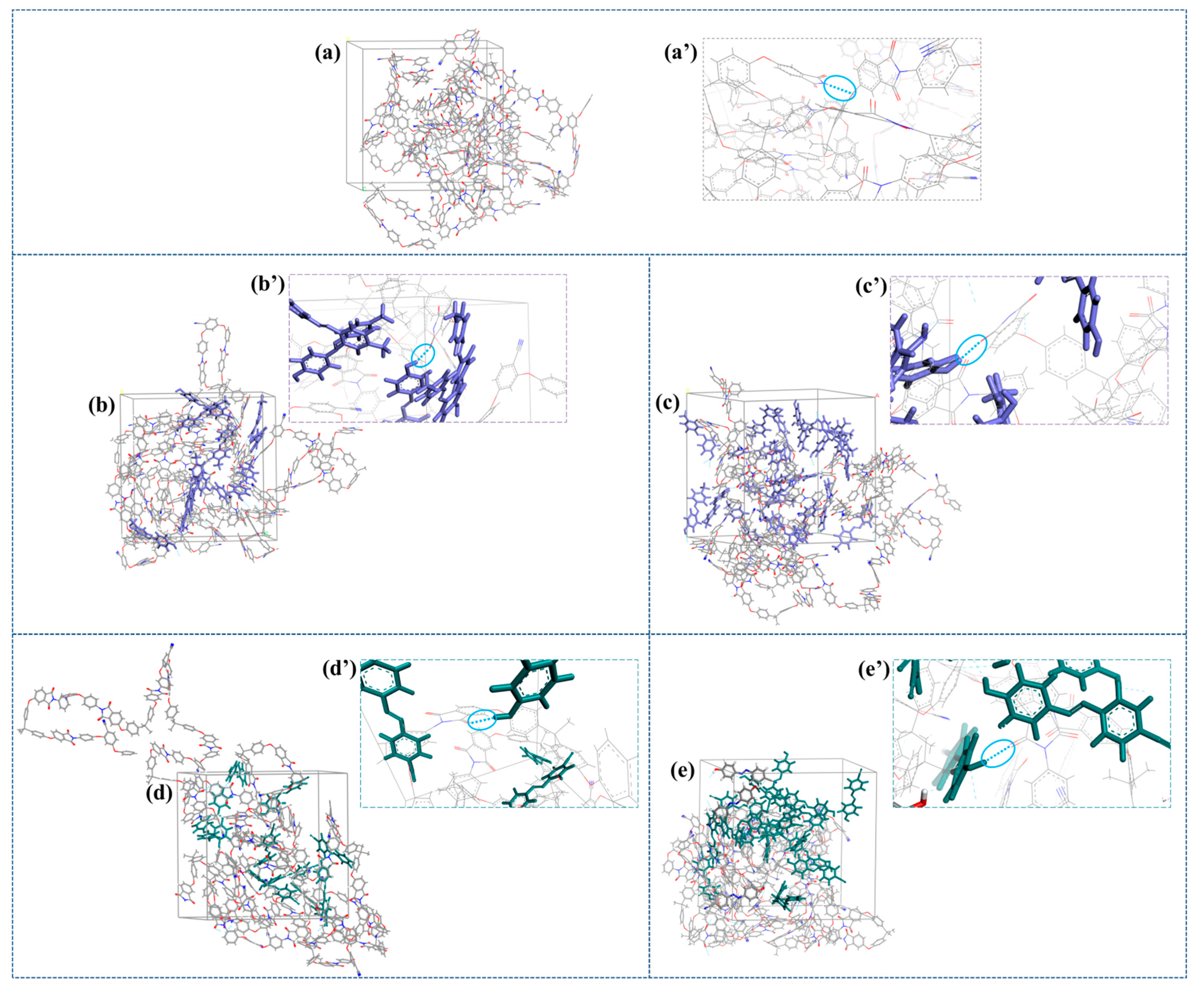
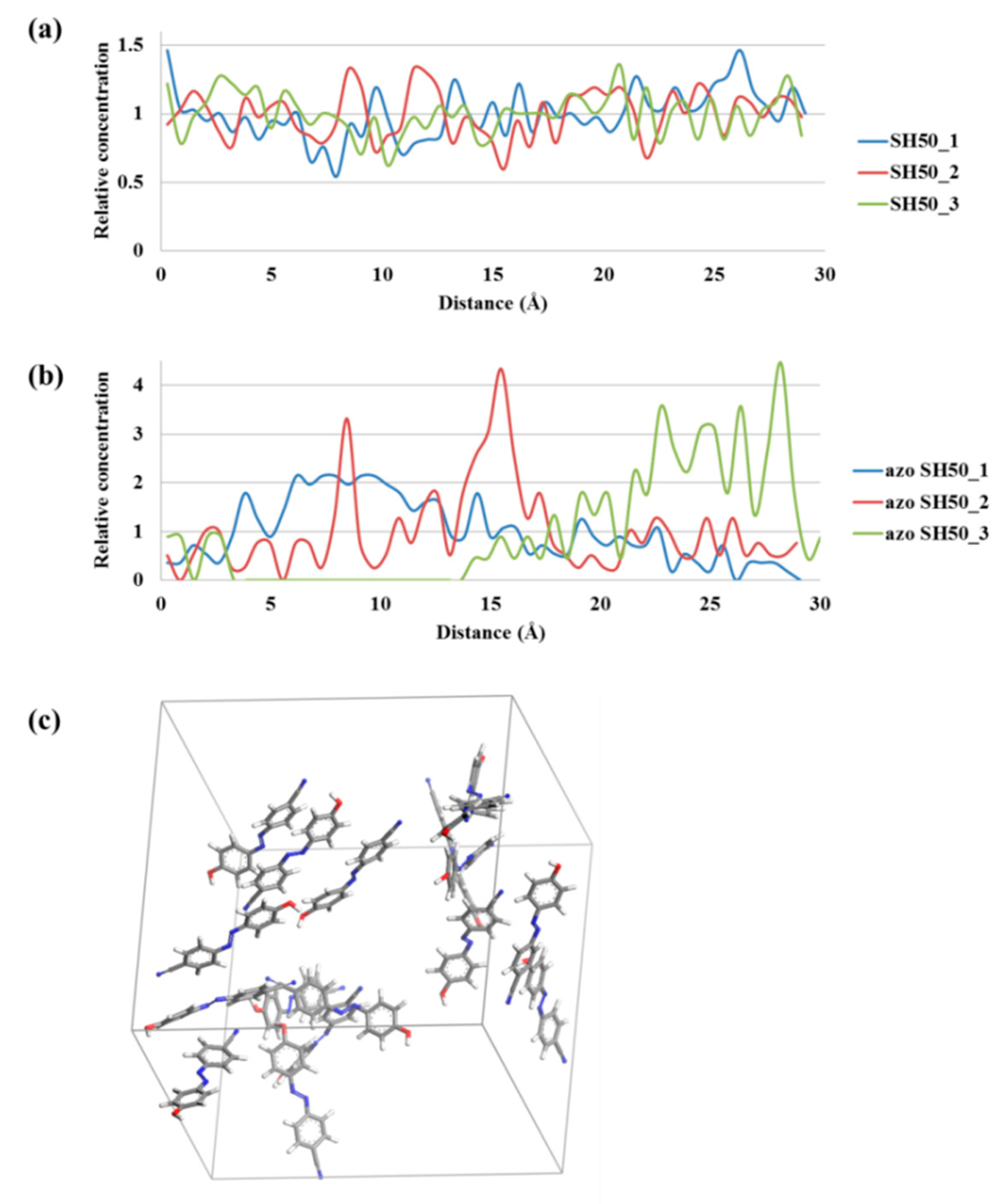
2.4. Molecular Modeling
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Polyamidic Acid (PAA)
3.3. Synthesis of Polyimide-Based Supramolecular Systems
3.4. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B

Appendix C
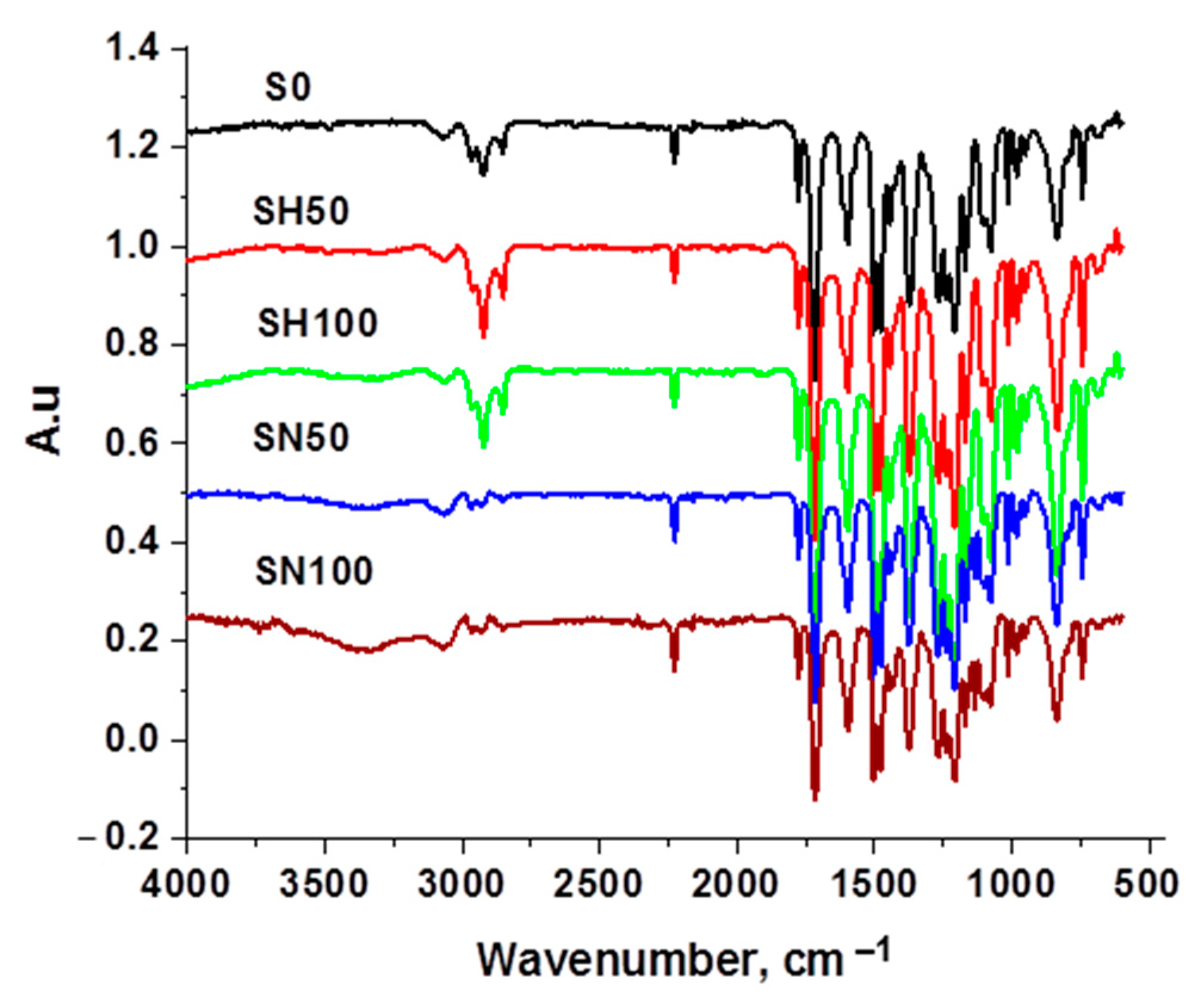

Appendix D



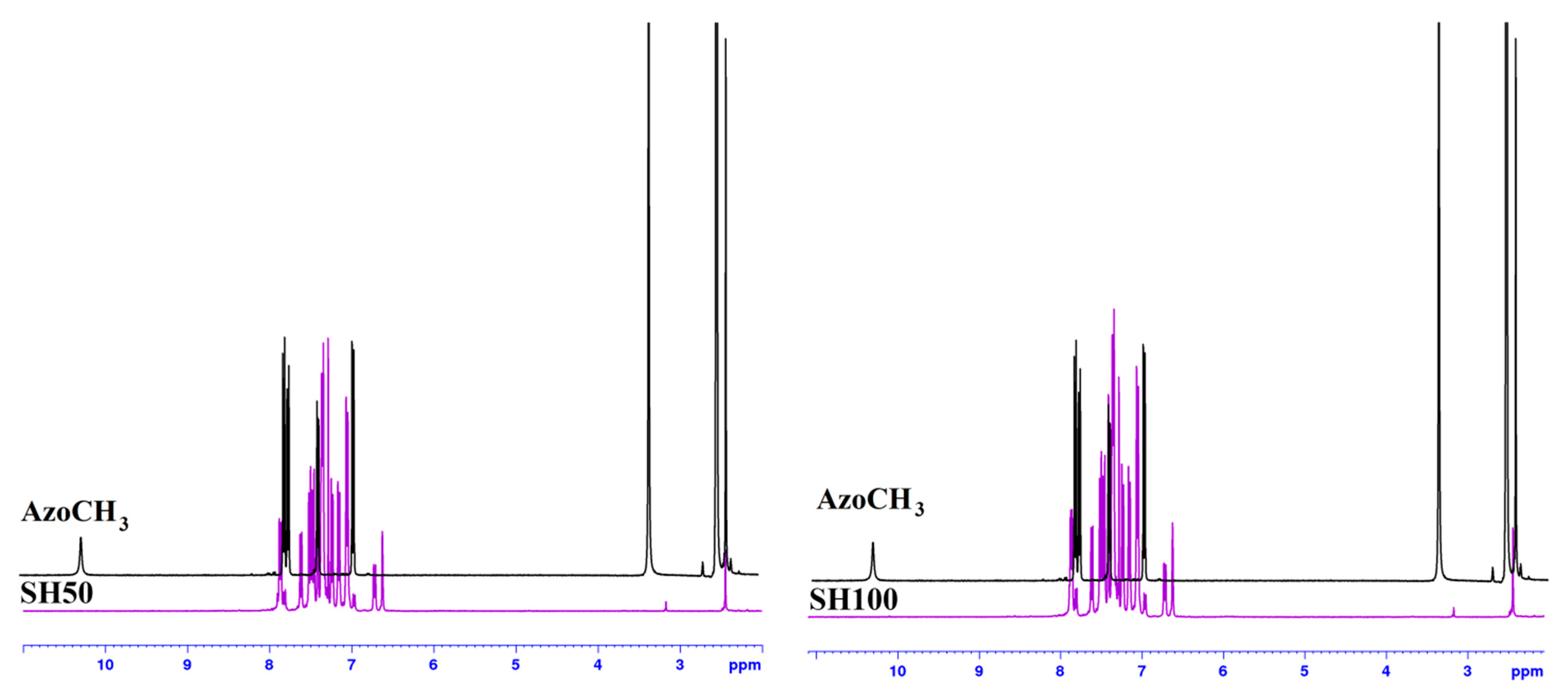
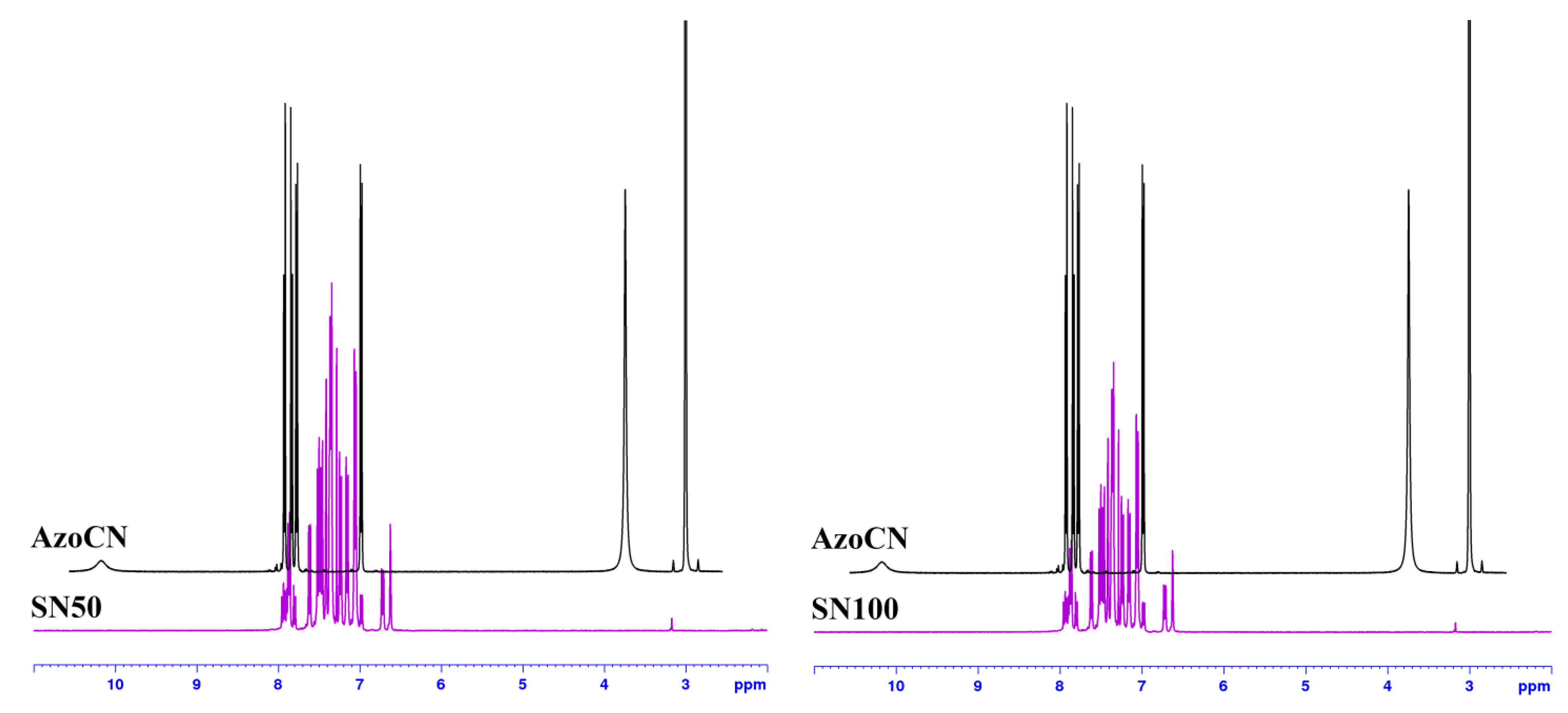
References
- Blom, P.W.M. Polymer Electronics: To Be or Not to Be? Adv. Mater. Technol. 2020, 5, 2000144. [Google Scholar] [CrossRef]
- Onorato, J.; Pakhnyuk, V.; Luscombe, C.K. Structure and design of polymers for durable, stretchable organic electronics. Polym. J. 2017, 49, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; Lim, G.; Park, B.; Reichmanis, E. Control of Molecular Ordering, Alignment, and Charge Transport in Solution-Processed Conjugated Polymer Thin Films. Polymers 2017, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Yang, M.; Zhang, X.; Du, Z.; Fu, Q.; Gao, X.; Gong, J. Control of Polymer Properties by Entanglement: A Review. Macromol. Mater. Eng. 2021, 306, 2100536. [Google Scholar] [CrossRef]
- Huang, B.-Y.; Yu, K.-Y.; Huang, S.-Y.; Kuo, C.-T. The investigation of the two-dimensional surface relief grating on dye-doped polymer film. Opt. Mater. Express 2014, 4, 308. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X. Amphiphilic azo polymers: Molecular engineering, self-assembly and photoresponsive properties. Prog. Polym. Sci. 2013, 38, 271–301. [Google Scholar] [CrossRef]
- Yang, B.; Yu, M.; Yu, H. Azopolymer-Based Nanoimprint Lithography: Recent Developments in Methodology and Applications. Chempluschem 2020, 85, 2166–2176. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Schab-Balcerzak, E. Supramolecular azopolymers based on hydrogen bonds. Polimery 2015, 60, 425–434. [Google Scholar] [CrossRef]
- Cimrová, V.; Neher, D.; Kostromine, S.; Bieringer, T. Optical Anisotropy in Films of Photoaddressable Polymers. Macromolecules 1999, 32, 8496–8503. [Google Scholar] [CrossRef]
- Vapaavuori, J.; Bazuin, C.G.; Priimagi, A. Supramolecular design principles for efficient photoresponsive polymer-azobenzene complexes. J. Mater. Chem. C 2018, 6, 2168–2188. [Google Scholar] [CrossRef]
- Priimagi, A.; Shevchenko, A. Azopolymer-based micro- and nanopatterning for photonic applications. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 163–182. [Google Scholar] [CrossRef]
- Luca, A.R.; Moleavin, I.-A.; Hurduc, N.; Hamel, M.; Rocha, L. Mass transport in low Tg azo-polymers: Effect on the surface relief grating induction and stability of additional side chain groups able to generate physical interactions. Appl. Surf. Sci. 2014, 290, 172–179. [Google Scholar] [CrossRef]
- Oliveira, O.N.; Li, L.; Kumar, J.; Tripathy, S.K. Surface-Relief Gratings on Azobenzene-Containing Films. In Photoreactive Organic Thin Films; Elsevier: Amsterdam, The Netherlands, 2002; p. 429-I. [Google Scholar]
- Kim, K.-H.; Jeong, Y.-C. One-step fabrication of hierarchical multiscale surface relief gratings by holographic lithography of azobenzene polymer. Opt. Express 2018, 26, 5711. [Google Scholar] [CrossRef]
- Sava, I.; Stoica, I.; Topala, I.; Mihaila, I.; Barzic, A.I. Photodesign and fabrication of surface relief gratings on films of polyimide-based supramolecular systems obtained using host-guest strategy. Polymer 2022, 249, 124829. [Google Scholar] [CrossRef]
- Ikeda, T.; Tsutsumi, O. Optical Switching and Image Storage by Means of Azobenzene Liquid-Crystal Films. Science 1995, 268, 1873–1875. [Google Scholar] [CrossRef]
- Siitonen, S.; Pietarinen, J.; Laakkonen, P.; Jefimovs, K.; Kuittinen, M.; Alajoki, T.; Mönkkönen, K.; Pääkkönen, E.J.; Tervonen, A. Replicated Polymer Light Guide Interconnector with Depth Modified Surface Relief Grating Couplers. Opt. Rev. 2007, 14, 304–309. [Google Scholar] [CrossRef]
- Hagen, R.; Bieringer, T. Photoaddressable Polymers for Optical Data Storage. Adv. Mater. 2001, 13, 1805–1810. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, L.-N.; Wei, W.-H.; Guan, W.; Dai, Y.-Z.; Sun, X.-C.; Gao, B.-R. Shape memory of a polymer grating surface fabricated by two-beam interference lithography. Appl. Opt. 2022, 61, 792. [Google Scholar] [CrossRef]
- Atmah, N.R.A.; Caliman, W.R.; Pawlicka, A.; Sabat, R.G.; Nunzi, J.-M. Surface Relief Grating on Chitosan-N,N-dimethyl-4-(2-pyridylazo)aniline Thin Film. Polymers 2022, 14, 791. [Google Scholar] [CrossRef]
- Jelken, J.; Henkel, C.; Santer, S. Formation of half-period surface relief gratings in azobenzene containing polymer films. Appl. Phys. B 2020, 126, 149. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Huang, B.-Y.; Hung, W.-C.; Yu, K.-Y.; Cheng, W.-S.; Kuo, C.-T. Temperature and orientation dependence of surface relief gratings based on dye-doped polymer film with the interface of nematic liquid crystals. Opt. Commun. 2011, 284, 934–937. [Google Scholar] [CrossRef]
- Kitamura, I.; Hara, M.; Nagano, S.; Seki, T. Photo-triggered surface relief formation of polystyrene films based on the Marangoni flow driven by a surface photoresponsive skin layer. Mol. Cryst. Liq. Cryst. 2021, 727, 52–64. [Google Scholar] [CrossRef]
- Ubukata, T.; Seki, T.; Ichimura, K. Surface relief grating in hybrid films composed of azobenzene polymer and liquid crystal molecule. Colloids Surf. A Physicochem. Eng. Asp. 2002, 198–200, 113–117. [Google Scholar] [CrossRef]
- Sava, I.; Burescu, A.; Stoica, I.; Musteata, V.; Cristea, M.; Mihaila, I.; Pohoata, V.; Topala, I. Properties of some azo-copolyimide thin films used in the formation of photoinduced surface relief gratings. RSC Adv. 2015, 5, 10125–10133. [Google Scholar] [CrossRef]
- Barzic, A.I.; Neha Kanwar Rawat, A.K.H. (Eds.) Imidic Polymers and Green Polymer Chemistry New Technology and Developments in Process and Product, 1st ed.; Apple Academic Press: New York, NY, USA, 2021; ISBN 9781774637678. [Google Scholar] [CrossRef]
- Ghosh, M. Polyimides: Fundamentals and Applications; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996; ISBN 0-8247-9466-4. [Google Scholar]
- Chen, J.P.; Labarthet, F.L.; Natansohn, A.; Rochon, P. Highly Stable Optically Induced Birefringence and Holographic Surface Gratings on a New Azocarbazole-Based Polyimide. Macromolecules 1999, 32, 8572–8579. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Lu, Q. Effect of irradiation history on the preparation of laser induced periodic microstructure on polyimide surface. Appl. Surf. Sci. 2007, 253, 3690–3695. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Sobolewska, A.; Stumpe, J.; Hamryszak, L.; Bujak, P. Surface relief gratings in azobenzene supramolecular systems based on polyimides. Opt. Mater. 2012, 35, 155–167. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Konieczkowska, J.; Siwy, M.; Sobolewska, A.; Wojtowicz, M.; Wiacek, M. Comparative studies of polyimides with covalently bonded azo-dyes with their supramolecular analoges: Thermo-optical and photoinduced properties. Opt. Mater. 2014, 36, 892–902. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Flakus, H.; Jarczyk-Jedryka, A.; Konieczkowska, J.; Siwy, M.; Bijak, K.; Sobolewska, A.; Stumpe, J. Photochromic supramolecular azopolyimides based on hydrogen bonds. Opt. Mater. 2015, 47, 501–511. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Janeczek, H.; Małecki, J.; Trzebicka, B.; Szmigiel, D.; Kozanecka-Szmigiel, A.; Schab-Balcerzak, E. Noncovalent azopoly(ester imide)s: Experimental study on structure-property relations and theoretical approach for prediction of glass transition temperature and hydrogen bond formation. Polymer 2017, 113, 53–66. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Bujak, K.; Nocoń, K.; Schab-Balcerzak, E. The large and stable photomechanical effect in the glassy guest-host azopolymers. Dye. Pigment. 2019, 171, 107659. [Google Scholar] [CrossRef]
- Bujak, K.; Orlikowska, H.; Sobolewska, A.; Schab-Balcerzak, E.; Janeczek, H.; Bartkiewicz, S.; Konieczkowska, J. Azobenzene vs. azopyridine and matrix molar masses effect on photoinduced phenomena. Eur. Polym. J. 2019, 115, 173–184. [Google Scholar] [CrossRef]
- Stoica, I.; Sava, I.; Epure, E.-L.; Tiron, V.; Konieczkowska, J.; Schab-Balcerzak, E. Advanced morphological, statistical and molecular simulations analysis of laser-induced micro/nano multiscale surface relief gratings. Surf. Interf. 2022, 29, 101743. [Google Scholar] [CrossRef]
- Stoica, I.; Epure, E.-L.; Constantin, C.-P.; Damaceanu, M.-D.; Ursu, E.-L.; Mihaila, I.; Sava, I. Evaluation of Local Mechanical and Chemical Properties via AFM as a Tool for Understanding the Formation Mechanism of Pulsed UV Laser-Nanoinduced Patterns on Azo-Naphthalene-Based Polyimide Films. Nanomaterials 2021, 11, 812. [Google Scholar] [CrossRef]
- Bujak, K.; Sava, I.; Stoica, I.; Tiron, V.; Topala, I.; Węgłowski, R.; Schab-Balcerzak, E.; Konieczkowska, J. Photoinduced properties of “T-type” polyimides with azobenzene or azopyridine moieties. Eur. Polym. J. 2020, 126, 109563. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Rusu, R.-D.; Olaru, M.; Stoica, I.; Bruma, M. Nanostructured polyimide films by UV excimer laser irradiation. Rom. J. Inf. Sci. Technol. 2010, 13, 368–377. [Google Scholar]
- Damaceanu, M.-D.; Rusu, R.-D.; Olaru, M.A.; Timpu, D.; Bruma, M. KrF Pulsed Laser Ablation of Thin Films Made from Fluorinated Heterocyclic Poly(naphthyl-imide)s. Microsc. Microanal. 2012, 18, 545–557. [Google Scholar] [CrossRef]
- Obilor, A.F.; Pacella, M.; Wilson, A.; Silberschmidt, V.V. Micro-texturing of polymer surfaces using lasers: A review. Int. J. Adv. Manuf. Technol. 2022, 120, 103–135. [Google Scholar] [CrossRef]
- Sdr Parameters and its Applications in Characterizing Surface Data. Available online: https://www.azonano.com/article.aspx?ArticleID=5137 (accessed on 31 October 2022).
- ISO 25178-2:2012; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/42785.html (accessed on 31 October 2022).
- Lagugné Labarthet, F.; Buffeteau, T.; Sourisseau, C. Molecular Orientations in Azopolymer Holographic Diffraction Gratings as Studied by Raman Confocal Microspectroscopy. J. Phys. Chem. B 1998, 102, 5754–5765. [Google Scholar] [CrossRef]
- Hurduc, N.; Donose, B.C.; Macovei, A.; Paius, C.; Ibanescu, C.; Scutaru, D.; Hamel, M.; Branza-Nichita, N.; Rocha, L. Direct observation of athermal photofluidisation in azo-polymer films. Soft Matter 2014, 10, 4640–4647. [Google Scholar] [CrossRef]
- Damian, V.; Resmerita, E.; Stoica, I.; Ibanescu, C.; Sacarescu, L.; Rocha, L.; Hurduc, N. Surface relief gratings induced by pulsed laser irradiation in low glass-transition temperature azopolysiloxanes. J. Appl. Polym. Sci. 2014, 131, 41015. [Google Scholar] [CrossRef] [Green Version]
- Heath, D.R.; Wirth, J.G. Aminophenoxy Benzonitriles. US Patent 3,763,211, 2 October 1973. [Google Scholar]
- Bruma, M.; Schulz, B.; Mercer, F.W. Polyamide Copolymers Containing Hexafluoroisopropylene Groups. J. Macromol. Sci. Part A 1995, 32, 259–286. [Google Scholar] [CrossRef]
- Saxena, A.; Prabhakaran, P.; Rao, V.L.; Ninan, K. Synthesis and characterization of polyamides and poly(amide-imide)s derived from 2,6-bis(3-aminophenoxy)benzonitrile or 2,6-bis(4-aminophenoxy)benzonitrile. Polym. Int. 2005, 54, 544–552. [Google Scholar] [CrossRef]
- Helmut Ringsdorf, H.-W.S. Electro-optical effects of azo dye containing liquid crystalline copolymers. Die Makromol. Chemie 1984, 185, 1327–1334. [Google Scholar] [CrossRef]
- Sava, I.; Köpnick, T. Synthesis and characterization of new diamines containing side substituted azobenzene groups. Rev. Roum. Chim. 2014, 59, 585–592. [Google Scholar]
- Materials Studio 4.0. DMol3, Synthia, Discover, Forcite and Amorphous Cell Module; Accelrys Software Inc.: San Diego, CA, USA, 2005. [Google Scholar]
- Singh, M.; Sethi, S.K.; Manik, G. Pressure-sensitive adhesives based on acrylated epoxidized linseed oil: A computational approach. Int. J. Adhes. Adhes. 2022, 112, 103031. [Google Scholar] [CrossRef]
- Bujak, K.; Orlikowska, H.; Małecki, J.G.; Schab-Balcerzak, E.; Bartkiewicz, S.; Bogucki, J.; Sobolewska, A.; Konieczkowska, J. Fast dark cis-trans isomerization of azopyridine derivatives in comparison to their azobenzene analogues: Experimental and computational study. Dye. Pigment. 2019, 160, 654–662. [Google Scholar] [CrossRef]
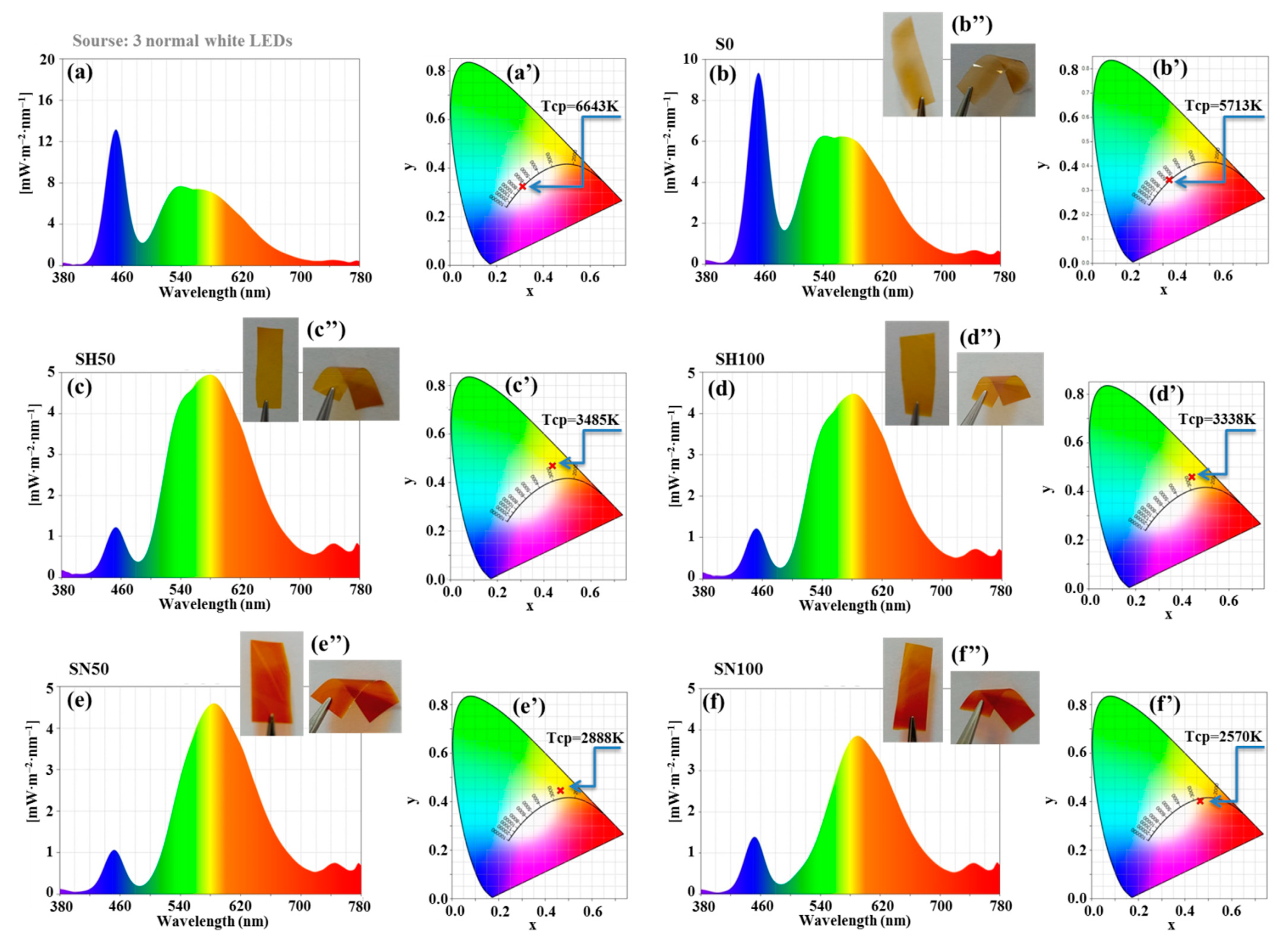




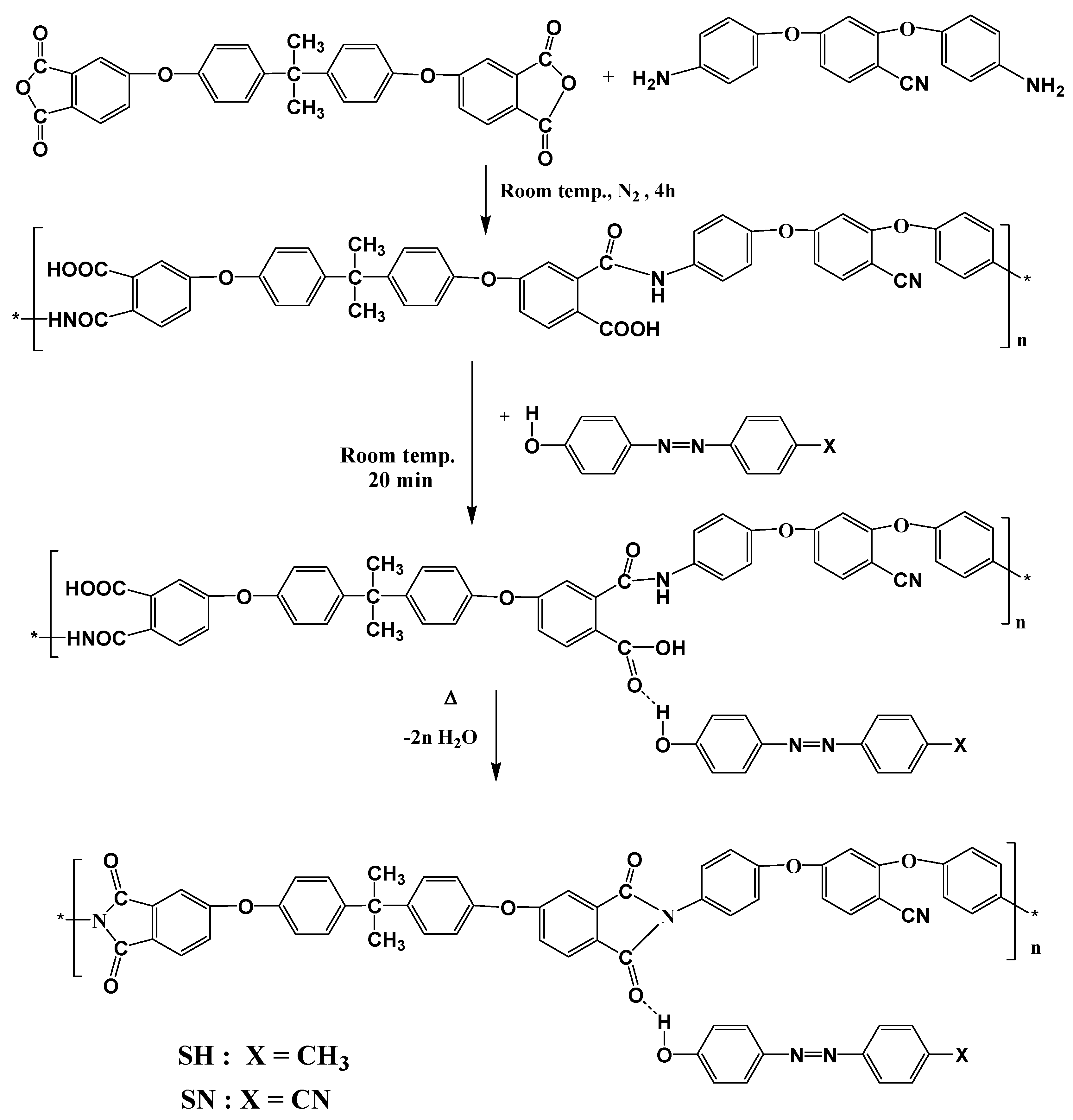
| Sample | λpk (nm) | Tcp (K) | Ev (lx) | T (%) |
|---|---|---|---|---|
| three normal light LEDs | 486 | 6643 | 465 | - |
| S0 | 452 | 5713 | 388 | 83 |
| SH50 | 579 | 3485 | 278 | 59 |
| SH100 | 582 | 3338 | 246 | 52 |
| SN50 | 585 | 2888 | 224 | 48 |
| SN100 | 590 | 2570 | 170 | 36 |
| Sample | Tonset, °C | T5, °C | Tmax, °C |
|---|---|---|---|
| S0 | 512 | - | 546 |
| SH100 | 228 | 308 | 537 |
| SN100 | 247 | 308 | 532 |
| Sample | Type of Structuration | H (nm) | W (nm) | Sq (nm) | Sdr (%) | Stdi | Str |
|---|---|---|---|---|---|---|---|
| S0 | Cone-like structures | 45 ± 15 | 667 ± 10 | 6.8 | 0.366 | 0.556 | 0.483 |
| SH50 | Surface relief gratings | 53 ± 2 | 592 ± 55 | 11.7 | 0.672 | 0.286 | 0.074 |
| SH100 | Surface relief gratings | 99 ± 6 | 532 ± 27 | 18.7 | 2.041 | 0.179 | 0.052 |
| SN50 | Surface relief gratings | 88 ± 3 | 670 ± 5 | 22.1 | 1.752 | 0.189 | 0.078 |
| SN100 | Surface relief gratings | 198 ± 11 | 672 ± 7 | 52.9 | 8.866 | 0.162 | 0.076 |
| System | S0 | SH50_1 | SH50_2 | SH50_3 | SN50_1 | SN50_2 | SN50_3 |
|---|---|---|---|---|---|---|---|
| % cis | - | 0 | 30 | 80 | 0 | 20 | 80 |
| Density (g/cm3) | 1.235 | 1.186 | 1.207 | 1.218 | 1.238 | 1.229 | 1.232 |
| System | SH100_1 | SH100_2 | SH100_3 | SN100_1 | SN100_2 | SN100_3 | |
| % cis | 0 | 30 | 80 | 40 | 0 | 75 | |
| Density (g/cm3) | 1.201 | 1.219 | 1.205 | 1.223 | 1.215 | 1.227 |
| System | S0 | SH50_1 | SH50_2 | SH50_3 | SN50_1 | SN50_2 | SN50_3 |
|---|---|---|---|---|---|---|---|
| MSD | 1.71 | 1.03 | 1.46 | 1.84 | 0.73 | 1.27 | 2.94 |
| System | SH100_1 | SH100_2 | SH100_3 | SN100_1 | SN100_2 | SN100_3 | |
| MSD | 1.13 | 0.91 | 1.25 | 0.80 | 1.21 | 3.11 |
| Sample Code | Polyamidic Acid mol (g) | Azo Monomer AzoCH3 mol (g) | Azo Monomer AzoCN mol (g) |
|---|---|---|---|
| SH50 | 0.9768 × 10−4 (0.0818) | 0.4889 × 10−4 (0.010354) | - |
| SH100 | 0.9768 × 10−4 (0.0818) | 0.9768 × 10−4 (0.020708) | - |
| SN50 | 0.9768 × 10−4 (0.0818) | - | 0.4889 × 10−4 (0.01089) |
| SN100 | 0.9768 × 10−4 (0.0818) | - | 0.9768 × 10−4 (0.02178) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, I.; Epure, E.-L.; Barzic, A.I.; Mihaila, I.; Constantin, C.-P.; Sava, I. The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics. Int. J. Mol. Sci. 2022, 23, 15223. https://doi.org/10.3390/ijms232315223
Stoica I, Epure E-L, Barzic AI, Mihaila I, Constantin C-P, Sava I. The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics. International Journal of Molecular Sciences. 2022; 23(23):15223. https://doi.org/10.3390/ijms232315223
Chicago/Turabian StyleStoica, Iuliana, Elena-Luiza Epure, Andreea Irina Barzic, Ilarion Mihaila, Catalin-Paul Constantin, and Ion Sava. 2022. "The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics" International Journal of Molecular Sciences 23, no. 23: 15223. https://doi.org/10.3390/ijms232315223
APA StyleStoica, I., Epure, E.-L., Barzic, A. I., Mihaila, I., Constantin, C.-P., & Sava, I. (2022). The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics. International Journal of Molecular Sciences, 23(23), 15223. https://doi.org/10.3390/ijms232315223