Thermo-Transient Receptor Potential Channels: Therapeutic Potential in Gastric Cancer
Abstract
1. Introduction
2. Thermo-TRPs
3. Structures and Functions
4. Thermo-TRPs Are Involved in Tumor Onset and Progression
4.1. Gastric Cancer
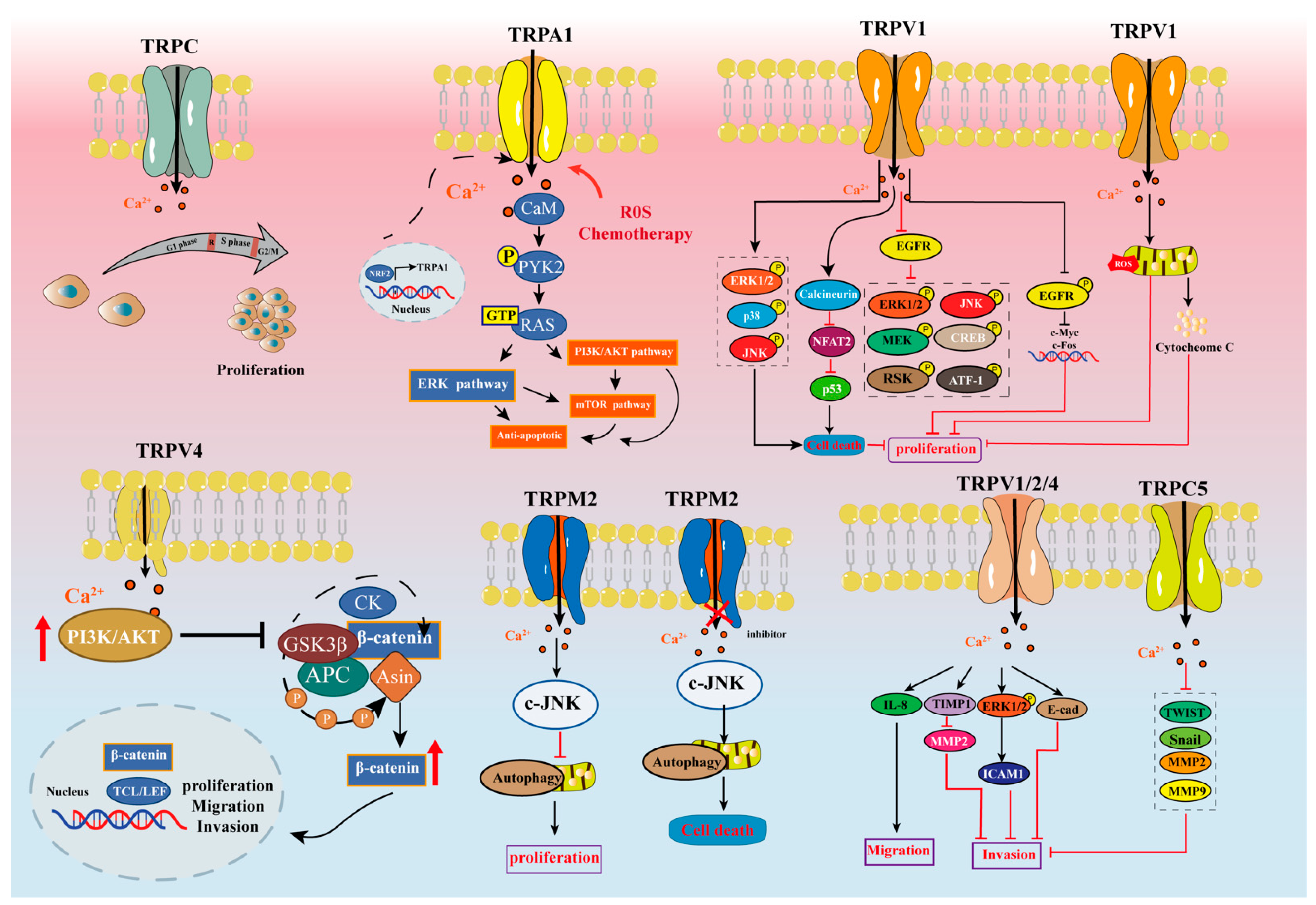
4.2. Cell Death and Proliferation
4.2.1. TRPV
4.2.2. TRPM
4.2.3. TRPA
4.2.4. TRPC
4.3. Cell Migration, Invasion, and Metastasis
4.3.1. TRPV
4.3.2. TRPM
4.3.3. TRPA
4.3.4. TRPC
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liang, H. [The Precised Management of Surgical Treatment for Gastric Cancer: Interpretation of the 5th edition of Japanese Gastric Cancer Treatment Guideline and the 15th edition of Japanese Classification for Gastric Cancer]. Zhonghua Zhong Liu Za Zhi 2019, 41, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.D.; Zhu, Z.X.; Zhang, X.; Liu, J. Targeted therapy for gastric cancer: Current status and future directions (Review). Oncol. Rep. 2016, 35, 1245–1254. [Google Scholar] [CrossRef]
- Shapovalov, G.; Ritaine, A.; Skryma, R.; Prevarskaya, N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016, 38, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luan, Y.; Yu, R.; Zhang, Z.; Zhang, J.; Wang, W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci. Trends 2014, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fels, B.; Bulk, E.; Petho, Z.; Schwab, A. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals 2018, 11, 48. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Fu, L.; Wang, Y.; He, M.; Zhao, L.; Liao, X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5322–5348. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Qiu, L. Transient receptor potential vanilloid 1 promotes EGFR ubiquitination and modulates EGFR/MAPK signalling in pancreatic cancer cells. Cell Biochem. Funct. 2020, 38, 401–408. [Google Scholar] [CrossRef]
- Domotor, A.; Peidl, Z.; Vincze, A.; Hunyady, B.; Szolcsanyi, J.; Kereskay, L.; Szekeres, G.; Mozsik, G. Immunohistochemical distribution of vanilloid receptor, calcitonin-gene related peptide and substance P in gastrointestinal mucosa of patients with different gastrointestinal disorders. Inflammopharmacology 2005, 13, 161–177. [Google Scholar] [CrossRef]
- Yee, N.S. TRPM8 Ion Channels as Potential Cancer Biomarker and Target in Pancreatic Cancer. Adv. Protein. Chem. Struct. Biol. 2016, 104, 127–155. [Google Scholar] [CrossRef]
- Almasi, S.; Kennedy, B.E.; El-Aghil, M.; Sterea, A.M.; Gujar, S.; Partida-Sanchez, S.; El Hiani, Y. TRPM2 channel-mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway. J. Biol. Chem. 2018, 293, 3637–3650. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Nizamuddin, P.B.; Uddin, S.; Al-Thani, M.; Frenneaux, M.P.; Janahi, I.A.; Steinhoff, M.; Azizi, F. TRPV2: A Cancer Biomarker and Potential Therapeutic Target. Dis. Markers 2020, 2020, 8892312. [Google Scholar] [CrossRef] [PubMed]
- Minke, B. TRP channels and Ca2+ signaling. Cell Calcium 2006, 40, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Giorgi, C.; Galluzzi, L.; Pinton, P. Ca(2+) Fluxes and Cancer. Mol. Cell 2020, 78, 1055–1069. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta 2007, 1772, 937–946. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Santoni, G. Oncogenic and anti-oncogenic effects of transient receptor potential channels. Curr. Top. Med. Chem. 2013, 13, 344–366. [Google Scholar] [CrossRef]
- Smani, T.; Shapovalov, G.; Skryma, R.; Prevarskaya, N.; Rosado, J.A. Functional and physiopathological implications of TRP channels. Biochim. Biophys. Acta 2015, 1853, 1772–1782. [Google Scholar] [CrossRef]
- Holzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011, 131, 142–170. [Google Scholar] [CrossRef]
- Moran, M.M.; McAlexander, M.A.; Biro, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V.; Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Amantini, C. The role of transient receptor potential vanilloid type-2 ion channels in innate and adaptive immune responses. Front. Immunol. 2013, 4, 34. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Huang, S.; Ding, Y.; Wang, W.; Wang, A.; Lu, Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef]
- Vriens, J.; Nilius, B.; Voets, T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014, 15, 573–589. [Google Scholar] [CrossRef]
- Zimmermann, K.; Lennerz, J.K.; Hein, A.; Link, A.S.; Kaczmarek, J.S.; Delling, M.; Uysal, S.; Pfeifer, J.D.; Riccio, A.; Clapham, D.E. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 18114–18119. [Google Scholar] [CrossRef]
- Gavva, N.R.; Davis, C.; Lehto, S.G.; Rao, S.; Wang, W.; Zhu, D.X. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol. Pain 2012, 8, 36. [Google Scholar] [CrossRef]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef]
- Krakowiak, M.S.; Noto, J.M.; Piazuelo, M.B.; Hardbower, D.M.; Romero-Gallo, J.; Delgado, A.; Chaturvedi, R.; Correa, P.; Wilson, K.T.; Peek, R.M., Jr. Matrix metalloproteinase 7 restrains Helicobacter pylori-induced gastric inflammation and premalignant lesions in the stomach by altering macrophage polarization. Oncogene 2015, 34, 1865–1871. [Google Scholar] [CrossRef]
- Beceiro, S.; Radin, J.N.; Chatuvedi, R.; Piazuelo, M.B.; Horvarth, D.J.; Cortado, H.; Gu, Y.; Dixon, B.; Gu, C.; Lange, I.; et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal. Immunol. 2017, 10, 493–507. [Google Scholar] [CrossRef]
- Weber, L.V.; Al-Refae, K.; Wolk, G.; Bonatz, G.; Altmuller, J.; Becker, C.; Gisselmann, G.; Hatt, H. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer Dove Med. Press 2016, 8, 243–252. [Google Scholar] [CrossRef]
- Fan, H.; Shen, Y.X.; Yuan, Y.F. Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac. J. Cancer Prev. 2014, 15, 2559–2563. [Google Scholar] [CrossRef]
- Kato, S.; Shiozaki, A.; Kudou, M.; Shimizu, H.; Kosuga, T.; Ohashi, T.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. TRPV2 Promotes Cell Migration and Invasion in Gastric Cancer via the Transforming Growth Factor-beta Signaling Pathway. Ann. Surg. Oncol. 2022, 29, 2944–2956. [Google Scholar] [CrossRef]
- Laurino, S.; Mazzone, P.; Ruggieri, V.; Zoppoli, P.; Calice, G.; Lapenta, A.; Ciuffi, M.; Ignomirelli, O.; Vita, G.; Sgambato, A.; et al. Cationic Channel TRPV2 Overexpression Promotes Resistance to Cisplatin-Induced Apoptosis in Gastric Cancer Cells. Front. Pharmacol. 2021, 12, 746628. [Google Scholar] [CrossRef]
- den Dekker, E.; Hoenderop, J.G.; Nilius, B.; Bindels, R.J. The epithelial calcium channels, TRPV5 & TRPV6: From identification towards regulation. Cell Calcium 2003, 33, 497–507. [Google Scholar] [CrossRef]
- Xie, R.; Xu, J.; Xiao, Y.; Wu, J.; Wan, H.; Tang, B.; Liu, J.; Fan, Y.; Wang, S.; Wu, Y.; et al. Calcium Promotes Human Gastric Cancer via a Novel Coupling of Calcium-Sensing Receptor and TRPV4 Channel. Cancer Res. 2017, 77, 6499–6512. [Google Scholar] [CrossRef]
- Saifeddine, M.; El-Daly, M.; Mihara, K.; Bunnett, N.W.; McIntyre, P.; Altier, C.; Hollenberg, M.D.; Ramachandran, R. GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br. J. Pharmacol. 2015, 172, 2493–2506. [Google Scholar] [CrossRef]
- Zaccor, N.W.; Sumner, C.J.; Snyder, S.H. The nonselective cation channel TRPV4 inhibits angiotensin II receptors. J. Biol. Chem. 2020, 295, 9986–9997. [Google Scholar] [CrossRef]
- Tang, B.; Wu, J.; Zhu, M.X.; Sun, X.; Liu, J.; Xie, R.; Dong, T.X.; Xiao, Y.; Carethers, J.M.; Yang, S.; et al. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene 2019, 38, 3946–3961. [Google Scholar] [CrossRef]
- Lopez-Carrillo, L.; Lopez-Cervantes, M.; Robles-Diaz, G.; Ramirez-Espitia, A.; Mohar-Betancourt, A.; Meneses-Garcia, A.; Lopez-Vidal, Y.; Blair, A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int. J. Cancer 2003, 106, 277–282. [Google Scholar] [CrossRef]
- Wong, K.K.; Banham, A.H.; Yaacob, N.S.; Nur Husna, S.M. The oncogenic roles of TRPM ion channels in cancer. J. Cell Physiol. 2019, 234, 14556–14573. [Google Scholar] [CrossRef]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. TRPM Family Channels in Cancer. Pharmaceuticals 2018, 11, 58. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, S.Y.; Lee, S.; Jeon, J.H.; Matsui, H.; Kwon, Y.K.; Kim, S.J.; So, I. The role of transient receptor potential channel blockers in human gastric cancer cell viability. Can. J. Physiol. Pharmacol. 2012, 90, 175–186. [Google Scholar] [CrossRef]
- Maeda, T.; Suzuki, A.; Koga, K.; Miyamoto, C.; Maehata, Y.; Ozawa, S.; Hata, R.I.; Nagashima, Y.; Nabeshima, K.; Miyazaki, K.; et al. TRPM5 mediates acidic extracellular pH signaling and TRPM5 inhibition reduces spontaneous metastasis in mouse B16-BL6 melanoma cells. Oncotarget 2017, 8, 78312–78326. [Google Scholar] [CrossRef]
- Hall, D.P.; Cost, N.G.; Hegde, S.; Kellner, E.; Mikhaylova, O.; Stratton, Y.; Ehmer, B.; Abplanalp, W.A.; Pandey, R.; Biesiada, J.; et al. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 2014, 26, 738–753. [Google Scholar] [CrossRef]
- Sacconi, A.; Biagioni, F.; Canu, V.; Mori, F.; Di Benedetto, A.; Lorenzon, L.; Ercolani, C.; Di Agostino, S.; Cambria, A.M.; Germoni, S.; et al. miR-204 targets Bcl-2 expression and enhances responsiveness of gastric cancer. Cell Death Dis. 2012, 3, e423. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, E.J.; Lee, J.H.; Jeon, J.H.; Kim, S.J.; So, I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008, 99, 2502–2509. [Google Scholar] [CrossRef]
- Kim, B.J.; Hong, C. Role of transient receptor potential melastatin type 7 channel in gastric cancer. Integr. Med. Res. 2016, 5, 124–130. [Google Scholar] [CrossRef]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003.e1007. [Google Scholar] [CrossRef]
- El-Salhy, M.; Solomon, T.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 5068–5085. [Google Scholar] [CrossRef]
- Manneck, D.; Manz, G.; Braun, H.S.; Rosendahl, J.; Stumpff, F. The TRPA1 Agonist Cinnamaldehyde Induces the Secretion of HCO3(-) by the Porcine Colon. Int. J. Mol. Sci. 2021, 22, 5198. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, M.; Zhang, S.; Miao, Z.; Wang, J.; Lu, X.; Zhao, X. TRPC5induced autophagy promotes the TMZresistance of glioma cells via the CAMMKbeta/AMPKalpha/mTOR pathway. Oncol. Rep. 2019, 41, 3413–3423. [Google Scholar] [CrossRef]
- Cai, R.; Ding, X.; Zhou, K.; Shi, Y.; Ge, R.; Ren, G.; Jin, Y.; Wang, Y. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int. J. Cancer 2009, 125, 2281–2287. [Google Scholar] [CrossRef]
- Coghlin, C.; Murray, G.I. Current and emerging concepts in tumour metastasis. J. Pathol. 2010, 222, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rubaiy, H.N.; Ludlow, M.J.; Henrot, M.; Gaunt, H.J.; Miteva, K.; Cheung, S.Y.; Tanahashi, Y.; Hamzah, N.; Musialowski, K.E.; Blythe, N.M.; et al. Picomolar, selective, and subtype-specific small-molecule inhibition of TRPC1/4/5 channels. J. Biol. Chem. 2017, 292, 8158–8173. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, J.; Shen, L.; Xu, X.M.; Yu, H.G. Overexpression of AKT decreases the chemosensitivity of gastric cancer cells to cisplatin in vitro and in vivo. Mol. Med. Rep. 2013, 7, 1387–1390. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vannucchi, M.G.; Spinelli, M.; Bizzoco, E.; Beneforti, P.; Turini, D.; Faussone-Pellegrini, M.S. Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder. Eur. Urol. 2005, 48, 691–698. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Sanchez, A.M.; Collado, B.; Malagarie-Cazenave, S.; Olea, N.; Carmena, M.J.; Prieto, J.C.; Diaz-Laviada, I.I. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur. J. Pharmacol. 2005, 515, 20–27. [Google Scholar] [CrossRef]
- Luo, L.; Yan, J.; Wang, X.; Sun, Z. The correlation between chili pepper consumption and gastric cancer risk: A meta-analysis. Asia Pac. J. Clin. Nutr. 2021, 30, 130–139. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, R.U.; Lopez-Carrillo, L. Diet and gastric cancer in Mexico and in the world. Salud Publica Mex. 2014, 56, 555–560. [Google Scholar]
- Szolcsanyi, J.; Sandor, Z. Multisteric TRPV1 nocisensor: A target for analgesics. Trends Pharmacol. Sci. 2012, 33, 646–655. [Google Scholar] [CrossRef]
- Nakanishi, M.; Morita, Y.; Hata, K.; Muragaki, Y. Acidic microenvironments induce lymphangiogenesis and IL-8 production via TRPV1 activation in human lymphatic endothelial cells. Exp. Cell Res. 2016, 345, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gong, L.; Liu, Y.; Hou, K.; Fan, Y.; Li, C.; Wen, T.; Qu, X.; Che, X. 4-phenylbutyric acid promotes migration of gastric cancer cells by histone deacetylase inhibition-mediated IL-8 upregulation. Epigenetics 2020, 15, 632–645. [Google Scholar] [CrossRef]
- Shikano, M.; Ueda, T.; Kamiya, T.; Ishida, Y.; Yamada, T.; Mizushima, T.; Shimura, T.; Mizoshita, T.; Tanida, S.; Kataoka, H.; et al. Acid inhibits TRPV4-mediated Ca(2)(+) influx in mouse esophageal epithelial cells. Neurogastroenterol. Motil. 2011, 23, 1020–1028.e1497. [Google Scholar] [CrossRef]
- Tennakoon, S.; Aggarwal, A.; Kallay, E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta 2016, 1863, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, J.; Sui, X. Correlation of COX-2 and MMP-13 expressions with gastric cancer and their effects on prognosis. J. BUON 2019, 24, 187–193. [Google Scholar]
- Laitinen, A.; Hagstrom, J.; Mustonen, H.; Kokkola, A.; Tervahartiala, T.; Sorsa, T.; Bockelman, C.; Haglund, C. Serum MMP-8 and TIMP-1 as prognostic biomarkers in gastric cancer. Tumour Biol. 2018, 40, 1010428318799266. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, P.; Vanlaeys, A.; Brassart, B.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Ouadid-Ahidouch, H.; Gautier, M. The Transient Receptor Potential Melastatin 7 Channel Regulates Pancreatic Cancer Cell Invasion through the Hsp90alpha/uPA/MMP2 pathway. Neoplasia 2017, 19, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, L.; Liang, R.; Chen, X.; Zhang, R.; Chen, W.; Zhu, J. CircDLST promotes the tumorigenesis and metastasis of gastric cancer by sponging miR-502-5p and activating the NRAS/MEK1/ERK1/2 signaling. Mol. Cancer 2019, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Almasi, S.; Sterea, A.M.; Fernando, W.; Clements, D.R.; Marcato, P.; Hoskin, D.W.; Gujar, S.; El Hiani, Y. TRPM2 ion channel promotes gastric cancer migration, invasion and tumor growth through the AKT signaling pathway. Sci. Rep. 2019, 9, 4182. [Google Scholar] [CrossRef] [PubMed]
- Starkus, J.G.; Fleig, A.; Penner, R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J. Physiol. 2010, 588, 1227–1240. [Google Scholar] [CrossRef]
- Miller, B.A.; Hoffman, N.E.; Merali, S.; Zhang, X.Q.; Wang, J.; Rajan, S.; Shanmughapriya, S.; Gao, E.; Barrero, C.A.; Mallilankaraman, K.; et al. TRPM2 channels protect against cardiac ischemia-reperfusion injury: Role of mitochondria. J. Biol. Chem. 2014, 289, 7615–7629. [Google Scholar] [CrossRef]
- Miller, B.A. TRPM2 in Cancer. Cell Calcium 2019, 80, 8–17. [Google Scholar] [CrossRef]
- Chen, S.J.; Hoffman, N.E.; Shanmughapriya, S.; Bao, L.; Keefer, K.; Conrad, K.; Merali, S.; Takahashi, Y.; Abraham, T.; Hirschler-Laszkiewicz, I.; et al. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2alpha. J. Biol. Chem. 2014, 289, 36284–36302. [Google Scholar] [CrossRef]
- Nayak, A.; Roy, A.D.; Rout, N.; Singh, S.P.; Bhattacharyya, A.; Roychowdhury, A. HIF1alpha-dependent upregulation of ATAD2 promotes proliferation and migration of stomach cancer cells in response to hypoxia. Biochem. Biophys. Res. Commun. 2020, 523, 916–923. [Google Scholar] [CrossRef]
- Khalil, M.; Babes, A.; Lakra, R.; Forsch, S.; Reeh, P.W.; Wirtz, S.; Becker, C.; Neurath, M.F.; Engel, M.A. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal. Immunol. 2016, 9, 1500–1513. [Google Scholar] [CrossRef]
- Nielsen, N.; Lindemann, O.; Schwab, A. TRP channels and STIM/ORAI proteins: Sensors and effectors of cancer and stroma cell migration. Br. J. Pharmacol. 2014, 171, 5524–5540. [Google Scholar] [CrossRef]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. ROS Promotes Cancer Cell Survival through Calcium Signaling. Cancer Cell 2018, 33, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lu, N.N.; Feng, L. Apigetrin inhibits gastric cancer progression through inducing apoptosis and regulating ROS-modulated STAT3/JAK2 pathway. Biochem. Biophys. Res. Commun. 2018, 498, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Semtner, M.; Schaefer, M.; Pinkenburg, O.; Plant, T.D. Potentiation of TRPC5 by protons. J. Biol. Chem. 2007, 282, 33868–33878. [Google Scholar] [CrossRef]
- Bong, A.H.L.; Monteith, G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1786–1794. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Y.; Qiao, H. CCL18 promotes the invasion and migration of gastric cancer cells via ERK1/2/NF-kappaB signaling pathway. Tumour. Biol. 2016, 37, 641–651. [Google Scholar] [CrossRef]
- Schwarz, E.C.; Qu, B.; Hoth, M. Calcium, cancer and killing: The role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim. Biophys. Acta 2013, 1833, 1603–1611. [Google Scholar] [CrossRef]
- Ge, P.; Wei, L.; Zhang, M.; Hu, B.; Wang, K.; Li, Y.; Liu, S.; Wang, J.; Li, Y. TRPC1/3/6 inhibition attenuates the TGF-beta1-induced epithelial-mesenchymal transition in gastric cancer via the Ras/Raf1/ERK signaling pathway. Cell Biol. Int. 2018, 42, 975–984. [Google Scholar] [CrossRef]
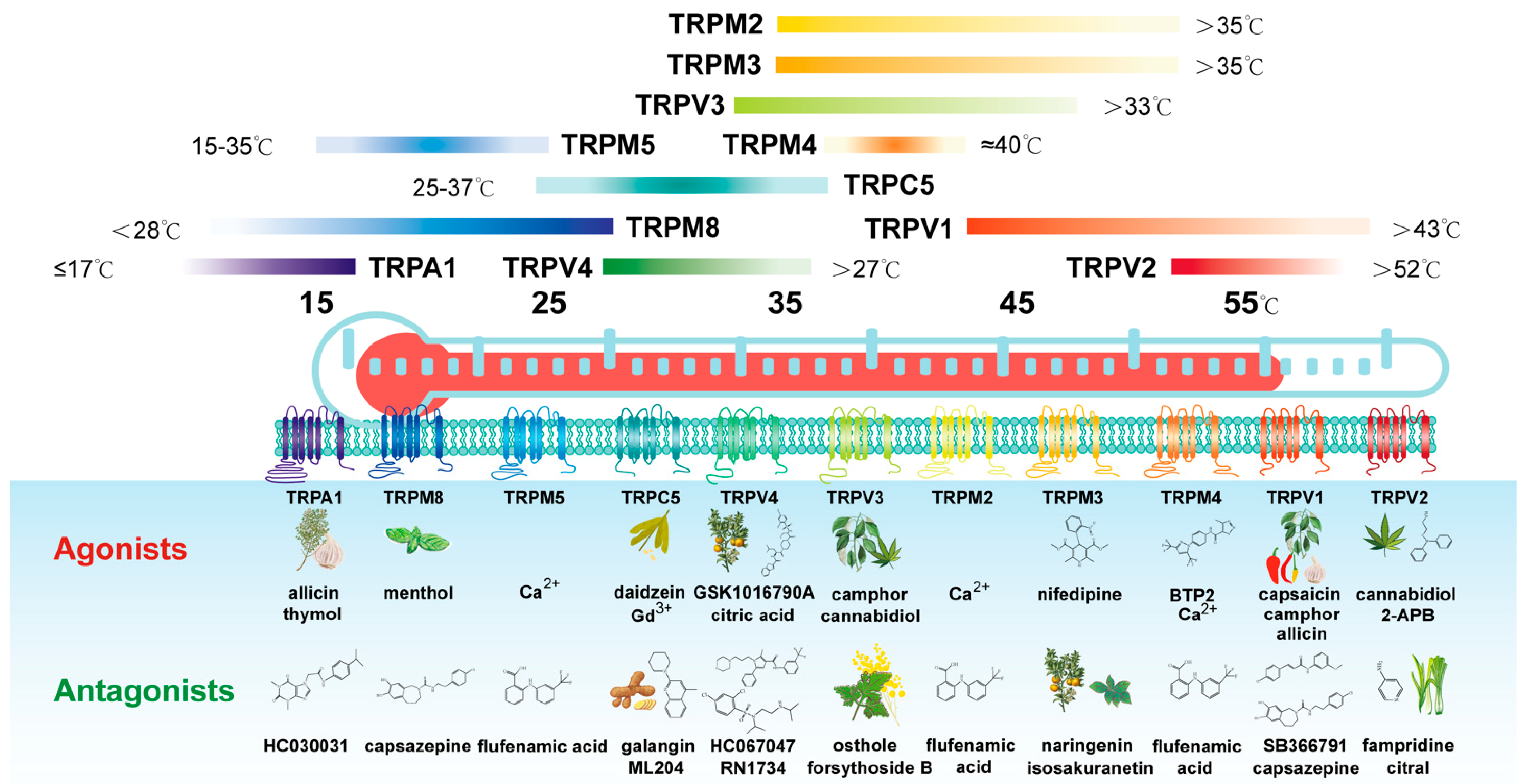

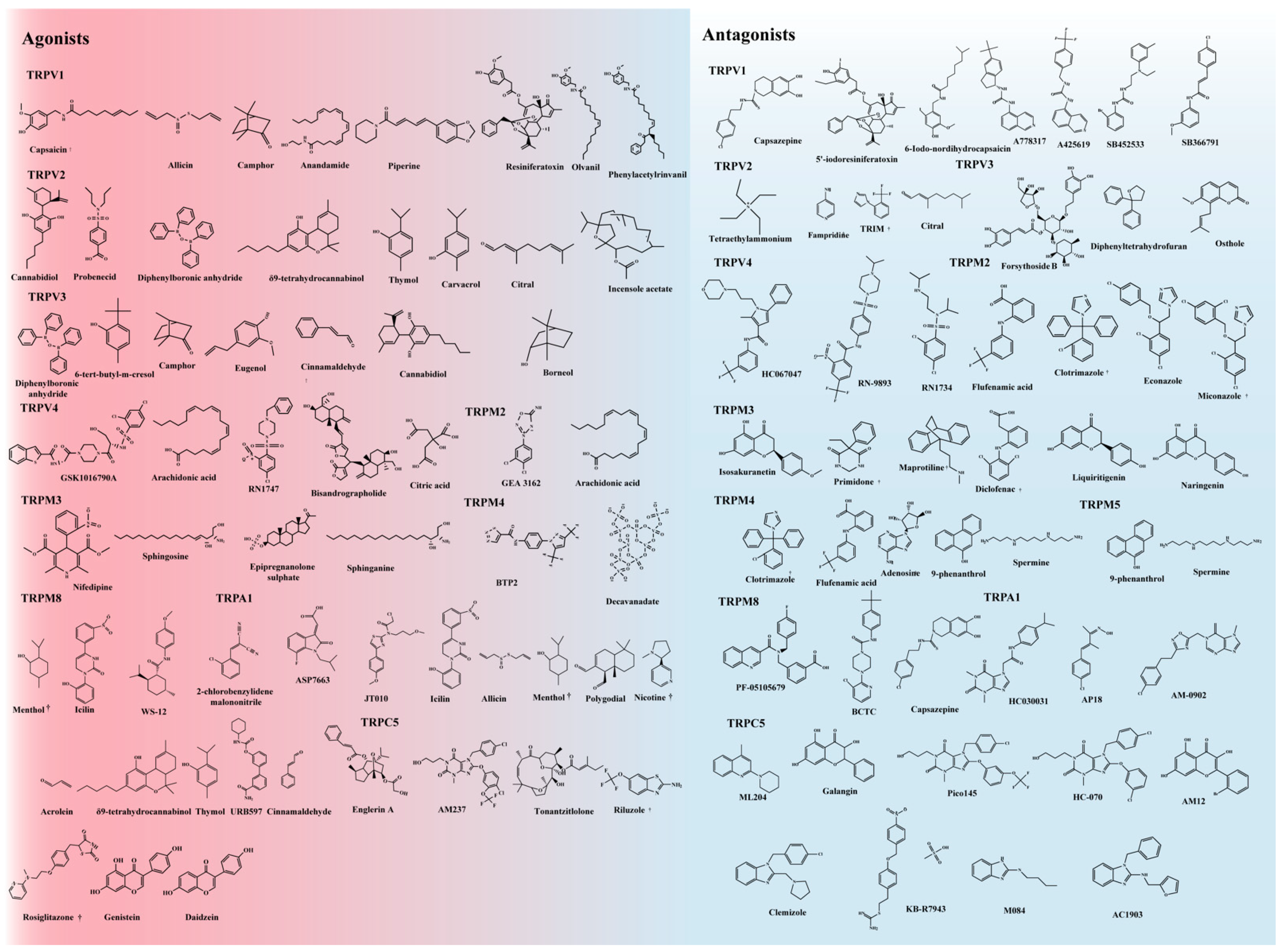
| Channel Subunit | Activation Temperature | Physiological Functions | Association with Patient Outcomes |
|---|---|---|---|
| TRPV subfamily | |||
| TRPV1 | >43 °C | Thermo-sensation (heat); autonomic thermos regulation; inflammatory hyperalgesia; nociception and pain management | negatively associated with the overall survival of GC patients (p < 0.0001) |
| TRPV2 | >52 °C | Thermo-sensation (noxious heat); nociception; mediated immune response | negatively associated with the overall survival of GC patients (p < 0.0001) |
| TRPV3 | >33 °C | Thermo-sensation (moderate heat); nociception; skin integrity, hair growth and sebocyte function, mood regulation | negatively associated with the overall survival of GC patients (p = 0.015) |
| TRPV4 | >27 °C | Thermo-sensation (moderate heat); mechano-sensation; osmo-sensation; nociception; endothelium vaso-motor control and shear stress sensor; modulation of cell migration; control adherens junctions in skin | negatively associated with the overall survival of GC patients (p < 0.0001) |
| TRPM subfamily | |||
| TRPM2 | >35 °C | Thermo-sensation (moderate heat); oxidative and nitrosative stress response; immunity cells infiltration; regulation of pancreas insulin release; apoptosis control | negatively associated with the overall survival of GC patients (p = 0.00054) |
| TRPM3 | >35 °C | Regulation of pancreas insulin release and glucose homeostasis; steroid hormone (pregnanolon) sensor | negatively associated with the overall survival of GC patients (p < 0.0001) |
| TRPM4 | ~40 °C | Regulation of catecholamine release from chromafn cells; involved in mast cell activation and dendritic cell migration; regulation of Ca2+ entry | negatively associated with the overall survival of GC patients (p = 0.015) |
| TRPM5 | 15–35 °C | A key component of taste (sweet, bitter, umami) transduction; regulator of glucose-induced insulin release | negatively associated with the overall survival of GC patients (p < 0.0001) |
| TRPM8 | <28 °C | Thermo-sensation (cold); autonomic thermos regulation (with TRPV1) | negatively associated with the overall survival of GC patients (p = 0.0015) |
| TRPA subfamily | |||
| TRPA1 | ≤17 °C | Thermo-sensation (noxious cold); mechano-sensation; chemo-sensor; nociception; inflammatory pain | negatively associated with the overall survival of GC patients (p < 0.0001) |
| TRPC subfamily | |||
| TRPC5 | 25–37 °C | Control of anxiety, fear and reward behaviors; promotion of brain development (together with TRPC1) | negatively associated with the overall survival of GC patients (p < 0.0001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, G.-F.; Deng, R.; Yu, S.-Y.; Wang, A.-Y.; Wei, Z.-H.; Zhao, Y.; Lu, Y. Thermo-Transient Receptor Potential Channels: Therapeutic Potential in Gastric Cancer. Int. J. Mol. Sci. 2022, 23, 15289. https://doi.org/10.3390/ijms232315289
Zong G-F, Deng R, Yu S-Y, Wang A-Y, Wei Z-H, Zhao Y, Lu Y. Thermo-Transient Receptor Potential Channels: Therapeutic Potential in Gastric Cancer. International Journal of Molecular Sciences. 2022; 23(23):15289. https://doi.org/10.3390/ijms232315289
Chicago/Turabian StyleZong, Gang-Fan, Rui Deng, Su-Yun Yu, Ai-Yun Wang, Zhong-Hong Wei, Yang Zhao, and Yin Lu. 2022. "Thermo-Transient Receptor Potential Channels: Therapeutic Potential in Gastric Cancer" International Journal of Molecular Sciences 23, no. 23: 15289. https://doi.org/10.3390/ijms232315289
APA StyleZong, G.-F., Deng, R., Yu, S.-Y., Wang, A.-Y., Wei, Z.-H., Zhao, Y., & Lu, Y. (2022). Thermo-Transient Receptor Potential Channels: Therapeutic Potential in Gastric Cancer. International Journal of Molecular Sciences, 23(23), 15289. https://doi.org/10.3390/ijms232315289






