Evolution of Treatment in Advanced Cholangiocarcinoma: Old and New towards Precision Oncology
Abstract
1. Introduction
2. The Chemotherapy Treatment for Advanced CCA
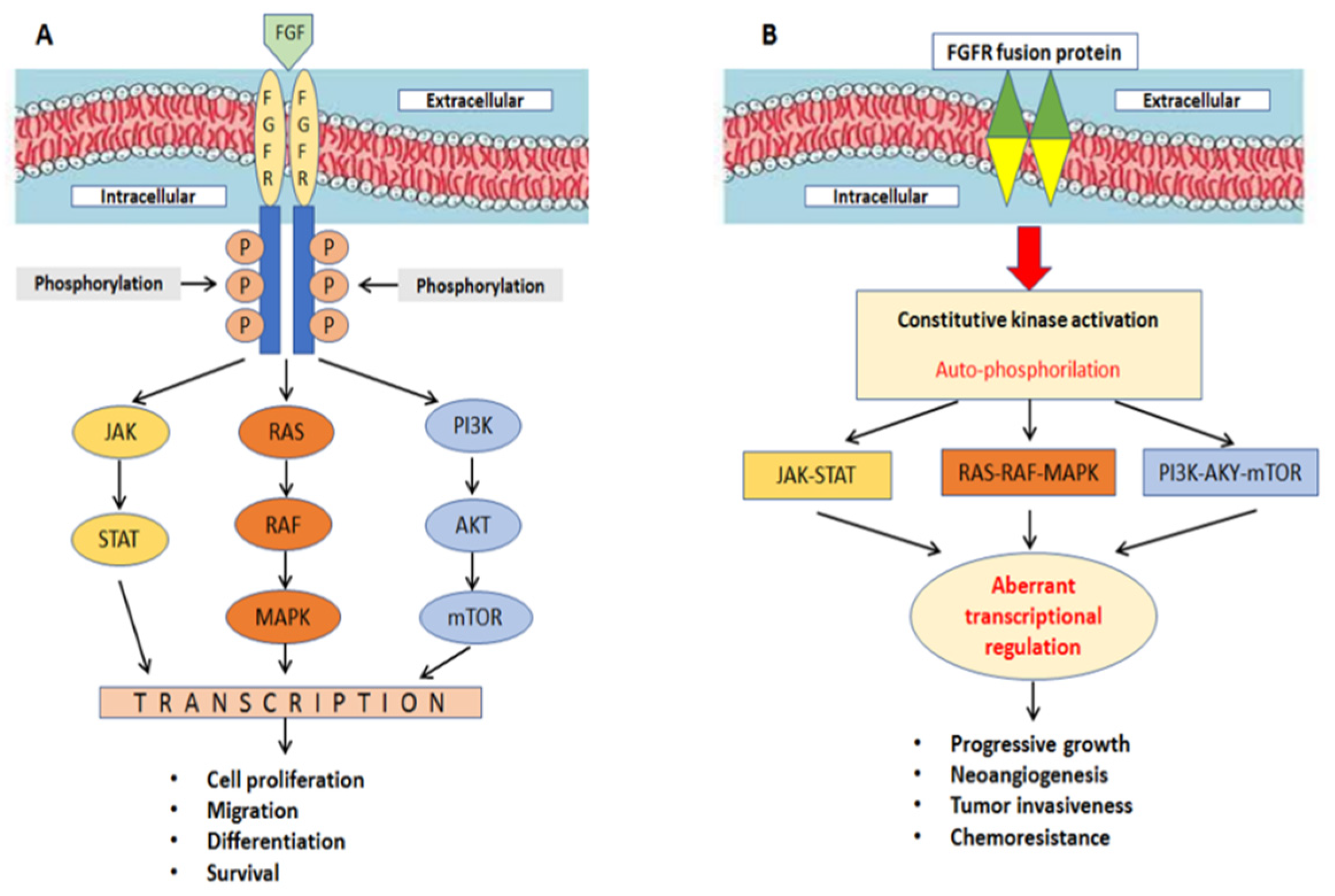
3. Targeting FGFRs in CCA
4. Targeting IDH-1/2 in CCA
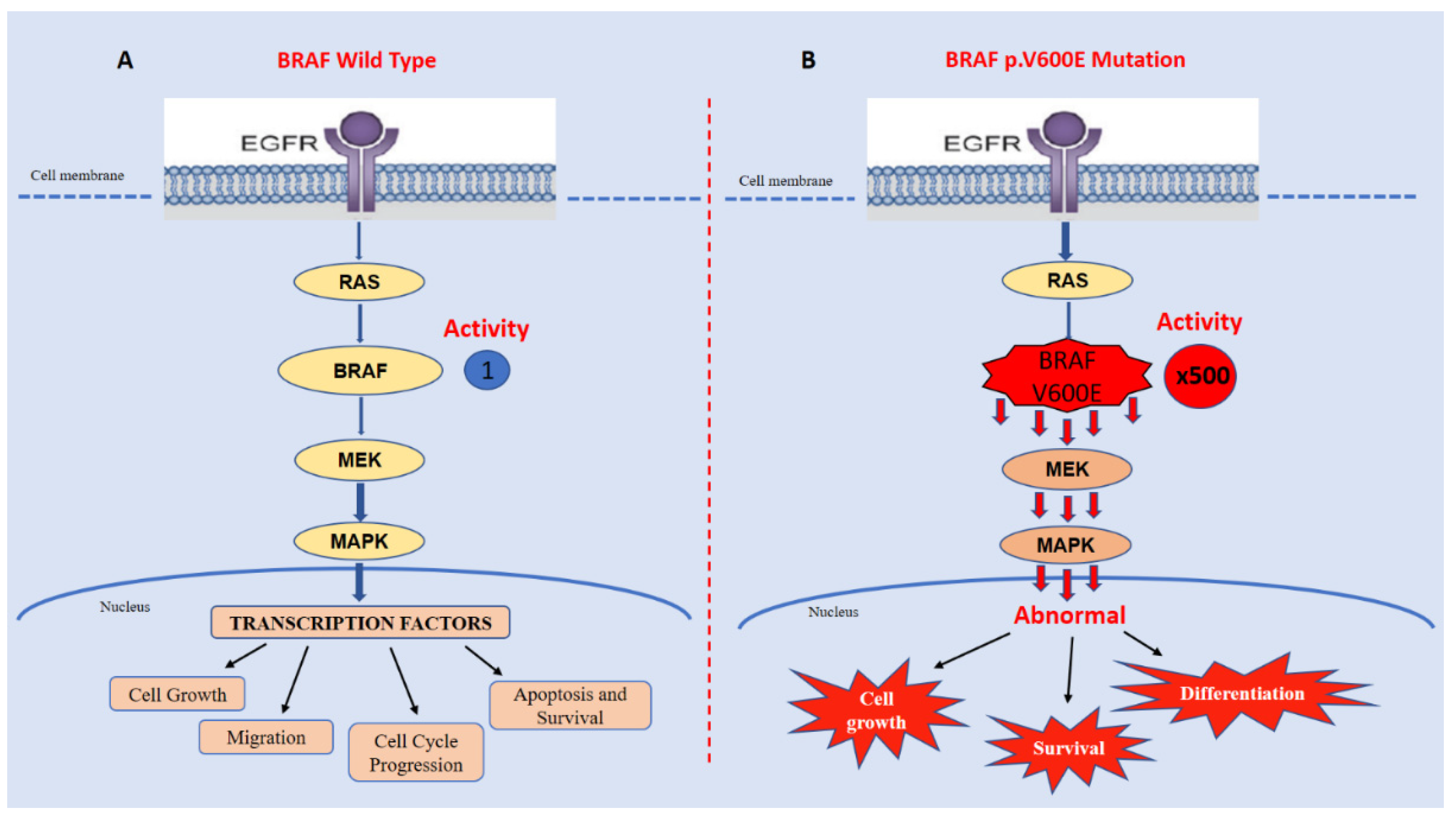
5. Inhibition of BRAF p.V600E in CCA
6. NTRK Fusions: Role in CCAs
7. HER2 Amplification in CCA
8. Overview of Target-Oriented Drugs in CCA
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzo, A.; Brandi, G. First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years After the ABC-02 Trial: “And Yet It Moves!”. Cancer Treat. Res. Commun. 2021, 27, 100335. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Woods, E.; Tsung, A.; Mittra, A. Biliary Tract Cancers: Treatment Updates and Future Directions in the Era of Precision Medicine and Immuno-Oncology. Front. Oncol. 2021, 11, 768009. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Raggi, C.; Fiaccadori, K.; Pastore, M.; Correnti, M.; Piombanti, B.; Forti, E.; Navari, N.; Abbadessa, G.; Hall, T.; Destro, A.; et al. Antitumor Activity of a Novel Fibroblast Growth Factor Receptor Inhibitor for Intrahepatic Cholangiocarcinoma. Am. J. Pathol. 2019, 189, 2090–2101. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Eswarakumar, V.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef]
- Presta, M.; Chiodelli, P.; Giacomini, A.; Rusnati, M.; Ronca, R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol. Ther. 2017, 179, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef]
- Jain, A.; Borad, M.J.; Kelley, R.K.; Wang, Y.; Abdel-Wahab, R.; Meric-Bernstam, F.; Baggerly, K.A.; Kaseb, A.O.; Al-Shamsi, H.O.; Ahn, D.H.; et al. Cholangiocarcinoma With FGFR Genetic Aberrations: A Unique Clinical Phenotype. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Losic, B.; Moeini, A.; Cabellos, L.; Hao, K.; Revill, K.; Bonal, D.M.; Miltiadous, O.; Zhang, Z.; Hoshida, Y.; et al. Massive parallel sequencing uncovers actionable FGFR2–PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat. Commun. 2015, 6, 6087. [Google Scholar] [CrossRef]
- Liu, P.C.C.; Koblish, H.; Wu, L.; Bowman, K.; Diamond, S.; DiMatteo, D.; Zhang, Y.; Hansbury, M.; Rupar, M.; Wen, X.; et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS ONE 2020, 15, e0231877. [Google Scholar] [CrossRef]
- Pu, X.; Ye, Q.; Cai, J.; Yang, X.; Fu, Y.; Fan, X.; Wu, H.; Chen, J.; Qiu, Y.; Yue, S. Typing FGFR2 translocation determines the response to targeted therapy of intrahepatic cholangiocarcinomas. Cell Death Dis. 2021, 12, 256. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, X.; Xu, B.; Wang, Y. [Ponatinib inhibits growth of patient-derived xenograft of cholangiocarcinoma expressing FGFR2-CCDC6 fusion protein in nude mice]. J. South. Med. Univ. 2020, 40, 1448–1456. (In Chinese) [Google Scholar]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Chen, C.-P.; Wu, C.-E. Precision Medicine in Cholangiocarcinoma: Past, Present, and Future. Life 2022, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Futibatinib, an investigational agent for the treatment of intrahepatic cholangiocarcinoma: Evidence to date and future perspectives. Expert Opin. Investig. Drugs 2021, 30, 317–324. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; He, Y.; Soni, N.; et al. FOENIX-CCA2: A phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J. Clin. Oncol. 2020, 38 (Suppl. 15), 108. [Google Scholar] [CrossRef]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a Major Antioxidant: When, What for and Why It Fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef]
- Bledea, R.; Vasudevaraja, V.; Patel, S.; Stafford, J.; Serrano, J.; Esposito, G.; Tredwin, L.M.; Goodman, N.; Kloetgen, A.; Golfinos, J.G.; et al. Functional and topographic effects on DNA methylation in IDH1/2 mutant cancers. Sci. Rep. 2019, 9, 16830. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744, Erratum in Nature 2009, 465, 966. [Google Scholar] [CrossRef]
- Han, C.-J.; Zheng, J.-Y.; Sun, L.; Yang, H.-C.; Cao, Z.-Q.; Zhang, X.-H.; Zheng, L.-T.; Zhen, X.-C. The oncometabolite 2-hydroxyglutarate inhibits microglial activation via the AMPK/mTOR/NF-κB pathway. Acta Pharmacol. Sin. 2019, 40, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Sesanto, R.; Kuehn, J.F.; Barber, D.L.; White, K.A. Low pH Facilitates Heterodimerization of Mutant Isocitrate Dehydrogenase IDH1-R132H and Promotes Production of 2-Hydroxyglutarate. Biochemistry 2021, 60, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Khurshed, M.; Molenaar, R.; van Linde, M.; Mathôt, R.; Struys, E.; van Wezel, T.; van Noorden, C.; Klümpen, H.-J.; Bovée, J.; Wilmink, J. A Phase Ib Clinical Trial of Metformin and Chloroquine in Patients with IDH1-Mutated Solid Tumors. Cancers 2021, 13, 2474. [Google Scholar] [CrossRef] [PubMed]
- Crispo, F.; Pietrafesa, M.; Condelli, V.; Maddalena, F.; Bruno, G.; Piscazzi, A.; Sgambato, A.; Esposito, F.; Landriscina, M. IDH1 Targeting as a New Potential Option for Intrahepatic Cholangiocarcinoma Treatment—Current State and Future Perspectives. Molecules 2020, 25, 3754. [Google Scholar] [CrossRef]
- Saha, S.K.; Parachoniak, C.A.; Ghanta, K.S.; Fitamant, J.; Ross, K.N.; Najem, M.S.; Gurumurthy, S.; Akbay, E.A.; Sia, D.; Cornella, H.; et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature 2014, 513, 110–114. [Google Scholar] [CrossRef]
- Molenaar, R.J.; Maciejewski, J.P.; Wilmink, J.W.; Van Noorden, C.J.F. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene 2018, 37, 1949–1960. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Emerging molecular therapeutic targets for cholangiocarcinoma. J. Hepatol. 2017, 67, 632–644. [Google Scholar] [CrossRef]
- Cho, Y.S.; Levell, J.R.; Liu, G.; Caferro, T.; Sutton, J.; Shafer, C.M.; Costales, A.; Manning, J.R.; Zhao, Q.; Sendzik, M.; et al. Discovery and Evaluation of Clinical Candidate IDH305, a Brain Penetrant Mutant IDH1 Inhibitor. ACS Med. Chem. Lett. 2017, 8, 1116–1121. [Google Scholar] [CrossRef]
- Caravella, J.A.; Lin, J.; Diebold, R.B.; Campbell, A.-M.; Ericsson, A.; Gustafson, G.; Wang, Z.; Castro, J.; Clarke, A.; Gotur, D.; et al. Structure-Based Design and Identification of FT-2102 (Olutasidenib), a Potent Mutant-Selective IDH1 Inhibitor. J. Med. Chem. 2020, 63, 1612–1623. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Chan, M.T.; Wu, W.K.K. The role of microRNAs in intrahepatic cholangiocarcinoma. J. Cell. Mol. Med. 2017, 21, 177–184. [Google Scholar] [CrossRef]
- Sarantis, P.; Tzanetatou, E.D.; Ioakeimidou, E.; Vallilas, C.; Androutsakos, T.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Papavassiliou, A.G.; Karamouzis, M.V. Cholangiocarcinoma: The role of genetic and epigenetic factors; current and prospective treatment with checkpoint inhibitors and immunotherapy. Am. J. Transl. Res. 2021, 13, 13246–13260. [Google Scholar] [PubMed]
- Iyer, P.; Chen, M.-H.; Goyal, L.; Denlinger, C.S. Targets for therapy in biliary tract cancers: The new horizon of personalized medicine. Chin. Clin. Oncol. 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Baik, C.; Kirkwood, J.M. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends Cancer 2020, 6, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-J.; Nussinov, R. Allosteric activation of RAF in the MAPK signaling pathway. Curr. Opin. Struct. Biol. 2018, 53, 100–106. [Google Scholar] [CrossRef]
- Rauch, J.; Kolch, W. Spatial regulation of ARAF controls the MST2-Hippo pathway. Small GTPases 2017, 10, 243–248. [Google Scholar] [CrossRef]
- Tas, F.; Erturk, K. BRAF V600E mutation as a prognostic factor in cutaneous melanoma patients. Dermatol. Ther. 2020, 33, e13270. [Google Scholar] [CrossRef]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef]
- Eng, C. BRAF Mutation in Colorectal Cancer: An Enigmatic Target. J. Clin. Oncol. 2021, 39, 259–261. [Google Scholar] [CrossRef]
- Grothey, A.; Fakih, M.; Tabernero, J. Management of BRAF-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann. Oncol. 2021, 32, 959–967. [Google Scholar] [CrossRef]
- Robertson, S.; Hyder, O.; Dodson, R.; Nayar, S.K.; Poling, J.; Beierl, K.; Eshleman, J.R.; Lin, M.-T.; Pawlik, T.M.; Anders, R.A. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum. Pathol. 2013, 44, 2768–2773. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Lavingia, V.; Fakih, M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma—2 case reports and a brief review. J. Gastrointest. Oncol. 2016, 6, E98–E102. [Google Scholar] [CrossRef] [PubMed]
- Bernocchi, O.; Sirico, M.; Corona, S.; Strina, C.; Milani, M.; Cappelletti, M.; Ferrero, G.; Ziglioli, N.; Cervoni, V.; Macchiavelli, A.; et al. Tumor Type Agnostic Therapy Carrying BRAF Mutation: Case Reports and Review of Literature. Pharmaceuticals 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.N.; Schlaepfer, D.D. Adaptive Resistance to Chemotherapy, A Multi–FAK-torial Linkage. Mol. Cancer Ther. 2018, 17, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gao, C.; Gao, X.; Zhang, D.H.; Kuan, S.-F.; Burns, T.F.; Hu, J. Wnt/β-Catenin Pathway Activation Mediates Adaptive Resistance to BRAF Inhibition in Colorectal Cancer. Mol. Cancer Ther. 2018, 17, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, J.; Árokszállási, A.; András, C.; Balogh, I.; Béres, E.; Déri, J.; Peták, I.; Jánváry, L.; Horváth, Z. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: Dramatic clinical and radiological response with a confusing synchronic new liver lesion. J. Gastrointest. Oncol. 2017, 8, E32–E38. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Hsiao, S.J.; Zehir, A.; Sireci, A.N.; Aisner, D.L. Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy. J. Mol. Diagn. 2019, 21, 553–571. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016, 1, e000023. [Google Scholar] [CrossRef]
- Vaishnavi, A.; Le, A.T.; Doebele, R.C. TRKing Down an Old Oncogene in a New Era of Targeted Therapy. Cancer Discov. 2015, 5, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; Lassen, U.N. TRK Inhibition: A New Tumor-Agnostic Treatment Strategy. Target. Oncol. 2018, 13, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Woods, E.; Le, D.; Jakka, B.K.; Manne, A. Changing Landscape of Systemic Therapy in Biliary Tract Cancer. Cancers 2022, 14, 2137. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Gay, L.; Al-Rohil, R.; Rand, J.V.; Jones, D.M.; Lee, H.J.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; et al. New Routes to Targeted Therapy of Intrahepatic Cholangiocarcinomas Revealed by Next-Generation Sequencing. Oncologist 2014, 19, 235–242. [Google Scholar] [CrossRef]
- Solomon, J.P.; Benayed, R.; Hechtman, J.F.; Ladanyi, M. Identifying patients with NTRK fusion cancer. Ann. Oncol. 2019, 30 (Suppl. 8), viii16–viii22. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Albagoush, S.A.; Limaiem, F. HER2; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.N.; Skariah, S.; Denlinger, C.S.; Astsaturov, I. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin. Investig. Drugs 2017, 26, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Rausch, M.; Rössler, S.; Albrecht, M.; Braun, J.D.; Geissler, V.; Mehrabi, A.; Vogel, M.N.; Pathil-Warth, A.; Mechtersheimer, G.; et al. HER2 gene (ERBB2) amplification is a rare event in non-liver-fluke associated cholangiocarcinogenesis. BMC Cancer 2019, 19, 1191. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Chung, J.-Y.; Hewitt, S.; Yu, E.; Hong, S.-M. HER3 overexpression is a prognostic indicator of extrahepatic cholangiocarcinoma. Virchows Arch. 2012, 461, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Ogo, Y.; Nio, Y.; Yano, S.; Toga, T.; Koike, M.; Hashimoto, K.; Itakura, M.; Maruyama, R. Immunohistochemical expression of HER-1 and HER-2 in extrahepatic biliary carcinoma. Anticancer Res. 2006, 26, 763–770. [Google Scholar] [PubMed]
- Wang, W.; Zhang, J.; Zhan, X.; Lin, T.; Yang, M.; Hu, J.; Han, B.; Hu, S. SOX4 is associated with poor prognosis in cholangiocarcinoma. Biochem. Biophys. Res. Commun. 2014, 452, 614–621. [Google Scholar] [CrossRef]
- Fernandes, V.T.O.; Silva, M.B.; Begnami, M.D.; Saito, A. Prognosis of HER2 expression in cholangiocarcinoma when evaluated using gastric cancer methodology of immunohistochemistry. J. Clin. Oncol. 2015, 33, e15203. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Wang, C.; Wang, L.; Yang, M.; Qi, M.; Su, H.; Sun, X.; Liu, Z.; Zhang, J.; et al. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol. Rep. 2014, 32, 700–708. [Google Scholar] [CrossRef]
- Javle, M.M.; Hainsworth, J.D.; Swanton, C.; Burris, H.A.; Kurzrock, R.; Sweeney, C.; Meric-Bernstam, F.; Spigel, D.R.; Bose, R.; Guo, S.; et al. Pertuzumab + trastuzumab for HER2-positive metastatic biliary cancer: Preliminary data from MyPathway. J. Clin. Oncol. 2017, 35, 402. [Google Scholar] [CrossRef]
- Nam, A.-R.; Kim, J.-W.; Cha, Y.; Ha, H.; Park, J.E.; Bang, J.-H.; Jin, M.H.; Lee, K.-H.; Kim, T.-Y.; Han, S.-W.; et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget 2016, 7, 58007–58021. [Google Scholar] [CrossRef]
- Law, L.Y. Dramatic Response to Trastuzumab and Paclitaxel in a Patient With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Cholangiocarcinoma. J. Clin. Oncol. 2012, 30, e271–e273. [Google Scholar] [CrossRef] [PubMed]
- Gajria, D.; Chandarlapaty, S. HER2-amplified breast cancer: Mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev. Anticancer. Ther. 2011, 11, 263–275. [Google Scholar] [CrossRef]
- Yang, W.; Sun, Y. Promising Molecular Targets for the Targeted Therapy of Biliary Tract Cancers: An Overview. OncoTargets Ther. 2021, 14, 1341–1366. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, D.; Fritsch, R. Targeting HER2 in Biliary Tract Carcinomas: Challenges and Opportunities. Oncol. Res. Treat. 2021, 44, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320. [Google Scholar] [CrossRef]
- Madej, T.; Lanczycki, C.J.; Zhang, D.; Thiessen, P.A.; Geer, R.C.; Marchler-Bauer, A.; Bryant, S.H. MMDB and VAST+: Tracking structural similarities between macromolecular complexes. Nucleic Acids Res. 2014, 42, D297–D303. [Google Scholar] [CrossRef]



| Acronym | Full Name | Incidence (%) |
|---|---|---|
| APC | Adenomatous polyposis coli | 15 |
| ARID1A | AT-rich interaction domain 1A | 25 |
| AKT1 | Homologue retrovirus kinase isolated from AKT-8(-1) | <5 |
| AXIN1 | Axis inhibition protein 1 | 40 |
| BAP1 | BRCA1-associated protein 1 | 25 |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 | 20 |
| BRCA1 | Breast Cancer gene 1 | <1 |
| BRCA2 | Breast Cancer gene 2 | 3 |
| CDKN2A/B | Cyclin-dependent kinase inhibitor 2A/B | 15 |
| CTNNB1 | Catenin Beta 1 | 8 |
| c-MET | Cellular-mesenchymal epithelial transition factor | <5 |
| EGFR | Epidermal growth factor receptor | 20 |
| FGFR2 | Fibroblast growth factor receptor 2 | 15 |
| HER2 | Human epidermal growth factor receptor 2 | <10 |
| IDH1/2 | Isocitrate dehydrogenase 1/2 | 15 |
| KRAS | Kirsten rat sarcoma viral oncogene homolog | 20 |
| MEK | Mapk/erk kinase | 15 |
| NTRK | Neurotrophic tyrosine receptor kinase | <1 |
| PIK3CA | Phosphatidylinotitol 3-kinase catalytic subunit alpha | 15 |
| ROS1 | ROS proto-oncogene 1 | 10 |
| SMAD4 | Mothers against decapentaplegic homolog 4 | 25 |
| TP53 | Tumor protein 53 | 35 |
| VEGF | Vascular endothelial growth factor | 15 |
| Gene/Fusion Partner | Chromosomal Localization | Ref. |
|---|---|---|
| NTRK1/LMNA (Lamin A) | 1q22 | [64,67] |
| NTRK1/TPM3 (Tropomyosin 3) | 1q21.3 | [64] |
| NTRK1/RABGAP1L (RAB GTPase Activating Protein 1 Like) | 1q25.1 | [65,67] |
| NTRK1/PLEKHA6 (Pleckstrin Homology Domain Containing A6) | 1q32.1 | [66] |
| Target | Drug | Molecular Weight (Da) | Type of Inhibition | IC50 * | Most Common Clinical Toxicities (All Grades) |
|---|---|---|---|---|---|
| FGFR | Pemigatinib | 487.50 | Reversible | 0.5 nM/L * | Diarrhea, fatigue, alopecia, and eye tox |
| Infigratinib | 560.48 | Reversible | 1.4 nM/L * | Eye tox, stomatitis, and fatigue | |
| Derazantinib | 468.57 | Reversible | 1.8 nM/L * | Eye tox, stomatitis, and fatigue | |
| Futibatinib | 418.45 | Irreversible | 1.4 nM/L * | Nail tox, fatigue, and musculoskeletal tox | |
| IDH1 | Ivosidenib | 582.96 | Reversible | 70.0 nM/L | Diarrhea, neutropenia, leukocytosis, and fatigue |
| BRAF | Vemurafenib | 482.92 | Reversible | 31 nM/L | Alopecia, arthralgia, fatigue, and eye tox |
| Dabrafenib | 519.56 | Reversible | 0.7 nM/L | Fever, neutropenia, and fatigue | |
| NTRK | Larotrectinib | 526.51 | Reversible | 11 nM/L ** | Anemia, fatigue, and nausea |
| Entrectinib | 560.63 | Reversible | 1 nM/L ** | Dysgeusia, fatigue, and diarrhea | |
| HER2 | Trastuzumab | 145,531.5 | Reversible | 1 mg/mL | Fever, nausea, allergy, and diarrhea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capuozzo, M.; Santorsola, M.; Landi, L.; Granata, V.; Perri, F.; Celotto, V.; Gualillo, O.; Nasti, G.; Ottaiano, A. Evolution of Treatment in Advanced Cholangiocarcinoma: Old and New towards Precision Oncology. Int. J. Mol. Sci. 2022, 23, 15124. https://doi.org/10.3390/ijms232315124
Capuozzo M, Santorsola M, Landi L, Granata V, Perri F, Celotto V, Gualillo O, Nasti G, Ottaiano A. Evolution of Treatment in Advanced Cholangiocarcinoma: Old and New towards Precision Oncology. International Journal of Molecular Sciences. 2022; 23(23):15124. https://doi.org/10.3390/ijms232315124
Chicago/Turabian StyleCapuozzo, Maurizio, Mariachiara Santorsola, Loris Landi, Vincenza Granata, Francesco Perri, Venere Celotto, Oreste Gualillo, Guglielmo Nasti, and Alessandro Ottaiano. 2022. "Evolution of Treatment in Advanced Cholangiocarcinoma: Old and New towards Precision Oncology" International Journal of Molecular Sciences 23, no. 23: 15124. https://doi.org/10.3390/ijms232315124
APA StyleCapuozzo, M., Santorsola, M., Landi, L., Granata, V., Perri, F., Celotto, V., Gualillo, O., Nasti, G., & Ottaiano, A. (2022). Evolution of Treatment in Advanced Cholangiocarcinoma: Old and New towards Precision Oncology. International Journal of Molecular Sciences, 23(23), 15124. https://doi.org/10.3390/ijms232315124








