Natural-Product-Mediated Autophagy in the Treatment of Various Liver Diseases
Abstract
:1. Introduction
2. Overview of Autophagy and Its Regulation
3. Potential Therapeutic Effects of Natural Products on Liver Diseases by Modulating Autophagy
3.1. Multiple Roles of Natural-Product-Mediated Autophagy Regulation in HCC
3.1.1. Natural Products Protect against HCC by Inhibiting Autophagy
3.1.2. Natural Products Exert Anti-HCC Effects by Inducing Autophagy
3.1.3. Autophagy Activation Is Considered as a Side Effect Associated with Anti-HCC Activities of Natural Products
3.2. Potential Application of Natural Products in Fatty Liver Disease (FLD) Treatment by Modulating Autophagy
3.3. Effects of Natural-Product-Mediated Autophagy on Liver Fibrosis (LF)
3.4. Potential Therapeutic Effects of Natural-Product-Mediated Autophagy on Viral Hepatitis
3.5. Potential Therapeutic Benefits of Natural Products on Acute Liver Injury (ALI) by Modulating Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFCP1 | double FYVE domain–containing protein 1 |
| ULK | Unc51-like kinase |
| PI3K | phosphatidylinositol 3-kinase |
| LC3 | microtubule-associated protein light chain 3 |
| FIP200 | FAK family kinase-interacting protein of 200 kD |
| VAPA/VAPB | (Vesicle-associated membrane protein)-associated Protein A/B |
| VPS34 | vacuolar protein sorting 34 |
| IMATs | isolation membrane-associated tubular structures |
| ERGIC | ER-Golgi intermediate compartment |
| ESCRT | endosomal sorting complexes required for transport |
| SNARE | endosomal sorting complexes required for transport |
| HOPS | homotypic fusion and protein sorting |
| AMPK | Adenosine 5‘-monophosphate (AMP)-activated protein kinase |
| AKT | Protein kinase B |
| mTOR | mammalian target of rapamycin |
| MAPK | mitogen-activated protein kinase |
| PINK1 | PTEN-induced kinase 1 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Nrf2 | erythroid 2-related factor 2 |
| ERK | extracellular regulated protein kinases |
| LPS | lipopolysaccharide |
| D-GalN | D-galactosamine |
References
- Wang, F.S.; Fan, J.G.; Zhang, Z.; Gao, B.; Wang, H.Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Wang, F.; Wong, N.K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.; Henry, L. Contribution of Alcoholic and Nonalcoholic Fatty Liver Disease to the Burden of Liver-Related Morbidity and Mortality. Gastroenterology 2016, 150, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, S.; Guo, P.; Zhang, X.; Tang, Y.; Dong, C.; Zhao, S.; Li, L.; Al-Dhamin, Z.; Ai, R.; et al. Identification of potential plasma markers for hepatitis B virus-related chronic hepatitis and liver fibrosis/cirrhosis. J. Med. Virol. 2022, 94, 3900–3910. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhou, M.; Wang, F.; Mubarik, S.; Wang, Y.; Meng, R.; Shi, F.; Wen, H.; Yu, C. Secular Trend of Cancer Death and Incidence in 29 Cancer Groups in China, 1990–2017: A Joinpoint and Age-Period-Cohort Analysis. Cancer Manag. Res. 2020, 12, 6221–6238. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Gores, G.J. Non-alcoholic steatohepatitis pathogenesis: Sublethal hepatocyte injury as a driver of liver inflammation. Gut 2018, 67, 963–972. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Chen, X.; Tan, M.; Chen, Y.; Lu, D.; Zhang, X.; Dean, J.M.; Razani, B.; Lodhi, I.J. Acetyl-CoA Derived from Hepatic Peroxisomal beta-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation. Mol. Cell 2020, 79, 30–42. [Google Scholar] [CrossRef]
- Parlati, L.; Regnier, M.; Guillou, H.; Postic, C. New targets for NAFLD. JHEP Rep. 2021, 3, 100346. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Teng, J.; Chen, J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy 2021, 17, 2680–2688. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Sun, Y.; Berleth, N.; Deitersen, J.; Schlütermann, D.; Stuhldreier, F.; Wallot-Hieke, N.; José Mendiburo, M.; Cox, J.; et al. TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy 2021, 17, 3992–4009. [Google Scholar] [CrossRef]
- Ueno, T.; Komatsu, M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef]
- Allaire, M.; Rautou, P.E.; Codogno, P.; Lotersztajn, S. Autophagy in liver diseases: Time for translation? J. Hepatol. 2019, 70, 985–998. [Google Scholar] [CrossRef] [Green Version]
- Rautou, P.E.; Mansouri, A.; Lebrec, D.; Durand, F.; Valla, D.; Moreau, R. Autophagy in liver diseases. J. Hepatol. 2010, 53, 1123–1134. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.L.; Liu, H.M.; Liu, X.M.; Yuan, X.Y.; Xu, C.; Wang, F.; Lin, J.Z.; Xu, R.C.; Zhang, D.K. Screening S protein—ACE2 blockers from natural products: Strategies and advances in the discovery of potential inhibitors of COVID-19. Eur. J. Med. Chem. 2021, 226, 113857. [Google Scholar] [CrossRef]
- Cheng, X.; Ma, X.; Zhu, Q.; Song, D.; Ding, X.; Li, L.; Jiang, X.; Wang, X.; Tian, R.; Su, H.; et al. Pacer Is a Mediator of mTORC1 and GSK3-TIP60 Signaling in Regulation of Autophagosome Maturation and Lipid Metabolism. Mol. Cell 2019, 73, 788–802.e7. [Google Scholar] [CrossRef]
- Tooze, S.A.; Yoshimori, T. The origin of the autophagosomal membrane. Nat. Cell Biol. 2010, 12, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi-Nishino, M.; Fujita, N.; Noda, T.; Yamaguchi, A.; Yoshimori, T.; Yamamoto, A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009, 11, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, N.; Miao, G.; Chen, Y.; Zhao, H.; Zhang, H. The ER Contact Proteins VAPA/B Interact with Multiple Autophagy Proteins to Modulate Autophagosome Biogenesis. Curr. Biol. 2018, 28, 1234–1245.e4. [Google Scholar] [CrossRef]
- Itakura, E.; Kishi, C.; Inoue, K.; Mizushima, N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 2008, 19, 5360–5372. [Google Scholar] [CrossRef] [Green Version]
- Dooley, H.C.; Razi, M.; Polson, H.E.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef] [Green Version]
- Fracchiolla, D.; Chang, C.; Hurley, J.H.; Martens, S. A PI3K-WIPI2 positive feedback loop allosterically activates LC3 lipidation in autophagy. J. Cell Biol. 2020, 219, e201912098. [Google Scholar] [CrossRef]
- Slobodkin, M.R.; Elazar, Z. The Atg8 family: Multifunctional ubiquitin-like key regulators of autophagy. Essays Biochem. 2013, 55, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. ATG4s: Above and beyond the Atg8-family protein lipidation system. Autophagy 2021, 17, 2648–2650. [Google Scholar] [CrossRef]
- Uemura, T.; Yamamoto, M.; Kametaka, A.; Sou, Y.S.; Yabashi, A.; Yamada, A.; Annoh, H.; Kametaka, S.; Komatsu, M.; Waguri, S. A cluster of thin tubular structures mediates transformation of the endoplasmic reticulum to autophagic isolation membrane. Mol. Cell. Biol. 2014, 34, 1695–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shima, T.; Kirisako, H.; Nakatogawa, H. COPII vesicles contribute to autophagosomal membranes. J. Cell Biol. 2019, 218, 1503–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yan, R.; Xu, J.; Zhao, S.; Ma, X.; Sun, Q.; Zhang, M.; Li, Y.; Liu, J.G.; Chen, L.; et al. A new type of ERGIC-ERES membrane contact mediated by TMED9 and SEC12 is required for autophagosome biogenesis. Cell Res. 2022, 32, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Klionsky, D.J. Autophagic membrane delivery through ATG9. Cell Res. 2017, 27, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Puri, C.; Renna, M.; Bento, C.F.; Moreau, K.; Rubinsztein, D.C. ATG16L1 meets ATG9 in recycling endosomes: Additional roles for the plasma membrane and endocytosis in autophagosome biogenesis. Autophagy 2014, 10, 182–184. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Otomo, C.; Leitner, A.; Ohashi, K.; Aebersold, R.; Lander, G.C.; Otomo, T. Insights into autophagosome biogenesis from structural and biochemical analyses of the ATG2A-WIPI4 complex. Proc. Natl. Acad. Sci. USA 2018, 115, E9792–E9801. [Google Scholar] [CrossRef] [Green Version]
- Laraia, L.; Friese, A.; Corkery, D.P.; Konstantinidis, G.; Erwin, N.; Hofer, W.; Karatas, H.; Klewer, L.; Brockmeyer, A.; Metz, M.; et al. The cholesterol transfer protein GRAMD1A regulates autophagosome biogenesis. Nat. Chem. Biol. 2019, 15, 710–720. [Google Scholar] [CrossRef] [Green Version]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 2010, 141, 656–667. [Google Scholar] [CrossRef] [Green Version]
- Andrejeva, G.; Gowan, S.; Lin, G.; Wong Te Fong, A.L.; Shamsaei, E.; Parkes, H.G.; Mui, J.; Raynaud, F.I.; Asad, Y.; Vizcay-Barrena, G.; et al. De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy. Autophagy 2020, 16, 1044–1060. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Spangenberg, H.; Munson, M.J.; Brech, A.; Schink, K.O.; Tan, K.W.; Sørensen, V.; Wenzel, E.M.; Radulovic, M.; Engedal, N.; et al. ESCRT-mediated phagophore sealing during mitophagy. Autophagy 2020, 16, 826–841. [Google Scholar] [CrossRef]
- Takahashi, Y.; He, H.; Tang, Z.; Hattori, T.; Liu, Y.; Young, M.M.; Serfass, J.M.; Chen, L.; Gebru, M.; Chen, C.; et al. An autophagy assay reveals the ESCRT-III component CHMP2A as a regulator of phagophore closure. Nat. Commun. 2018, 9, 2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Liang, X.; Hattori, T.; Tang, Z.; He, H.; Chen, H.; Liu, X.; Abraham, T.; Imamura-Kawasawa, Y.; Buchkovich, N.J.; et al. VPS37A directs ESCRT recruitment for phagophore closure. J. Cell Biol. 2019, 218, 3336–3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, R.; Guardia, C.M.; Pu, J.; Chen, Y.; Bonifacino, J.S. BORC coordinates encounter and fusion of lysosomes with autophagosomes. Autophagy 2017, 13, 1648–1663. [Google Scholar] [CrossRef] [Green Version]
- Pankiv, S.; Alemu, E.A.; Brech, A.; Bruun, J.A.; Lamark, T.; Overvatn, A.; Bjørkøy, G.; Johansen, T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol. 2010, 188, 253–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, J.; Guardia, C.M.; Keren-Kaplan, T.; Bonifacino, J.S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 2016, 129, 4329–4339. [Google Scholar] [CrossRef] [Green Version]
- Hegedűs, K.; Takáts, S.; Boda, A.; Jipa, A.; Nagy, P.; Varga, K.; Kovács, A.L.; Juhász, G. The Ccz1-Mon1-Rab7 module and Rab5 control distinct steps of autophagy. Mol. Biol. Cell 2016, 27, 3132–3142. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Miao, G.; Xue, X.; Guo, X.; Yuan, C.; Wang, Z.; Zhang, G.; Chen, Y.; Feng, D.; Hu, J.; et al. The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes. Mol. Cell 2016, 63, 781–795. [Google Scholar] [CrossRef] [Green Version]
- McEwan, D.G.; Popovic, D.; Gubas, A.; Terawaki, S.; Suzuki, H.; Stadel, D.; Coxon, F.P.; Miranda de Stegmann, D.; Bhogaraju, S.; Maddi, K.; et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell 2015, 57, 39–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocak, M.; Ezazi Erdi, S.; Jorba, G.; Maestro, I.; Farrés, J.; Kirkin, V.; Martinez, A.; Pless, O. Targeting autophagy in disease: Established and new strategies. Autophagy 2022, 18, 473–495. [Google Scholar] [CrossRef]
- Eskelinen, E.L.; Reggiori, F.; Baba, M.; Kovács, A.L.; Seglen, P.O. Seeing is believing: The impact of electron microscopy on autophagy research. Autophagy 2011, 7, 935–956. [Google Scholar] [CrossRef]
- Kim, H.; Seong, J. Fluorescent Protein-Based Autophagy Biosensors. Materials 2021, 14, 3019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, S.; Zhang, J.; Hou, J.; Wu, F.; Wang, W.; Han, X.; Gui, Y. Melatonin alleviates vascular endothelial cell damage by regulating an autophagy-apoptosis axis in Kawasaki disease. Cell Prolif. 2022, 55, e13251. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, P.L.; Lam, D.F.; Li, J.K.; Fu, X.Q.; Yin, C.L.; Chou, J.Y.; Wang, Y.P.; Liu, Y.X.; Chen, Y.J.; Wu, J.Y.; et al. Gomisin N Exerts Anti-liver Cancer Effects and Regulates PI3K-Akt and mTOR-ULK1 Pathways In Vitro. Biol. Pharm. Bull. 2020, 43, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, Z.; Wang, Y.; Guo, D.; Yang, J.; Chen, L.; Tan, N. Natural Cyclopeptide RA-XII, a New Autophagy Inhibitor, Suppresses Protective Autophagy for Enhancing Apoptosis through AMPK/mTOR/P70S6K Pathways in HepG2 Cells. Molecules 2017, 22, 1934. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Weng, Y.P.; Lin, H.Y.; Tang, S.W.; Chen, C.J.; Liang, C.J.; Ku, C.Y.; Lin, J.Y. Aqueous extract of Polygonum bistorta modulates proteostasis by ROS-induced ER stress in human hepatoma cells. Sci. Rep. 2017, 7, 41437. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Huang, M.; Yao, N.; Hu, J.; Li, Y.; Chen, L.; Hu, N.; Ye, W.; Chi-Shing Tai, W.; Zhang, D.; et al. The cycloartane triterpenoid ADCX impairs autophagic degradation through Akt overactivation and promotes apoptotic cell death in multidrug-resistant HepG2/ADM cells. Biochem. Pharmacol. 2017, 146, 87–100. [Google Scholar] [CrossRef]
- Nazim, U.M.; Park, S.Y. Attenuation of autophagy flux by 6-shogaol sensitizes human liver cancer cells to TRAIL-induced apoptosis via p53 and ROS. Int. J. Mol. Med. 2019, 43, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.D.; Zhang, C.; Shi, Y.M.; Xia, Y.Z.; Guo, C.; Yang, L.; Kong, L.Y. Icariside II-induced mitochondrion and lysosome mediated apoptosis is counterbalanced by an autophagic salvage response in hepatoblastoma. Cancer Lett. 2015, 366, 19–31. [Google Scholar] [CrossRef]
- Ji, Y.; Li, L.; Ma, Y.X.; Li, W.T.; Li, L.; Zhu, H.Z.; Wu, M.H.; Zhou, J.R. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J. Nutr. Biochem. 2019, 69, 108–119. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, X.; Wu, Z.; Shi, Y.; Wen, T. Uvangoletin, extracted from Sarcandra glabra, exerts anticancer activity by inducing autophagy and apoptosis and inhibiting invasion and migration on hepatocellular carcinoma cells. Phytomedicine 2022, 94, 153793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shang, L.; Jiang, W.; Wu, W. Shikonin induces apoptosis and autophagy via downregulation of pyrroline-5-carboxylate reductase1 in hepatocellular carcinoma cells. Bioengineered 2022, 13, 7904–7918. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, J.; Liu, X.; Li, Z.; Fang, K.; Zhang, L.; Han, M.; Zhang, Z.; Gong, Z.; Lin, X.; et al. Autophagy induction by xanthoangelol exhibits anti-metastatic activities in hepatocellular carcinoma. Cell Biochem. Funct. 2019, 37, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.D.; Lin, S.; Yang, P.T.; Bai, M.H.; Jin, Y.Y.; Min, W.L.; Ma, H.B.; Wang, B.F. Saikosaponin-d Increases the Radiosensitivity of Hepatoma Cells by Adjusting Cell Autophagy. J. Cancer 2019, 10, 4947–4953. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Wang, L.; Ren, L.; Li, J.; Li, S.; Cui, Q.; Li, S. Dihydroartemisinin, an antimalarial drug, induces absent in melanoma 2 inflammasome activation and autophagy in human hepatocellular carcinoma HepG2215 cells. Phytother. Res. 2019, 33, 1413–1425. [Google Scholar] [CrossRef]
- Yin, S.; Jin, W.; Qiu, Y.; Fu, L.; Wang, T.; Yu, H. Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. J. Hematol. Oncol. 2022, 15, 32. [Google Scholar] [CrossRef]
- Ciccarone, F.; Castelli, S.; Ciriolo, M.R. Oxidative Stress-Driven Autophagy acROSs Onset and Therapeutic Outcome in Hepatocellular Carcinoma. Oxid. Med. Cell. Longev. 2019, 2019, 6050123. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ren, R.; Li, J.; Cui, X. Recombinant Buckwheat Trypsin Inhibitor Induces Mitophagy by Directly Targeting Mitochondria and Causes Mitochondrial Dysfunction in Hep G2 Cells. J. Agric. Food Chem. 2015, 63, 7795–7804. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, J.; Hu, M.; Gao, Y.; Huang, L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano 2020, 14, 4816–4828. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, S.; Jiang, C.; Luo, X.; Guo, X.; Zhao, C.; Fan, L.; Meng, Y.; Lu, J.; Song, X.; et al. Involvement of autophagy induction in penta-1,2,3,4,6-O-galloyl-β-D-glucose-induced senescence-like growth arrest in human cancer cells. Autophagy 2014, 10, 296–310. [Google Scholar] [CrossRef]
- Sheng, X.; Zhu, P.; Qin, J.; Li, Q. The biological role of autophagy in regulating and controlling the proliferation of liver cancer cells induced by bufalin. Oncol. Rep. 2018, 39, 2931–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Q.; Bi, L.L.; Li, X.; Miao, S.; Zhang, J.; Zhang, S.; Yang, Q.; Xie, Y.H.; Zhang, J.; Wang, S.W. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 cells: Roles of apoptosis and autophagy. Int. J. Mol. Sci. 2013, 14, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Yang, J.S.; Peng, S.F.; Lu, C.C.; Chiang, J.H.; Chung, J.G.; Lin, M.W.; Lin, J.K.; Amagaya, S.; Wai-Shan Chung, C.; et al. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int. J. Oncol. 2012, 41, 1431–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.; Han, J.; Zhai, B.; Ming, X.; Zhuang, L.; Liu, Y.; Pan, S.; Liu, T. Blocking autophagy enhances the apoptosis effect of bufalin on human hepatocellular carcinoma cells through endoplasmic reticulum stress and JNK activation. Apoptosis 2014, 19, 210–223. [Google Scholar] [CrossRef]
- Tai, C.J.; Jassey, A.; Liu, C.H.; Tai, C.J.; Richardson, C.D.; Wong, S.H.; Lin, L.T. Targeting Autophagy Augments BBR-Mediated Cell Death in Human Hepatoma Cells Harboring Hepatitis C Virus RNA. Cells 2020, 9, 908. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Tang, X.; Liu, H.; Tang, J.; Yang, Y.; Jing, X.; Xiao, Q.; Wang, W.; Gou, X.; Wang, Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011, 102, 1287–1292. [Google Scholar] [CrossRef]
- Yao, N.; Wang, C.; Hu, N.; Li, Y.; Liu, M.; Lei, Y.; Chen, M.; Chen, L.; Chen, C.; Lan, P.; et al. Inhibition of PINK1/Parkin-dependent mitophagy sensitizes multidrug-resistant cancer cells to B5G1, a new betulinic acid analog. Cell Death Dis. 2019, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Z.Q.; Song, J.; Liu, Q.M.; Wang, C.; Huang, Z.; Chu, L.; Liang, H.F.; Zhang, B.X.; Chen, X.P. 18β-Glycyrrhetinic-acid-mediated unfolded protein response induces autophagy and apoptosis in hepatocellular carcinoma. Sci. Rep. 2018, 8, 9365. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Qiu, Y.; Pang, X.; Li, J.; Wu, S.; Yin, S.; Han, L.; Zhang, Y.; Jin, C.; Gao, X.; et al. Lycorine Promotes Autophagy and Apoptosis via TCRP1/Akt/mTOR Axis Inactivation in Human Hepatocellular Carcinoma. Mol. Cancer Ther. 2017, 16, 2711–2723. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, Q.; Lu, D.; Pang, X.; Yin, S.; Wang, K.; Wang, R.; Yang, S.; Zhang, Y.; Qiu, Y.; et al. HTBPI, an active phenanthroindolizidine alkaloid, inhibits liver tumorigenesis by targeting Akt. FASEB J. 2020, 34, 12255–12268. [Google Scholar] [CrossRef]
- Yin, S.; Qiu, Y.; Jin, C.; Wang, R.; Wu, S.; Liu, H.; Koo, S.; Han, L.; Zhang, Y.; Gao, X.; et al. 7-Deoxynarciclasine shows promising antitumor efficacy by targeting Akt against hepatocellular carcinoma. Int. J. Cancer 2019, 145, 3334–3346. [Google Scholar] [CrossRef]
- Tang, Z.H.; Li, T.; Chang, L.L.; Zhu, H.; Tong, Y.G.; Chen, X.P.; Wang, Y.T.; Lu, J.J. Glycyrrhetinic Acid triggers a protective autophagy by activation of extracellular regulated protein kinases in hepatocellular carcinoma cells. J. Agric. Food Chem. 2014, 62, 11910–11916. [Google Scholar] [CrossRef]
- Gong, L.; Di, C.; Xia, X.; Wang, J.; Chen, G.; Shi, J.; Chen, P.; Xu, H.; Zhang, W. AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 2016, 49, 2538–2548. [Google Scholar] [CrossRef] [Green Version]
- Kawano, Y.; Tanaka, M.; Fujishima, M.; Okumura, E.; Takekoshi, H.; Takada, K.; Uehara, O.; Abiko, Y.; Takeda, H. Acanthopanax senticosus Harms extract causes G0/G1 cell cycle arrest and autophagy via inhibition of Rubicon in human liver cancer cells. Oncol. Rep. 2021, 45, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Liu, J.S.; Deng, L.J.; Chen, M.F.; Yiu, A.; Cao, H.H.; Tian, H.Y.; Fung, K.P.; Kurihara, H.; Pan, J.X.; et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis 2013, 34, 1331–1342. [Google Scholar] [CrossRef] [Green Version]
- Baselli, G.A.; Jamialahmadi, O.; Pelusi, S.; Ciociola, E.; Malvestiti, F.; Saracino, M.; Santoro, L.; Cherubini, A.; Dongiovanni, P.; Maggioni, M.; et al. Rare ATG7 genetic variants predispose patients to severe fatty liver disease. J. Hepatol. 2022, 77, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Galle-Treger, L.; Helou, D.G.; Quach, C.; Howard, E.; Hurrell, B.P.; Muench, G.R.A.; Shafiei-Jahani, P.; Painter, J.D.; Iorga, A.; Dara, L.; et al. Autophagy impairment in liver CD11c(+) cells promotes non-alcoholic fatty liver disease through production of IL-23. Nat. Commun. 2022, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Hikita, H.; Tatsumi, T.; Sakamori, R.; Nozaki, Y.; Sakane, S.; Shiode, Y.; Nakabori, T.; Saito, Y.; Hiramatsu, N.; et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 2016, 64, 1994–2014. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Park, S.H.; Huh, Y.H.; Jung Kim, M.; Seo, H.D.; Ha, T.Y.; Ahn, J.; Jang, Y.J.; Jung, C.H. Iridoids of Valeriana fauriei contribute to alleviating hepatic steatosis in obese mice by lipophagy. Biomed. Pharmacother. 2020, 125, 109950. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Zhu, J.; Ruan, S.; Li, R.; Guo, B.; Lin, L. Honokiol attenuates lipotoxicity in hepatocytes via activating SIRT3-AMPK mediated lipophagy. Chin. Med. 2021, 16, 115. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, C.; Wang, Z.; Zhang, J.; Mao, X.; Chen, G.; Yao, X.; Liu, C. Cyclocarya paliurus extract attenuates hepatic lipid deposition in HepG2 cells by the lipophagy pathway. Pharm. Biol. 2020, 58, 838–844. [Google Scholar] [CrossRef]
- Parafati, M.; Lascala, A.; Morittu, V.M.; Trimboli, F.; Rizzuto, A.; Brunelli, E.; Coscarelli, F.; Costa, N.; Britti, D.; Ehrlich, J.; et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J. Nutr. Biochem. 2015, 26, 938–948. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, J.E.; Choi, H.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Seo, S.; Yang, S.Y.; An, B.S.; Lee, H.S.; et al. α-Linolenic Acid-Enriched Cold-Pressed Perilla Oil Suppress High-Fat Diet-Induced Hepatic Steatosis through Amelioration of the ER Stress-Mediated Autophagy. Molecules 2020, 25, 2662. [Google Scholar] [CrossRef]
- Lascala, A.; Martino, C.; Parafati, M.; Salerno, R.; Oliverio, M.; Pellegrino, D.; Mollace, V.; Janda, E. Analysis of proautophagic activities of Citrus flavonoids in liver cells reveals the superiority of a natural polyphenol mixture over pure flavones. J. Nutr. Biochem. 2018, 58, 119–130. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, H.Y.; Park, S.A.; Shin, T.S.; Chae, H.J. Eucommia ulmoides Leaf Extract Ameliorates Steatosis Induced by High-fat Diet in Rats by Increasing Lysosomal Function. Nutrients 2019, 11, 426. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Xu, L.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Zhao, Y.; Sun, H.; Yao, J.; Lin, Y.; et al. Potent effects of dioscin against obesity in mice. Sci. Rep. 2015, 5, 7973. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chan, H.; Zhang, L.; Liu, X.; Ho, I.H.T.; Zhang, X.; Ho, J.; Hu, W.; Tian, Y.; Kou, S.; et al. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and autophagic flux. J. Cell. Mol. Med. 2019, 23, 4290–4300. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Wu, Y.; Zhong, W.; Xia, G.; Xia, H.; Wang, L.; Wei, X.; Li, Y.; Shang, H.; He, H.; et al. Multiple anti-non-alcoholic steatohepatitis (NASH) efficacies of isopropylidenyl anemosapogenin via farnesoid X receptor activation and TFEB-mediated autophagy. Phytomedicine 2022, 102, 154148. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.C.; Yuan, H.X.; Guan, K.L. Autophagy regulation by nutrient signaling. Cell Res. 2014, 24, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Farah, B.L.; Sinha, R.A.; Wu, Y.; Singh, B.K.; Bay, B.H.; Yang, C.S.; Yen, P.M. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE 2014, 9, e87161. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, H.J.; You, M.; Kim, H.A. Red Pepper Seeds Inhibit Hepatic Lipid Accumulation by Inducing Autophagy via AMPK Activation. Nutrients 2022, 14, 4247. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, D.; Zhang, L.; Kang, X.; Li, Y.; Zhou, X.; Yuan, G. Catalpol induces autophagy and attenuates liver steatosis in ob/ob and high-fat diet-induced obese mice. Aging 2019, 11, 9461–9477. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, T.; Yang, L.; Wang, H.Y. Ginsenoside Rb2 Alleviates Hepatic Lipid Accumulation by Restoring Autophagy via Induction of Sirt1 and Activation of AMPK. Int. J. Mol. Sci. 2017, 18, 1063. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shi, Z.; Zhu, Y.; Shen, T.; Wang, H.; Shui, G.; Loor, J.J.; Fang, Z.; Chen, M.; Wang, X.; et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice. Br. J. Pharmacol. 2020, 177, 3591–3607. [Google Scholar] [CrossRef]
- Zhang, R.; Chu, K.; Zhao, N.; Wu, J.; Ma, L.; Zhu, C.; Chen, X.; Wei, G.; Liao, M. Corilagin Alleviates Nonalcoholic Fatty Liver Disease in High-Fat Diet-Induced C57BL/6 Mice by Ameliorating Oxidative Stress and Restoring Autophagic Flux. Front. Pharmacol. 2019, 10, 1693. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.T.; Lai, Y.H.; Lin, H.H.; Chen, J.H. Lotus Seedpod Extracts Reduced Lipid Accumulation and Lipotoxicity in Hepatocytes. Nutrients 2019, 11, 2895. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhou, Y.; Xu, W.; Wang, X.; Jin, H.; Bao, X.; Lu, C. Induction of Sestrin2 by pterostilbene suppresses ethanol-triggered hepatocyte senescence by degrading CCN1 via p62-dependent selective autophagy. Cell Biol. Toxicol. 2021. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Fu, Z.; Yin, J.; Wang, Y.; Sun, W.; Ren, H.; Zhang, Y. Kinsenoside Alleviates Alcoholic Liver Injury by Reducing Oxidative Stress, Inhibiting Endoplasmic Reticulum Stress, and Regulating AMPK-Dependent Autophagy. Front. Pharmacol. 2021, 12, 747325. [Google Scholar] [CrossRef]
- Zhang, M.H.; Li, J.; Zhu, X.Y.; Zhang, Y.Q.; Ye, S.T.; Leng, Y.R.; Yang, T.; Zhang, H.; Kong, L.Y. Physalin B ameliorates nonalcoholic steatohepatitis by stimulating autophagy and NRF2 activation mediated improvement in oxidative stress. Free Radic. Biol. Med. 2021, 164, 1–12. [Google Scholar] [CrossRef]
- Song, X.; Yin, S.; Huo, Y.; Liang, M.; Fan, L.; Ye, M.; Hu, H. Glycycoumarin ameliorates alcohol-induced hepatotoxicity via activation of Nrf2 and autophagy. Free Radic. Biol. Med. 2015, 89, 135–146. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Li, B.; Kang, X.J.; Gu, C.; Zhu, T.; Luo, Y.Y.; Pang, M.X.; Du, W.F.; Ge, W.H. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol. Lett. 2017, 274, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, R.; Wu, J.; Li, X. Self-eating: Friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics 2020, 10, 7993–8017. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhang, Z.; Chen, L.; Huang, W.; Zhang, F.; Wang, L.; Wang, Y.; Cao, P.; Zheng, S. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 2020, 36, 101600. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Yao, Z.; Wang, L.; Zhang, F.; Shao, J.; Chen, A.; Zheng, S. Activation of autophagy is required for Oroxylin A to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. Int. Immunopharmacol. 2018, 56, 148–155. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, Y.; Mo, C.; Zeng, T.; Huang, S.; Gao, L.; Sun, X.; Lv, Z. Betulinic acid attenuates liver fibrosis by inducing autophagy via the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. J. Nat. Med. 2019, 73, 179–189. [Google Scholar] [CrossRef]
- Liu, Y.M.; Cong, S.; Cheng, Z.; Hu, Y.X.; Lei, Y.; Zhu, L.L.; Zhao, X.K.; Mu, M.; Zhang, B.F.; Fan, L.D.; et al. Platycodin D alleviates liver fibrosis and activation of hepatic stellate cells by regulating JNK/c-JUN signal pathway. Eur. J. Pharmacol. 2020, 876, 172946. [Google Scholar] [CrossRef]
- Denardin, C.C.; Martins, L.A.; Parisi, M.M.; Vieira, M.Q.; Terra, S.R.; Barbé-Tuana, F.M.; Borojevic, R.; Vizzotto, M.; Emanuelli, T.; Guma, F.C. Autophagy induced by purple pitanga (Eugenia uniflora L.) extract triggered a cooperative effect on inducing the hepatic stellate cell death. Cell Biol. Toxicol. 2017, 33, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Ge, M.; Liu, H.; Zhang, Y.; Li, N.; Zhao, S.; Zhao, W.; Zhen, Y.; Yu, J.; He, H.; Shao, R.G. The anti-hepatic fibrosis effects of dihydrotanshinone I are mediated by disrupting the yes-associated protein and transcriptional enhancer factor D2 complex and stimulating autophagy. Br. J. Pharmacol. 2017, 174, 1147–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Liu, M.; You, Y.; Zhou, G.; Chen, Y.; Yuan, H.; Xie, L.; Han, S.; Zhu, K. Dual inhibition of reactive oxygen species and spleen tyrosine kinase as a therapeutic strategy in liver fibrosis. Free Radic. Biol. Med. 2021, 175, 193–205. [Google Scholar] [CrossRef]
- Chen, Y.; Que, R.; Zhang, N.; Lin, L.; Zhou, M.; Li, Y. Saikosaponin-d alleviates hepatic fibrosis through regulating GPER1/autophagy signaling. Mol. Biol. Rep. 2021, 48, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Regev, A.; Schiff, E.R. Viral hepatitis A, B, and C. Clin. Liver Dis. 2000, 4, 47–71. [Google Scholar] [CrossRef]
- Bruss, V. Revisiting the cytopathic effect of hepatitis B virus infection. Hepatology 2002, 36, 1327–1329. [Google Scholar] [CrossRef]
- Gonzalez-Peralta, R.P.; Davis, G.L.; Lau, J.Y. Pathogenetic mechanisms of hepatocellular damage in chronic hepatitis C virus infection. J. Hepatol. 1994, 21, 255–259. [Google Scholar] [CrossRef]
- Knolle, P.A.; Thimme, R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology 2014, 146, 1193–1207. [Google Scholar] [CrossRef]
- Trépo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef]
- Lauer, G.M.; Walker, B.D. Hepatitis C virus infection. N. Engl. J. Med. 2001, 345, 41–52. [Google Scholar] [CrossRef]
- Hepatitis B virus infection. Nat. Rev. Dis. Primers 2018, 4, 18036. [CrossRef] [Green Version]
- Vescovo, T.; Romagnoli, A.; Perdomo, A.B.; Corazzari, M.; Ciccosanti, F.; Alonzi, T.; Nardacci, R.; Ippolito, G.; Tripodi, M.; Garcia-Monzon, C.; et al. Autophagy protects cells from HCV-induced defects in lipid metabolism. Gastroenterology 2012, 142, 644–653.e3. [Google Scholar] [CrossRef]
- Ke, P.Y.; Chen, S.S. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Investig. 2011, 121, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Hu, J.; Shu, W.; Gao, B.; Xiong, S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015, 6, e1770. [Google Scholar] [CrossRef]
- Acerbi, G.; Montali, I.; Ferrigno, G.D.; Barili, V.; Schivazappa, S.; Alfieri, A.; Laccabue, D.; Loglio, A.; Borghi, M.; Massari, M.; et al. Functional reconstitution of HBV-specific CD8 T cells by in vitro polyphenol treatment in chronic hepatitis B. J. Hepatol. 2021, 74, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Liu, X.; Yang, Q.; Liu, H.; Liang, B.; Jiang, J.; Huang, J.; Ning, C.; Zang, N.; Zhou, B.; et al. Deguelin inhibits HCV replication through suppressing cellular autophagy via down regulation of Beclin1 expression in human hepatoma cells. Antivir. Res. 2020, 174, 104704. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; McGill, M.R.; Chao, X.; Du, K.; Williams, J.A.; Xie, Y.; Jaeschke, H.; Ding, W.X. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol. 2016, 65, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, M.; Jiang, W.; Zheng, H.; Qi, L.W.; Jiang, S. The role of Neu1 in the protective effect of dipsacoside B on acetaminophen-induced liver injury. Ann. Transl. Med. 2020, 8, 823. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Hu, C.; Wang, T.; Lu, J.; Wu, C.; Chen, L.; Jin, M.; Ji, G.; Cao, Q.; et al. Apigenin Prevents Acetaminophen-Induced Liver Injury by Activating the SIRT1 Pathway. Front. Pharmacol. 2020, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, C.; Li, X.; Liang, L.; Zhang, M.; Chen, B.; Liu, X.; Yang, D. Protective Effect of Dihydrokaempferol on Acetaminophen-Induced Liver Injury by Activating the SIRT1 Pathway. Am. J. Chin. Med. 2021, 49, 705–718. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, F.; Fan, G.; Liu, J.; Wang, Y.; Xue, X.; Lyu, X.; Lin, S.; Li, X. Ferulic acid ameliorates acetaminophen-induced acute liver injury by promoting AMPK-mediated protective autophagy. IUBMB Life 2022, 74, 880–895. [Google Scholar] [CrossRef]
- Yan, X.T.; Sun, Y.S.; Ren, S.; Zhao, L.C.; Liu, W.C.; Chen, C.; Wang, Z.; Li, W. Dietary α-Mangostin Provides Protective Effects against Acetaminophen-Induced Hepatotoxicity in Mice via Akt/mTOR-Mediated Inhibition of Autophagy and Apoptosis. Int. J. Mol. Sci. 2018, 19, 1335. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zheng, J.; Li, J.; Wang, Y.; Lu, X.; Fan, X. Integrating serum exosomal microRNA and liver microRNA profiles disclose the function role of autophagy and mechanisms of Fructus Meliae Toosendan-induced hepatotoxicity in mice. Biomed. Pharmacother. 2020, 123, 109709. [Google Scholar] [CrossRef]
- Hasnat, M.; Yuan, Z.; Naveed, M.; Khan, A.; Raza, F.; Xu, D.; Ullah, A.; Sun, L.; Zhang, L.; Jiang, Z. Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: A novel mechanism in triptolide-induced hepatotoxicity. Cell Biol. Toxicol. 2019, 35, 267–280. [Google Scholar] [CrossRef]
- Lee, A.Y.; Jang, Y.; Hong, S.H.; Chang, S.H.; Park, S.; Kim, S.; Kang, K.S.; Kim, J.E.; Cho, M.H. Ephedrine-induced mitophagy via oxidative stress in human hepatic stellate cells. J. Toxicol. Sci. 2017, 42, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhang, Y.; Zhang, Q.; Peng, S.; Shen, C.; Yu, Y.; Zhang, M.; Yang, W.; Wu, Q.; Zhang, Y.; et al. Content decline of SERCA inhibitors saikosaponin a and d attenuates cardiotoxicity and hepatotoxicity of vinegar-baked Radix bupleuri. Environ. Toxicol. Pharmacol. 2017, 52, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, J.; Wang, W.; Guo, J.; Yang, N.; Zhang, J.; Liu, Q.; Chen, Y.; Hu, T.; Rao, C. Zanthoxylum armatum DC. extract induces liver injury via autophagy suppression and oxidative damage by activation of mTOR/ULK1 pathway. Toxicon 2022, 217, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, H.; Wang, W.; Song, L.; Liu, M.; Cao, Y.; Qi, X.; Sun, J.; Gong, L. Prevention of D-GalN/LPS-induced ALI by 18β-glycyrrhetinic acid through PXR-mediated inhibition of autophagy degradation. Cell Death Dis. 2021, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, L.; Zhu, H.; Wang, F. The Protective Effect of Cordycepin on D-Galactosamine/Lipopolysaccharide-Induced Acute Liver Injury. Mediat. Inflamm. 2017, 2017, 3946706. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yin, H.; Zhao, Y.; Zhang, X.; Duan, C.; Liu, J.; Huang, C.; Liu, S.; Yang, S.; Li, X. Protective role of puerarin on LPS/D-Gal induced acute liver injury via restoring autophagy. Am. J. Transl. Res. 2018, 10, 957–965. [Google Scholar]
- Xia, Y.; Wang, P.; Yan, N.; Gonzalez, F.J.; Yan, T. Withaferin A alleviates fulminant hepatitis by targeting macrophage and NLRP3. Cell Death Dis. 2021, 12, 174. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Sun, B.; Liu, C.; Zhu, K.; Zhang, A. GBE attenuates arsenite-induced hepatotoxicity by regulating E2F1-autophagy-E2F7a pathway and restoring lysosomal activity. J. Cell. Physiol. 2021, 236, 4050–4065. [Google Scholar] [CrossRef]
- Zhou, X.L.; Wan, X.M.; Fu, X.X.; Xie, C.G. Puerarin prevents cadmium-induced hepatic cell damage by suppressing apoptosis and restoring autophagic flux. Biomed. Pharmacother. 2019, 115, 108929. [Google Scholar] [CrossRef]
- Yu, Z.M.; Wan, X.M.; Xiao, M.; Zheng, C.; Zhou, X.L. Puerarin induces Nrf2 as a cytoprotective mechanism to prevent cadmium-induced autophagy inhibition and NLRP3 inflammasome activation in AML12 hepatic cells. J. Inorg. Biochem. 2021, 217, 111389. [Google Scholar] [CrossRef]
- Seibler, P.; Burbulla, L.F.; Dulovic, M.; Zittel, S.; Heine, J.; Schmidt, T.; Rudolph, F.; Westenberger, A.; Rakovic, A.; Münchau, A.; et al. Iron overload is accompanied by mitochondrial and lysosomal dysfunction in WDR45 mutant cells. Brain 2018, 141, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.; Zhou, P.; Liu, L.; Jiang, W.; Xie, H.; Zhang, L.; Xie, D. Protective Effect of Astragaloside IV on Hepatic Injury Induced by Iron Overload. BioMed Res. Int. 2019, 2019, 3103946. [Google Scholar] [CrossRef] [PubMed]
- Cicchini, M.; Karantza, V.; Xia, B. Molecular pathways: Autophagy in cancer—A matter of timing and context. Clin. Cancer Res. 2015, 21, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
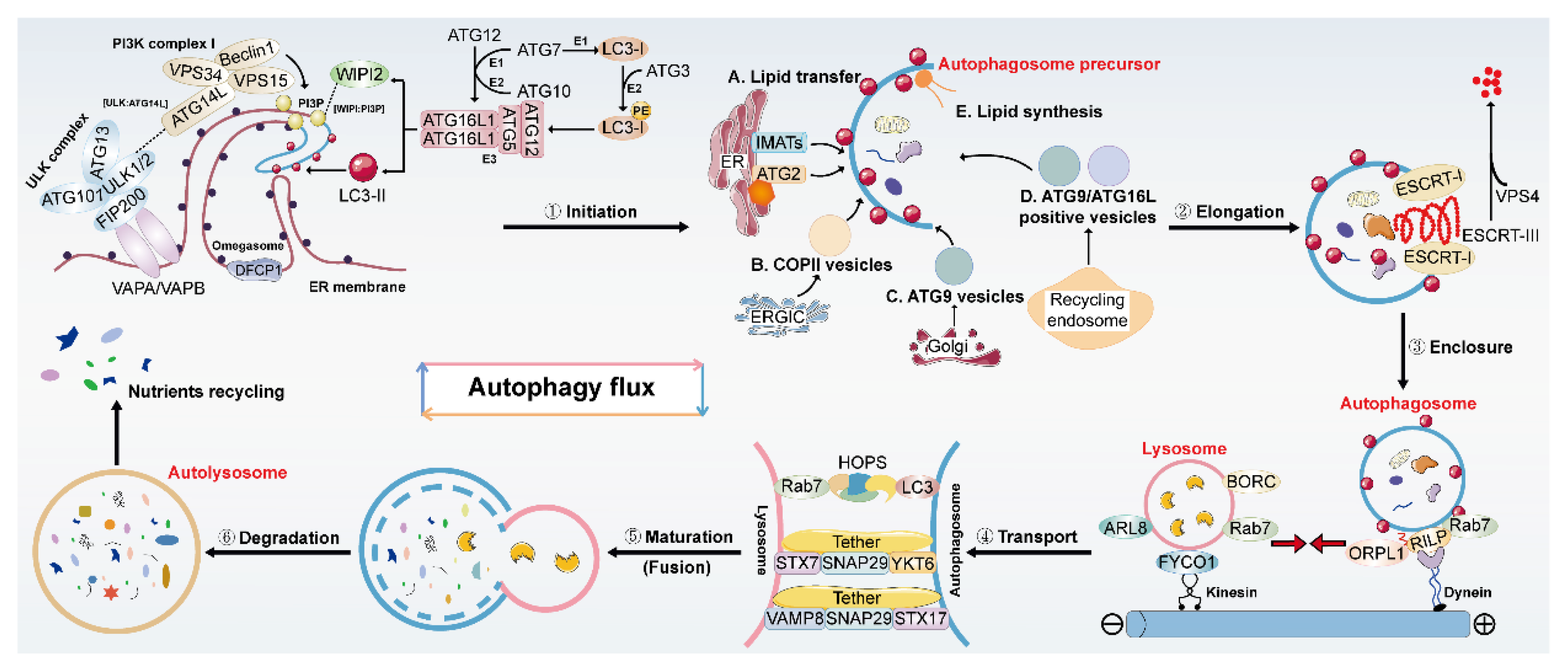
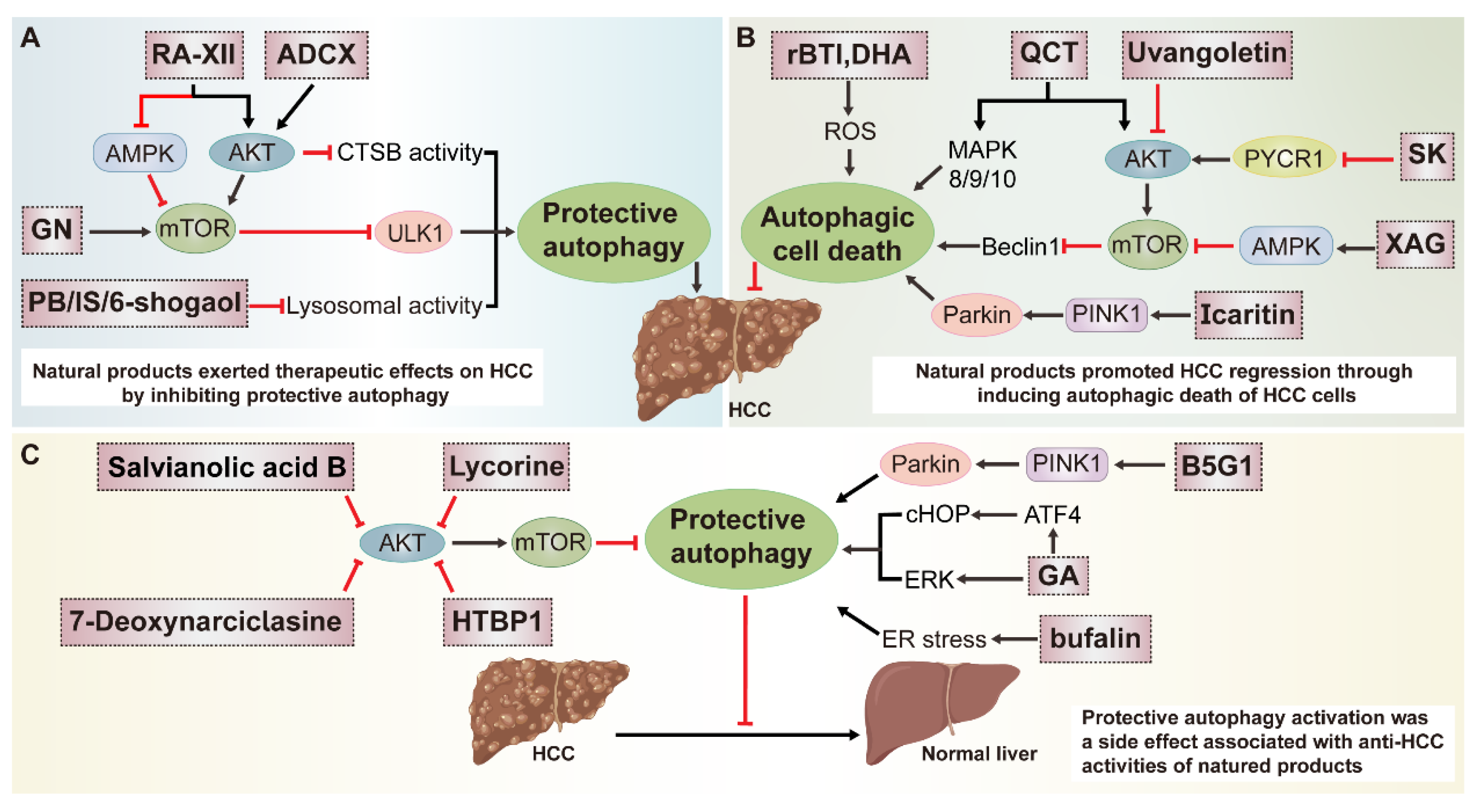
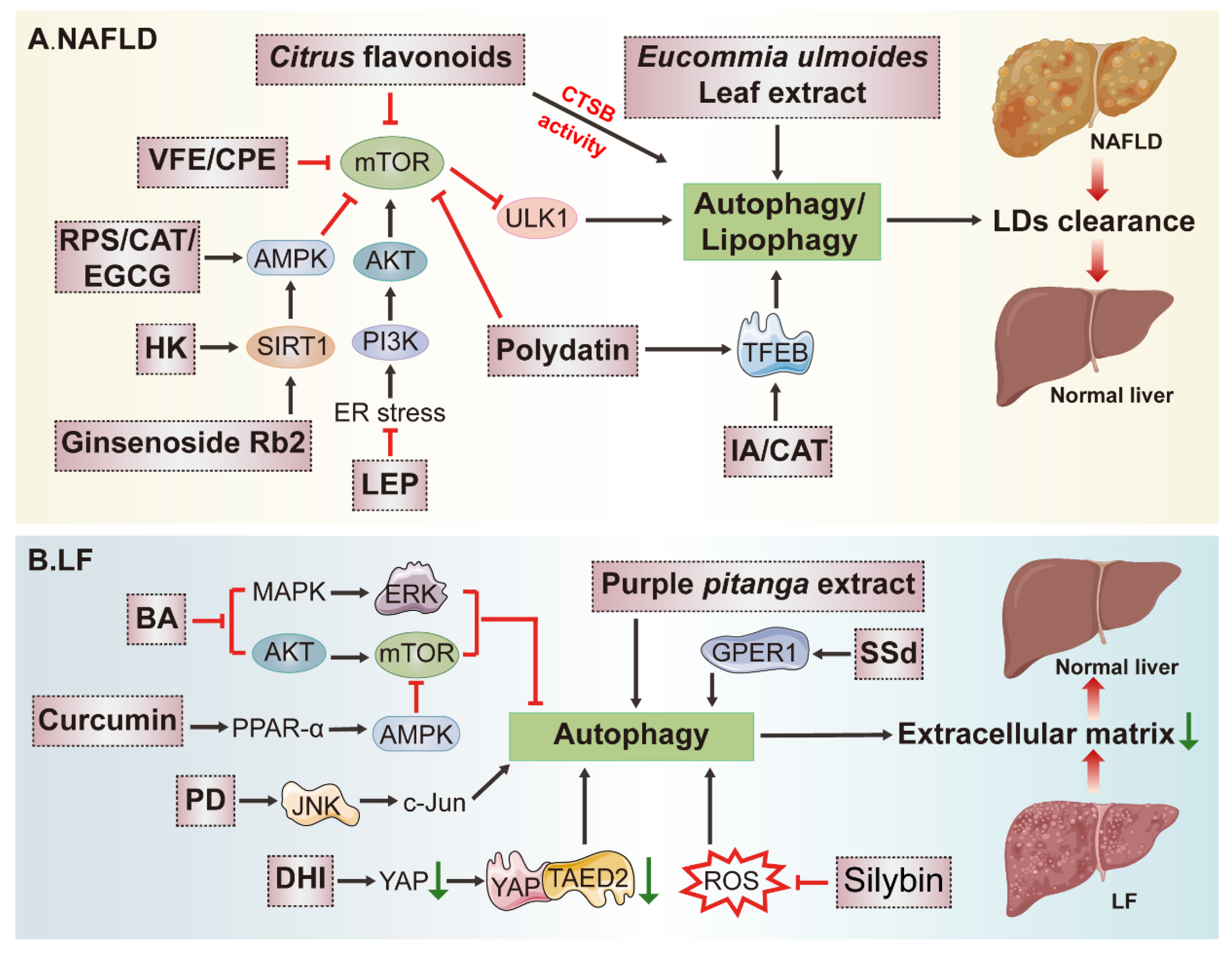
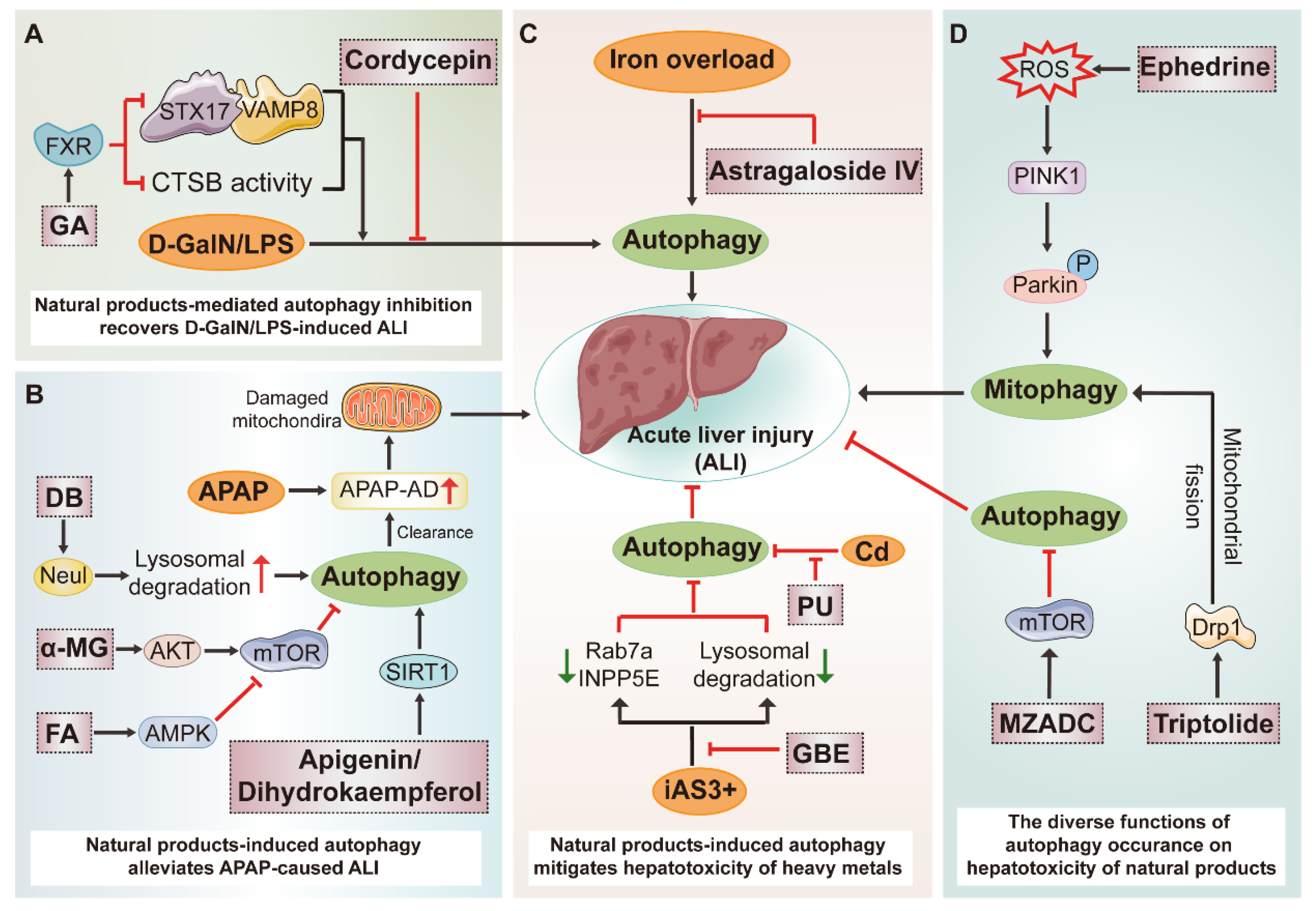


| Disease | Natural Product | Resource | Autophagy | Molecular Mechanism | Score | ||
|---|---|---|---|---|---|---|---|
| Autophagy Flux | Autophagosome Information | Degradation in Autolysosome | |||||
| HCC | ADCX | Cimicifugae rhizoma | (-) | (-) | NA | 7.5 | |
| IS | Epimedium koreanum Nakai | (-) | (-) | NA | 5 | ||
| RA-XII | Rubia yunnanensis | (-) | (-) | Inhibiting the AMPK-mTOR pathway | 3.5 | ||
| PB | Polygonum bistorta L. | (-) | (-) | NA | 2.5 | ||
| 6-shogaol | Ginger | (-) | (-) | NA | 2 | ||
| GN | Schisandra chinensis (Turca.) Baill | (-) | (-) | Activating the PI3K-AKT pathway | 1.5 | ||
| QCT | Wide distribution | (+) | (+) | Inhibiting the AKT-mTOR pathway | 5 | ||
| Uvangoletin | Sarcandra glabra (Thunb.) Nakai | (+) | (+) | Inhibiting the AKT-mTOR pathway | 4.5 | ||
| rBTI | Tartary buckwheat | (+) | (+) | NA | 4.5 | ||
| XAG | Angelica keiskei (Miq.) Koidz. | (+) | (+) | Activating the AMPK-mTOR pathway | 4 | ||
| PGG | Rhus chinensis Mill | (+) | (+) | NA | 3.5 | ||
| SSd | Radix bupleuri root | (+) | (+) | Inhibiting the mTOR pathway | 3 | ||
| DHA | Artemisia annua L. | (+) | (+) | Promoting the ROS production | 3 | ||
| SK | Lithospermum erythrorhizon | (+) | (+) | Down-regulating PYCR1, Inhibiting the AKT-mTOR pathway | 3 | ||
| Bufalin | Bufonid’s skin | (-) or (+) | (-) or (+) | NA | 2–4 | ||
| BBR | Berberis aristata | (-) or (+) | (-) or (+) | NA | 1–3 | ||
| SM | Solanum nigrum L. | (+) | (+) | NA | 1.5 | ||
| GA | Glycyrrhiza uralensis Fisch | (+) * | (+) | NA | 5.5 | ||
| Lycorine | Lycoris radiata | (+) * | (+) | NA | 5.5 | ||
| Arenobufagin | Bufonid’s skin | (+) * | (+) | NA | 5 | ||
| HTBPI | Tylophora ovata | (+) * | (+) | NA | 4.5 | ||
| 7-Deoxynarciclasine | Lycoris radiata | (+) * | (+) | NA | 4.5 | ||
| Salvianolic acid B | Salvia miltiorrhiza Bunge | (+) * | (+) | Inhibiting the AKT-mTOR pathway | 4.5 | ||
| FLD | Pterostilbene | Wide distribution | (+) | (+) | NA | 7.5 | |
| VFE | Valeriana fauriei Briq | (+) | (+) | Inhibiting the mTOR-ULK1 pathway | 6.5 | ||
| Corilagin | Wide distribution | (+) | (+) | NA | 6 | ||
| Cyanidin-3-O-glucoside | Black soybean testa | (+) | (+) | Activating the PINK1-Parkin pathway | 6 | ||
| Polydatin | Polygonum cuspidatum Sieb. et Zucc. | (+) | (+) | (+) | Activating the TFEB and inhibiting the mTOR | 5 | |
| EGCG | Green tea | (+) | (+) | Activating the AMPK | 5 | ||
| CAT | Rehmannia glutinosa DC. | (+) | (+) | (+) | Activating the AMPK-TFEB pathway | 5 | |
| HK | Magnolia genus L. | (+) | (+) | Activating the SIRT3-AMPK pathway | 4.5 | ||
| CPE | Cyclocarya paliurus | (+) | (+) | NA | 4.5 | ||
| RPS | Capsicum annuum L. | (+) | (+) | Activating the AMPK | 4 | ||
| Ginsenoside Rb2 | Panax ginseng C.A.Mey. | (+) | (+) | Activating the SIRT1-AMPK pathway | 4 | ||
| KD | Anoectochilus roxburghii (Wall.) Lindl. (Orchidaceae) | (+) | (+) | Activating the STRAD/LKB1-AMPK pathway | 3.5 | ||
| IA | Salvia miltiorrhiza Bunge | (+) | (+) | (+) | Activating the TFEB | 2 | |
| BPF | Citrus bergamia Risso et Poiteau | (+) | (+) | NA | 1.5 | ||
| LF | Curcumin | Curcuma zedoaria Roxb and… | (+) | (+) | Activating the AMPK-mTOR pathway | 7 | |
| BA | Wide distribution | (+) | (+) | Inhibiting the MAPK/ERK pathway | 5.5 | ||
| Silybin | Silybum marianum (L.) Gaertn. | (-) | (-) | Inhibiting the ROS production | 3.5 | ||
| Oroxylin A | Scutellariae radix | (+) | (+) | Inhibiting the MAPK-ERK and AKT-mTOR pathways | 3 | ||
| PD | Platycodon grandiflorum. | (+) | (+) | Inhibiting the JNK/c-JUN pathway | 3 | ||
| Purple pitanga extract | Eugenia uniflora L. | (+) | (+) | NA | 3 | ||
| SSd | Radix bupleuri root | (-) | (-) | Recovering the expression of GPER1 | 3 | ||
| DHI | Salvia miltiorrhiza Bunge | (+) | (+) | Down-regulating the expression of YAP | 2.5 | ||
| Virus infection | EGCG | Green tea | (+) | (+) | Recovering lysosomal acidification | 3 | |
| Deguelin | Mundulea sericea (Leguminosae) | (+) | (+) | NA | 2 | ||
| Resveratrol | Polygonum cuspidatum Siebold and Zucc. | (+) | (+) | NA | 1 | ||
| Oleuropein | Canarium album (Lour.) DC. | (+) | (+) | NA | 2 | ||
| ALI (APAP-induced-ALI) | Dihydrokaempferol | Wide distribution | (+) | (+) | Activating the SIRT1-AMPK pathway | 3.5 | |
| Apigenin | Matricaria chamomilla | (+) | (+) | Activating the SIRT1-AMPK pathway | 3.5 | ||
| FA | Wide distribution | (+) | (+) | Activating the AMPK | 3 | ||
| DB | Lonicera acuminata Wall. | (+) | (+) | Down-regulating the Neu1 | 2.5 | ||
| α-MG | Garcinia mangostana | (-) | (-) | augmenting the AKT-mTOR pathway | 1 | ||
| ALI (Chinese-herb-induced ALI) | Triptolide | Tripterygium wilfordii Hook. f. | (+) # | (+) | Promoting the Drp1-mediated mitochondrial fission | 5.5 | |
| Ephedrine | Chinese ephedra | (+) # | (+) | Evoking the PINK1-Parkin pathway | 4 | ||
| MZADC | Zanthoxylum armatum DC. | (-) # | (-) | Activating the mTOR-ULK1 pathway | 2 | ||
| SSa/SSd | Radix bupleuri root | (+) # | (+) | Inhibiting the activity of SERCA | 2 | ||
| ALI (D/L-induced ALI) | GA | Glycyrrhiza uralensis Fisch | (-) | (-) | Recovering the expression of PXR | 6 | |
| Puerarin | Radix puerariae | (+) | (+) | NA | 2.5 | ||
| Cordycepin | Cordyceps militaris | (-) | (-) | NA | 1.5 | ||
| WA | Withania Somnifera | (+) | (+) | NA | 1 | ||
| ALI (heavy-metal-induced ALI) | GBE | Ginkgo biloba | (+) | (+) | Inhibiting the E2F1/mTOR pathway | 8 | |
| Astragaloside IV | Astragalus aaronsohnianus Eig | (-) | (-) | NA | 1.5 | ||
| PU | Pueraria lobata | (+) | (+) | NA | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, G.; Li, F.; Wang, P.; Jin, X.; Liu, R. Natural-Product-Mediated Autophagy in the Treatment of Various Liver Diseases. Int. J. Mol. Sci. 2022, 23, 15109. https://doi.org/10.3390/ijms232315109
Fan G, Li F, Wang P, Jin X, Liu R. Natural-Product-Mediated Autophagy in the Treatment of Various Liver Diseases. International Journal of Molecular Sciences. 2022; 23(23):15109. https://doi.org/10.3390/ijms232315109
Chicago/Turabian StyleFan, Guifang, Fanghong Li, Ping Wang, Xuejing Jin, and Runping Liu. 2022. "Natural-Product-Mediated Autophagy in the Treatment of Various Liver Diseases" International Journal of Molecular Sciences 23, no. 23: 15109. https://doi.org/10.3390/ijms232315109
APA StyleFan, G., Li, F., Wang, P., Jin, X., & Liu, R. (2022). Natural-Product-Mediated Autophagy in the Treatment of Various Liver Diseases. International Journal of Molecular Sciences, 23(23), 15109. https://doi.org/10.3390/ijms232315109





