Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 1. Polyphosphodiesters
Abstract
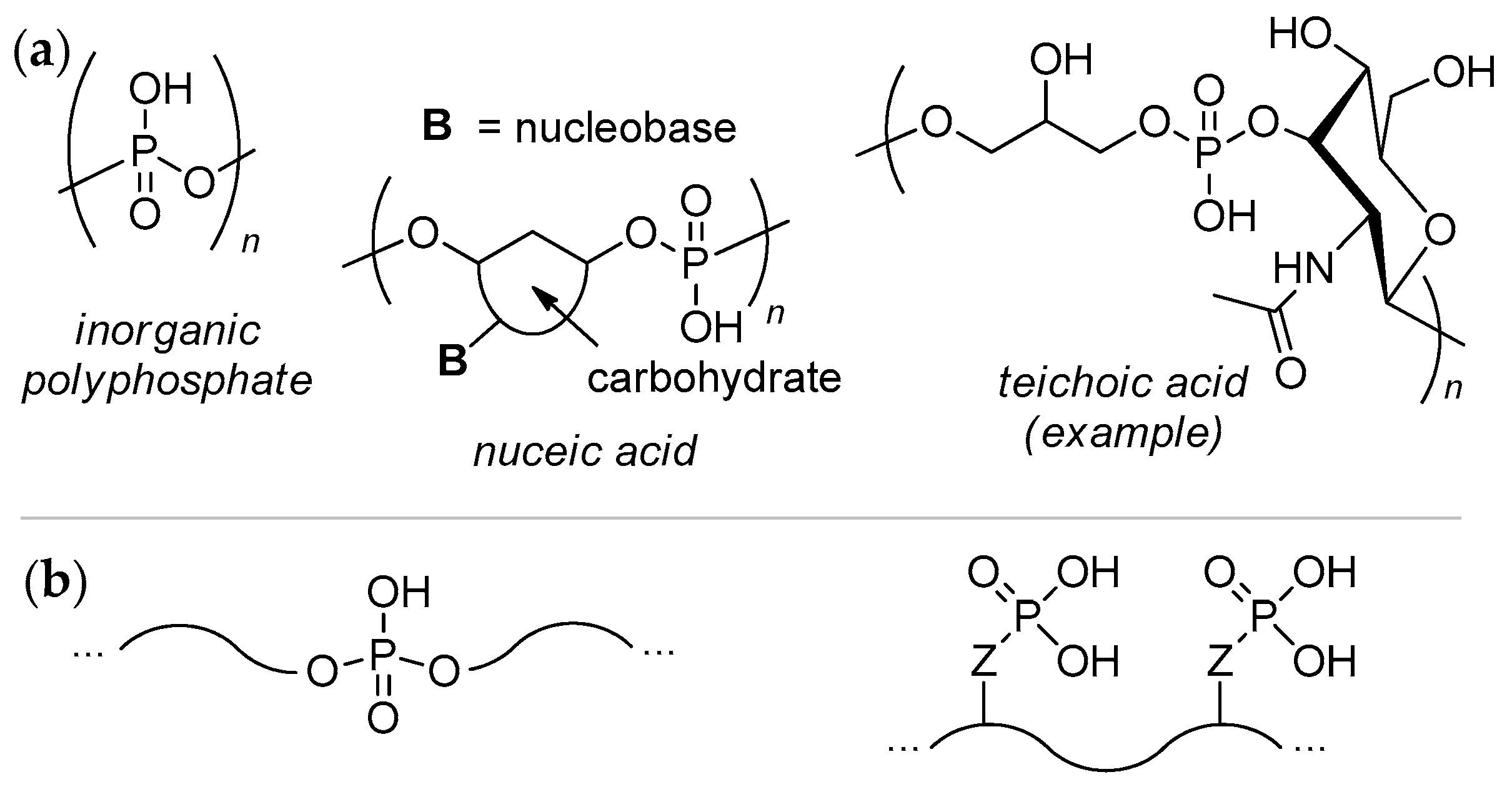
:1. Introduction
2. Design and Synthesis of Polyphosphodiesters
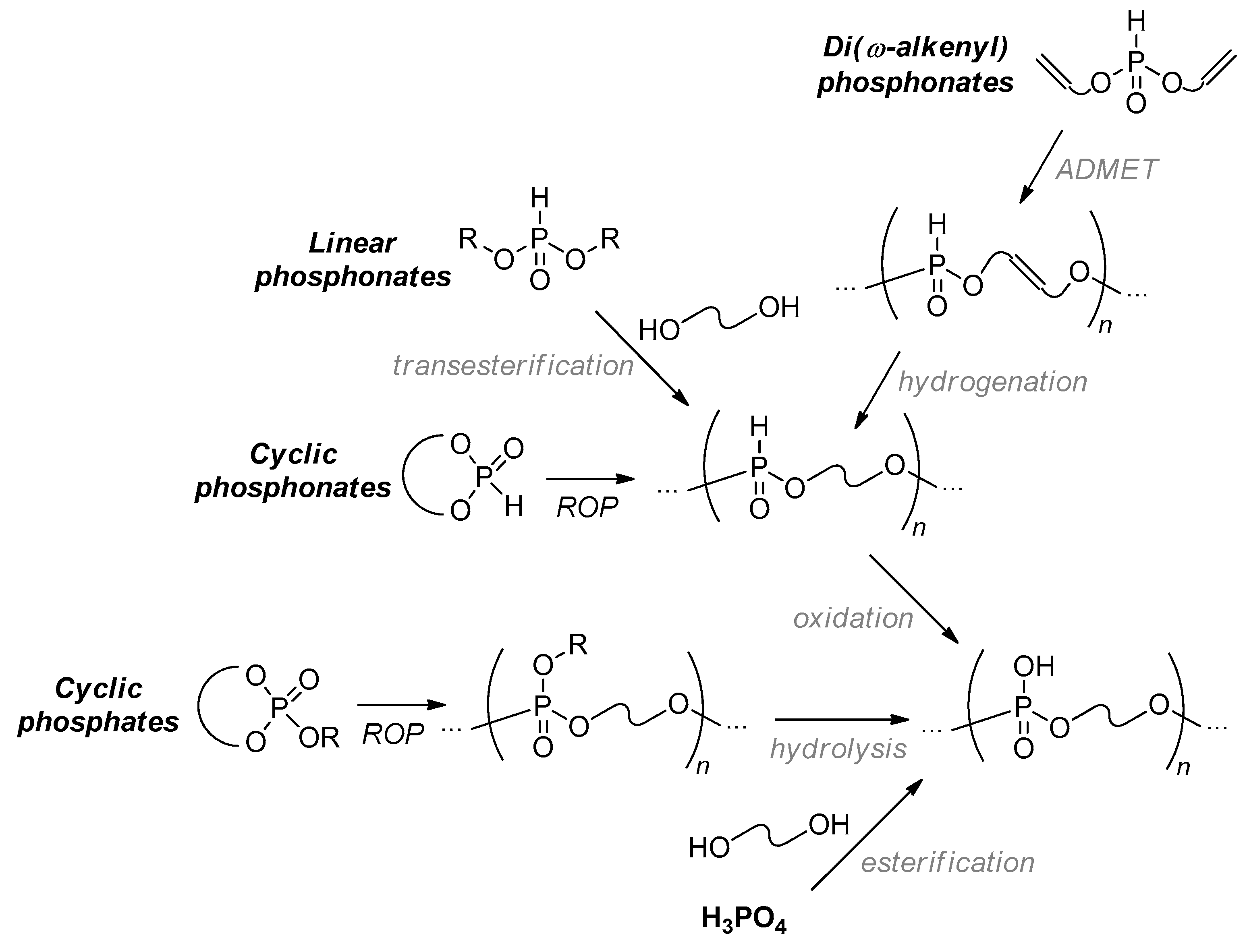
2.1. Synthetic Approaches to Polyphosphodiesters: An Overview
2.2. Polycondensation and Related Methods
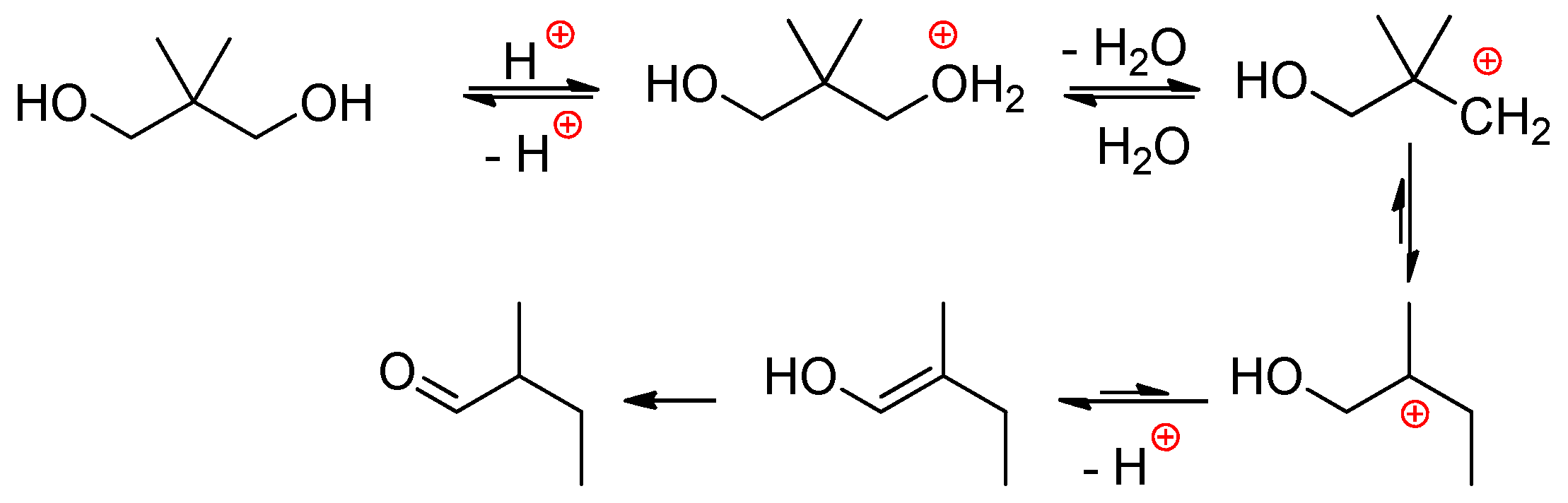
2.2.1. Reactions of H3PO4 with Diols and Polyols
- The reaction starts by the relatively slow dimerization of H3PO4 with a formation of H4P2O7 (and higher polyphosphoric acids) at 100 °C within 40 h, during this stage the water was removed either in the stream of neutral gas or azeotropically with heptane.
- After the addition of EG at 100 °C, H4P2O7 transformed to H3PO4 immediately, and the first phosphorylation reaction within additional 80 h was the formation of HOCH2CH2OP(O)(OH)2 and (HOCH2CH2O)2P(O)OH, triesters were formed in minimal amounts.
- Activation of the monophosphate esters (end groups) at any polymerization degree with H3PO4 proceeds via conversion of monoesters into pyrophosphoric acid esters –OCH2CH2OP(O)(OH)–OP(O)(OH)2 that represent reactive acidic sites.
- The polycondensation product is mostly linear with a structure of PEPA –(OCH2CH2OP(O)(OH))n–.
- Some branch points (triesters) are formed only at high temperature and prolonged polycondensation time.
2.2.2. The Reaction of Dichlorophosphates with Diols
2.2.3. Reaction of Dialkyl (or Diaryl) Phosphonates with Diols and Post-Modification
2.2.4. Polycondensation of (ω-Hydroxyalkyl)phosphonic Acids
2.3. ROP of Cyclic Phosphorus-Containing Monomers and Post-Modification
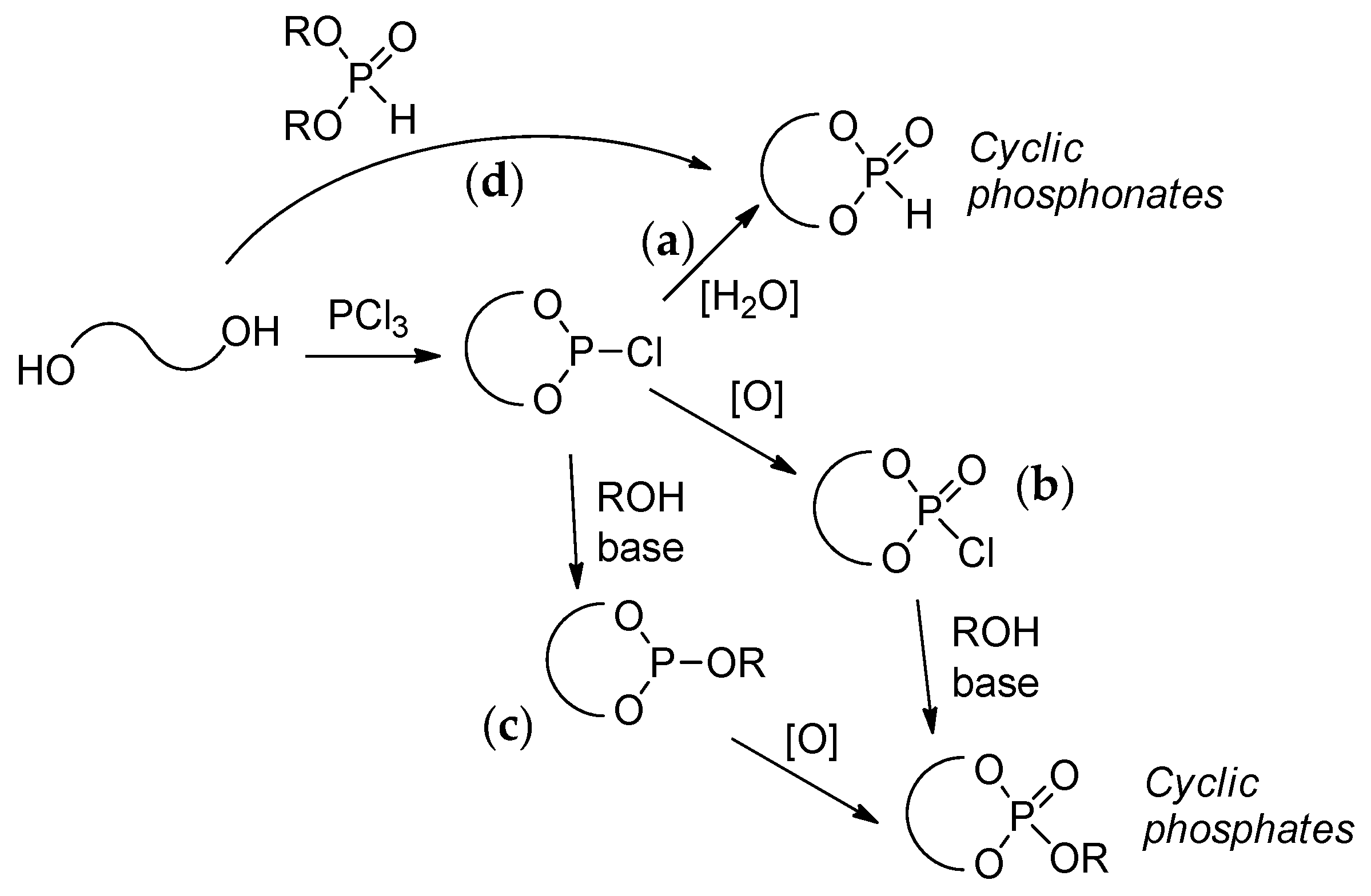
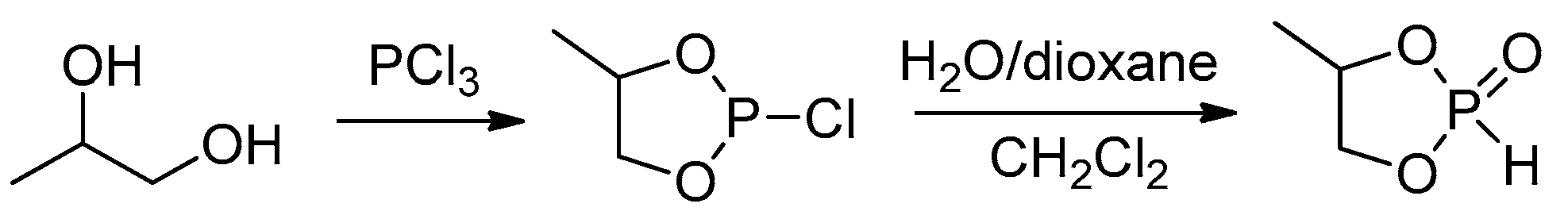
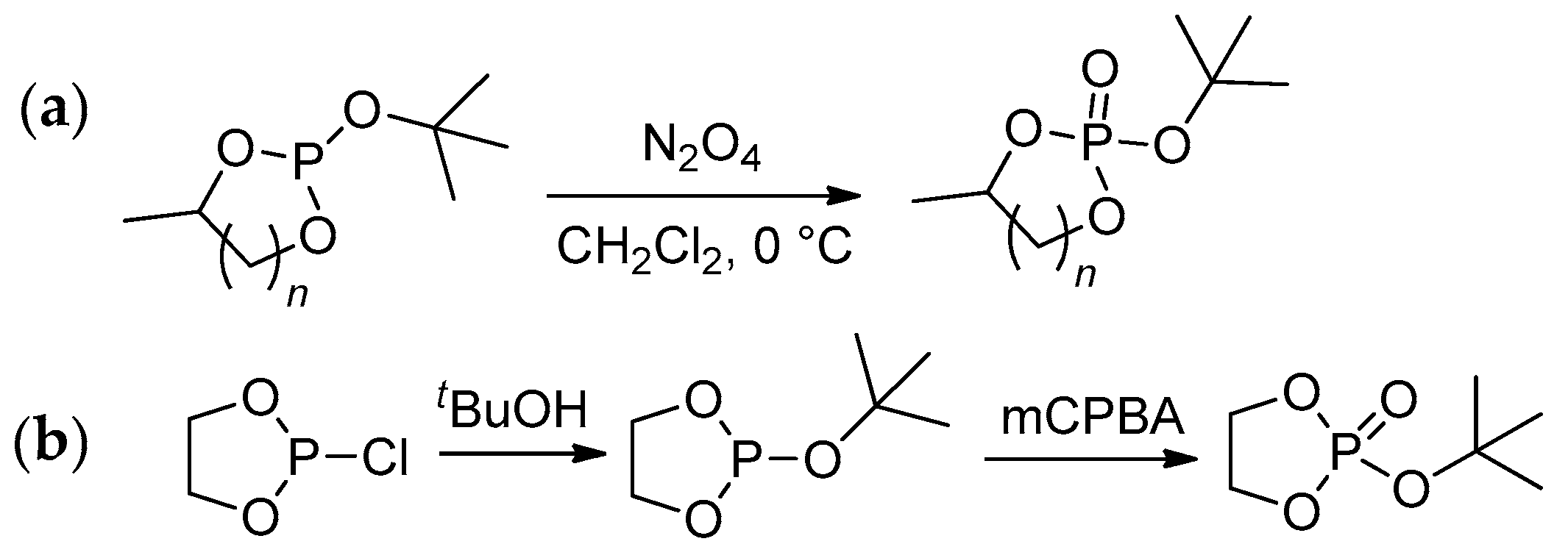
2.3.1. Synthesis of Cyclic Phosphorus-Containing Monomers
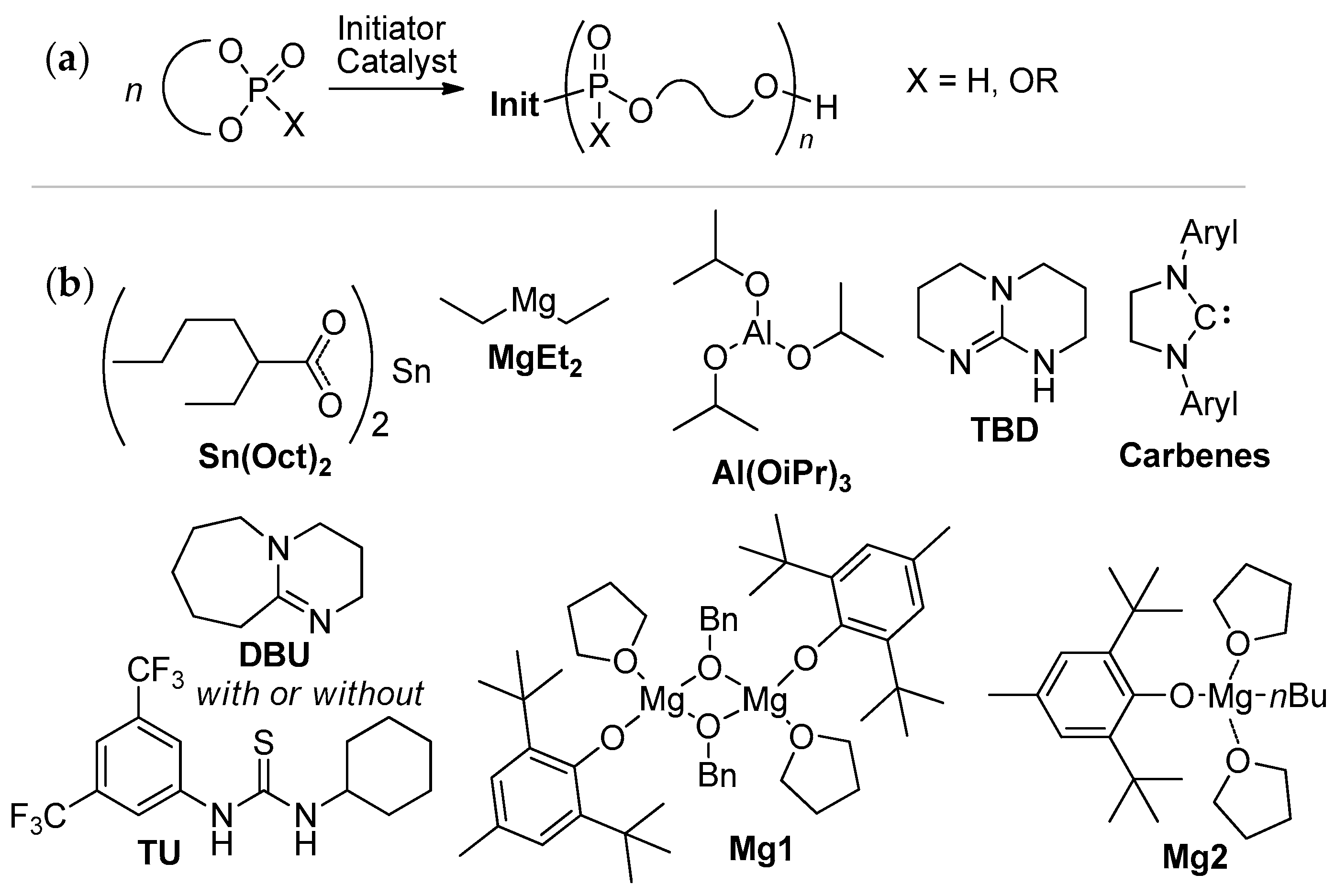
2.3.2. ROP of Cyclic Phosphorus-Containing Monomers
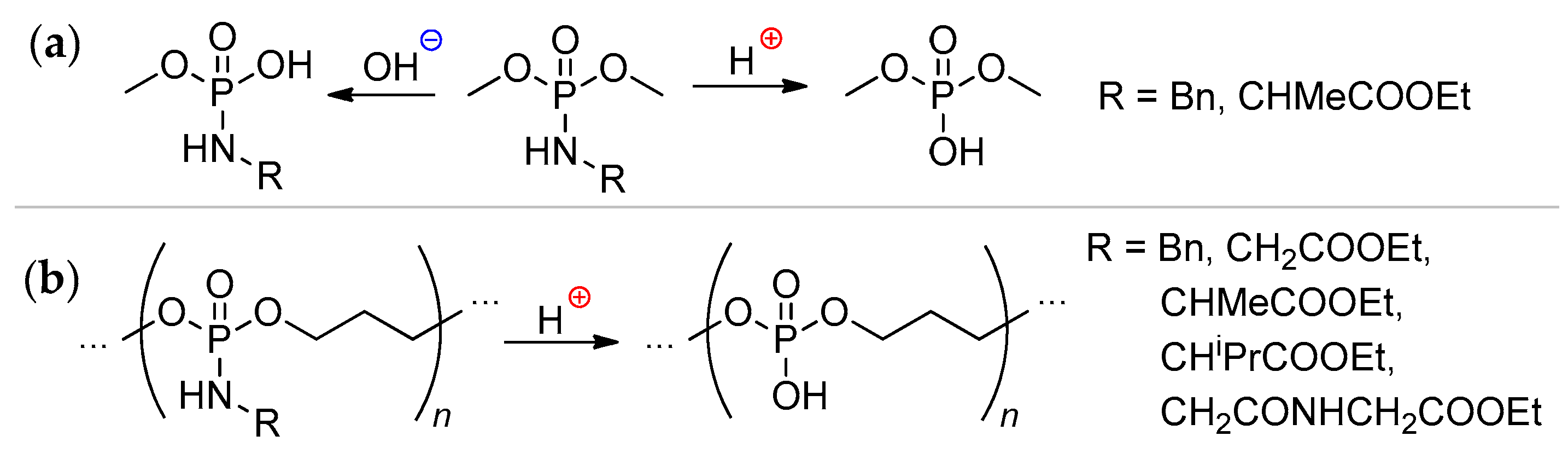
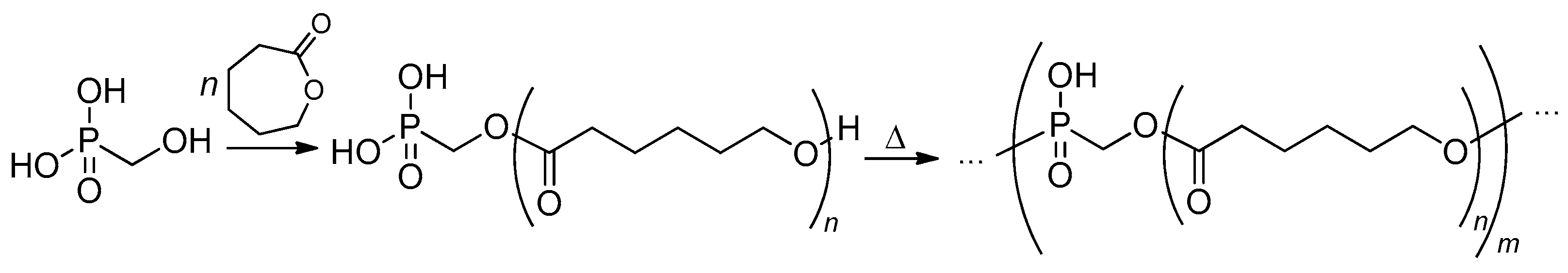
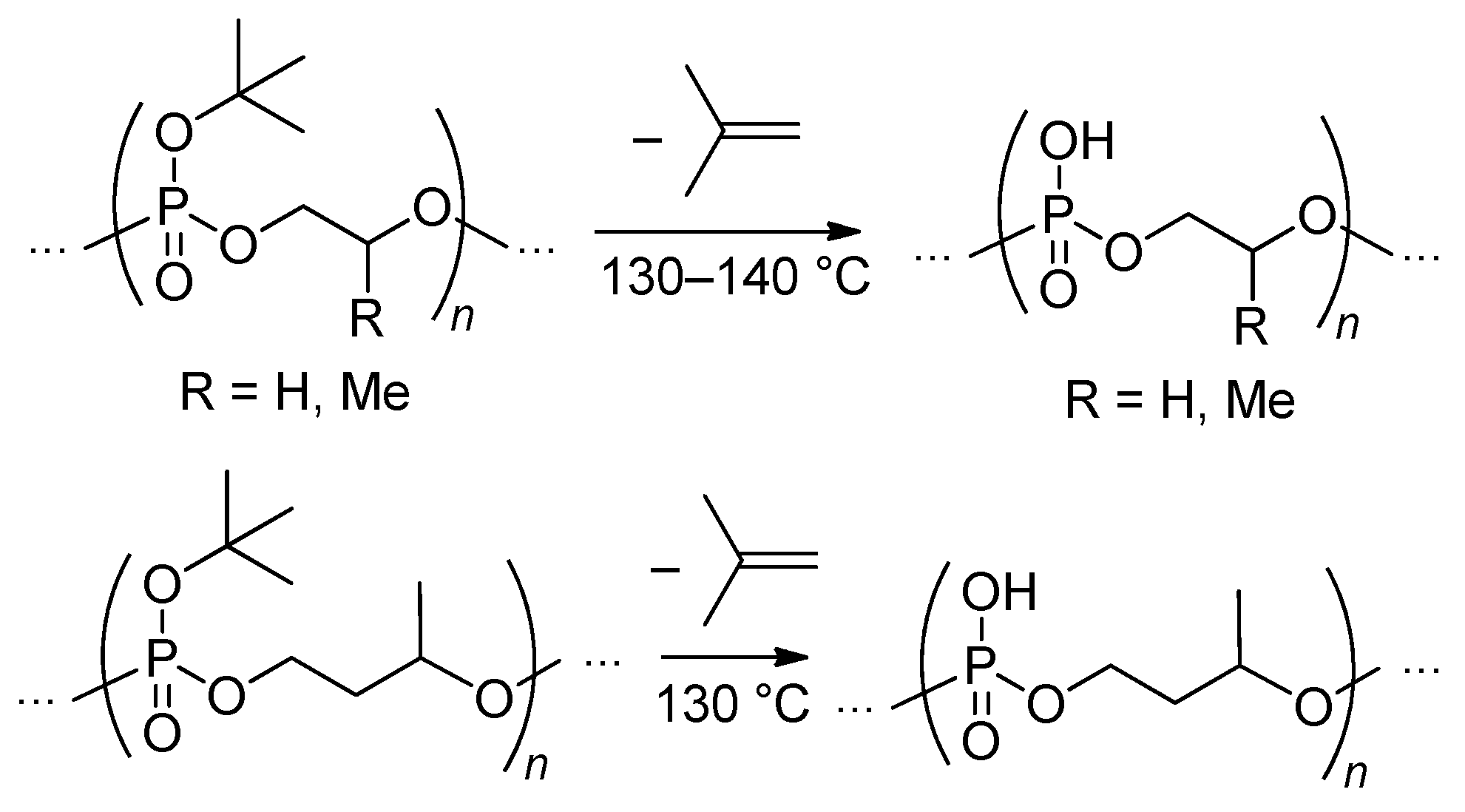
2.3.3. Post-Modification of the Poly(alkylene phosphonate)s
2.3.4. Post-Modification of Poly(alkylene phosphate)s
- Loss of control over polymer architecture and MWD: sterically non-hindered cyclic phosphates can form highly branched poly(alkylene phosphate)s. Switching between the ‘living’ (linear polymer, ĐM~1) and ‘immortal’ (transesterification of the polymer chain, branched polymer, ĐM > 1) ROP modes can occur at elevated temperatures and/or in case of wrong catalyst’ choice. Moreover, even in the presence of ‘good’ catalysts, complete conversion of the monomer greatly increases the risk of subsequent transesterification.
- This is why better chain control can be achieved when using sterically hindered cyclic phosphates, e.g., tBuOEP, despite its minor synthetic accessibility and very low reactivity that limits the use of this monomer in the synthesis of stat- and block-copolymers.
- The use of cyclic phosphonates eliminates the problem of branching and DPn control, but severe oxidation of the P–H bond at the final stage puts the end to a convenient option to introduce biomolecules or usable functional groups at the stages of ROP initiation or termination.
- The nature of the catalytic ROP imposes severe restrictions on the nature of the side substituent R in the molecule of cyclic phosphate (Scheme 16). So, for example, the –CH2CH2CN group, widely used in automated (!) synthesis of DNA analogs [103] and in synthesis of PCPAs with the use of ring-opening metathesis polymerization (ROMP) [104], has not found application in the ROP/deprotection approach to PCPAs, despite the fact that the synthesis of six-membered cyclic phosphate with this substituent was synthesized by Lapienis and Penczek back in 1977 [66].
- Additionally, in general, between fundamental studies of the ROP/deprotection approach to PCPAs in the late 1970s–1980s (conducted for the most part by the Penczek’ group) and relatively recent works (scientific groups of Wooley, Wurm, Iwasaki, Nifant’ev), a two-decades gap in investigations is clearly visible, which affected the progress in this scientific direction.
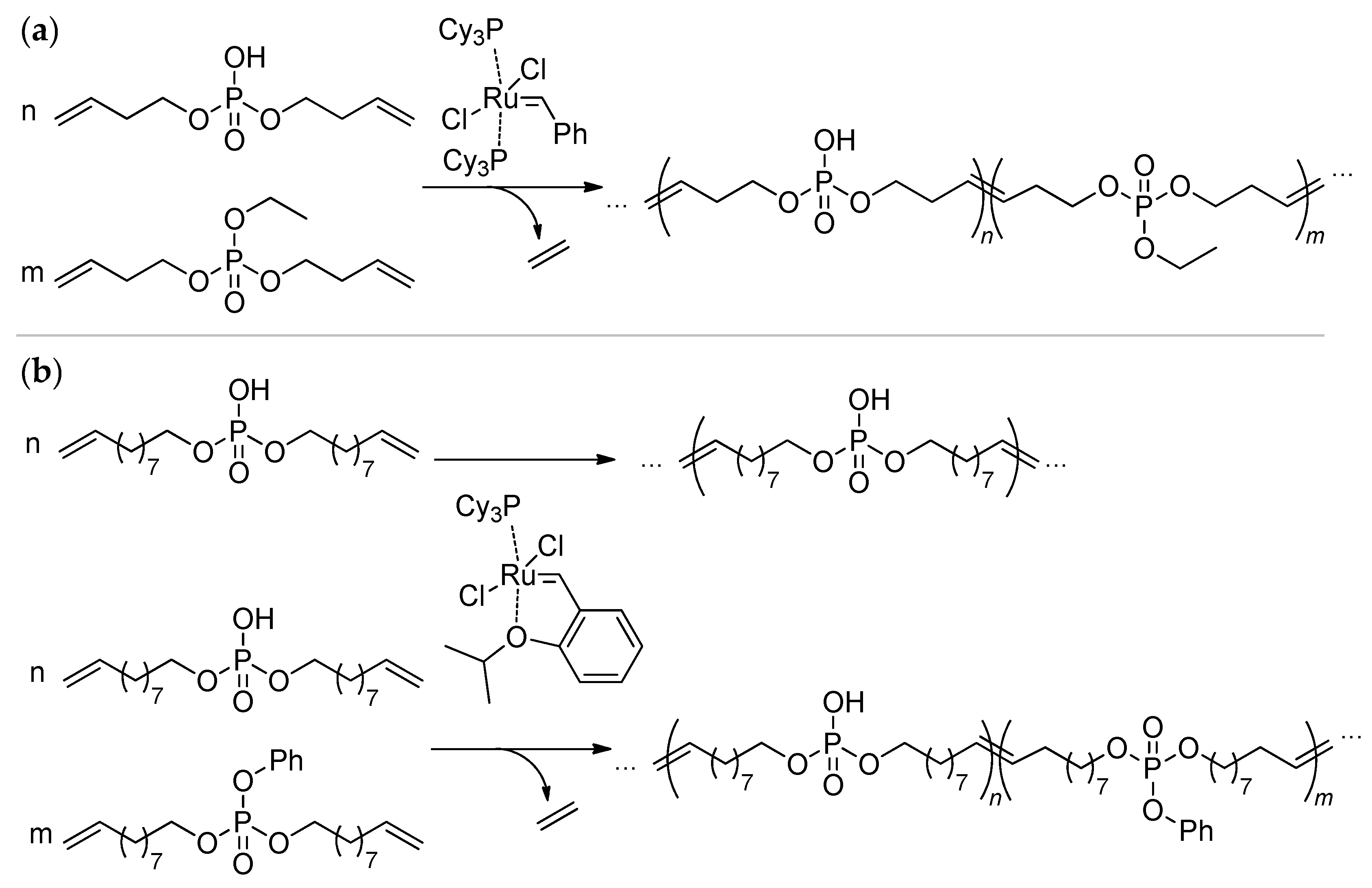
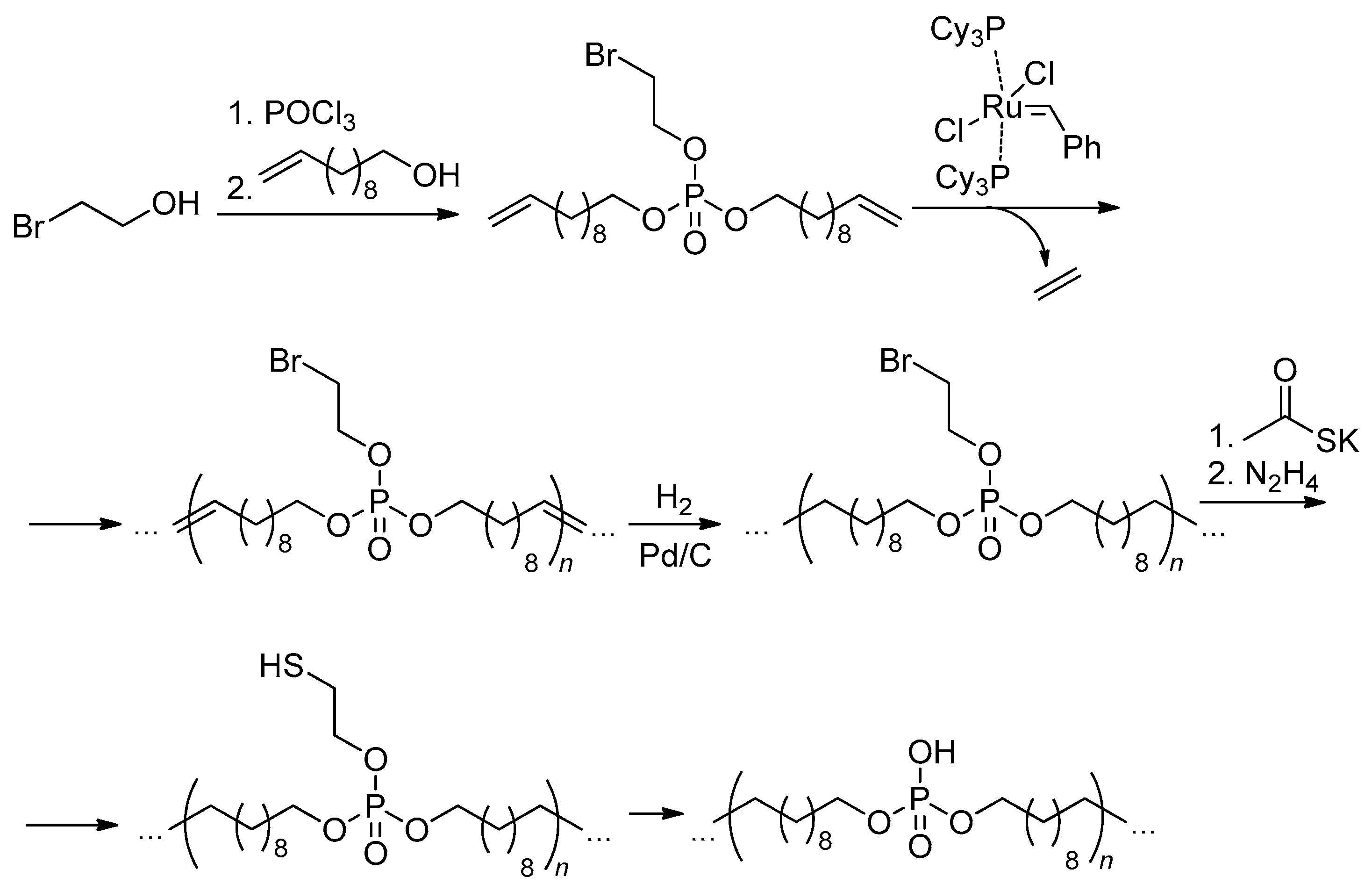
2.4. Metathesis Polycondensation
2.5. Other Synthetic Approaches to Polyphosphodiesters
2.5.1. The Use of Unsaturated 2-Cyanoethyl Phosphates
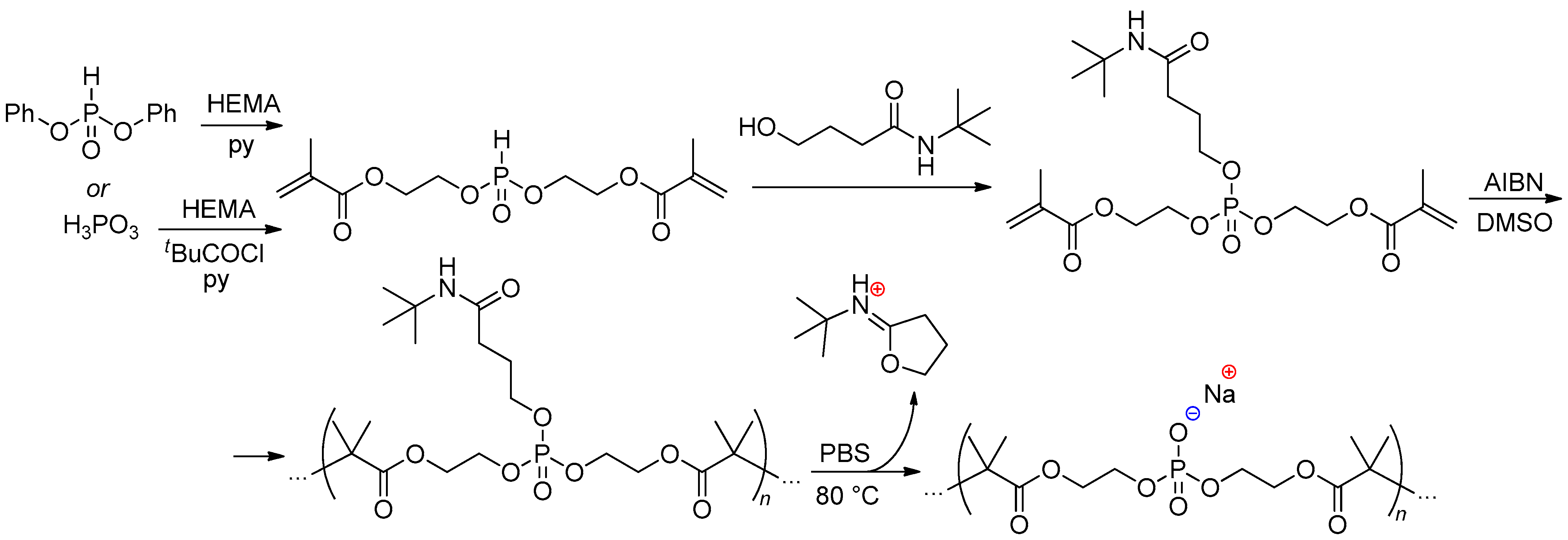
2.5.2. Bis(methacrylate) Phosphonates and Their Post-Modification
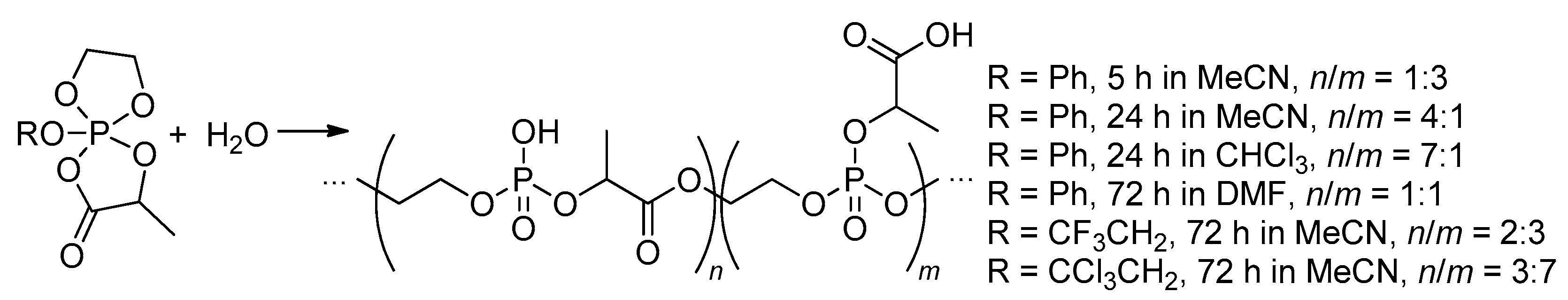
2.5.3. Hydrolytic Polymerization of Spiro(acylpentaoxy)phosphoranes
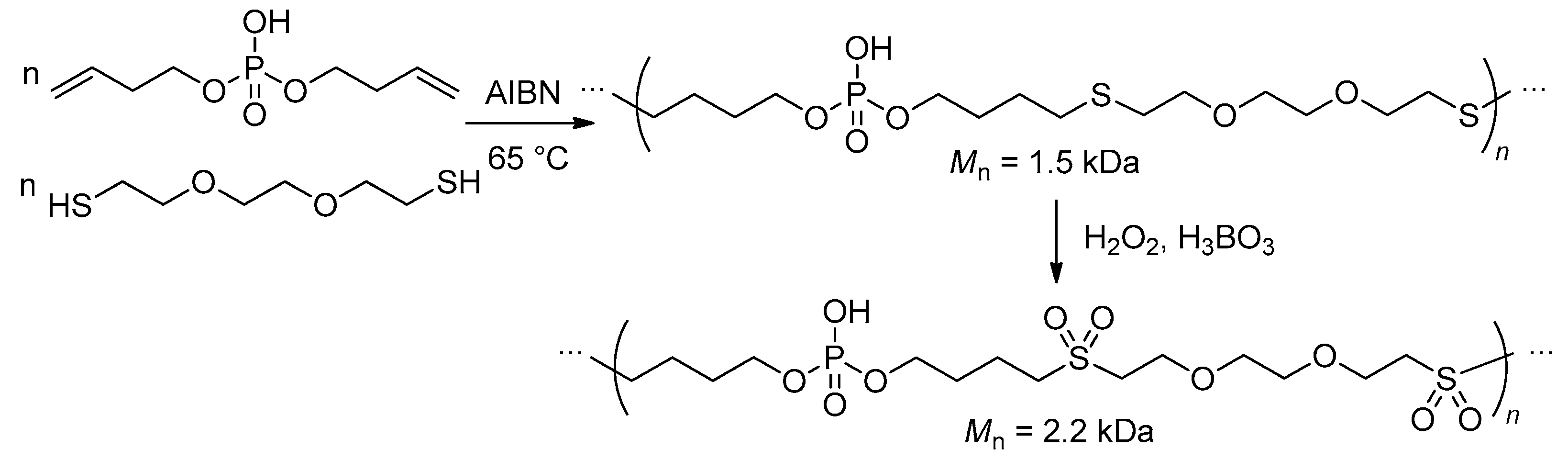
2.5.4. Thiol-Ene Polyaddition
2.5.5. Chain-End Vinyl Functionalization
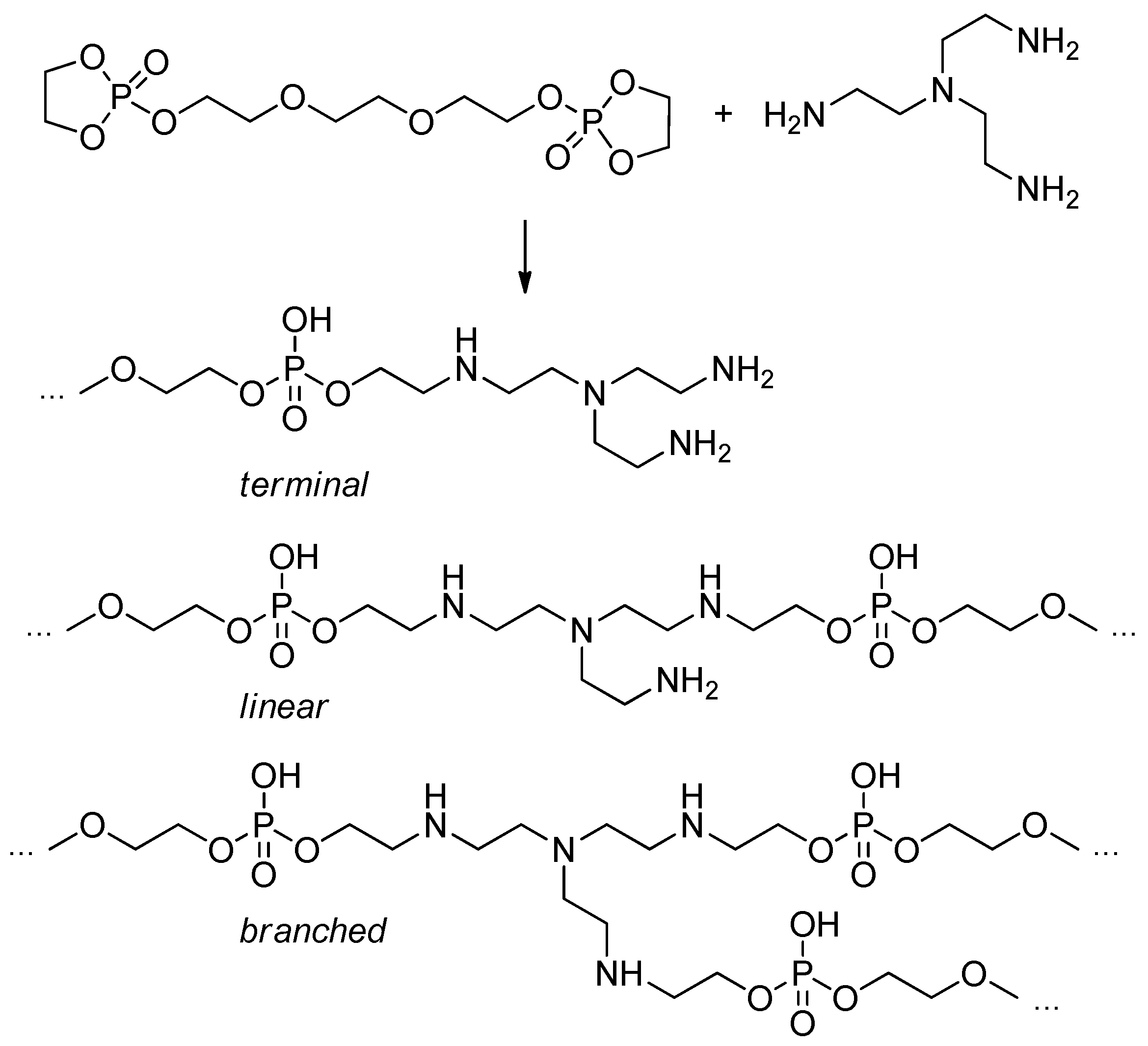
2.5.6. The Use of Bridged Cyclic Phosphates
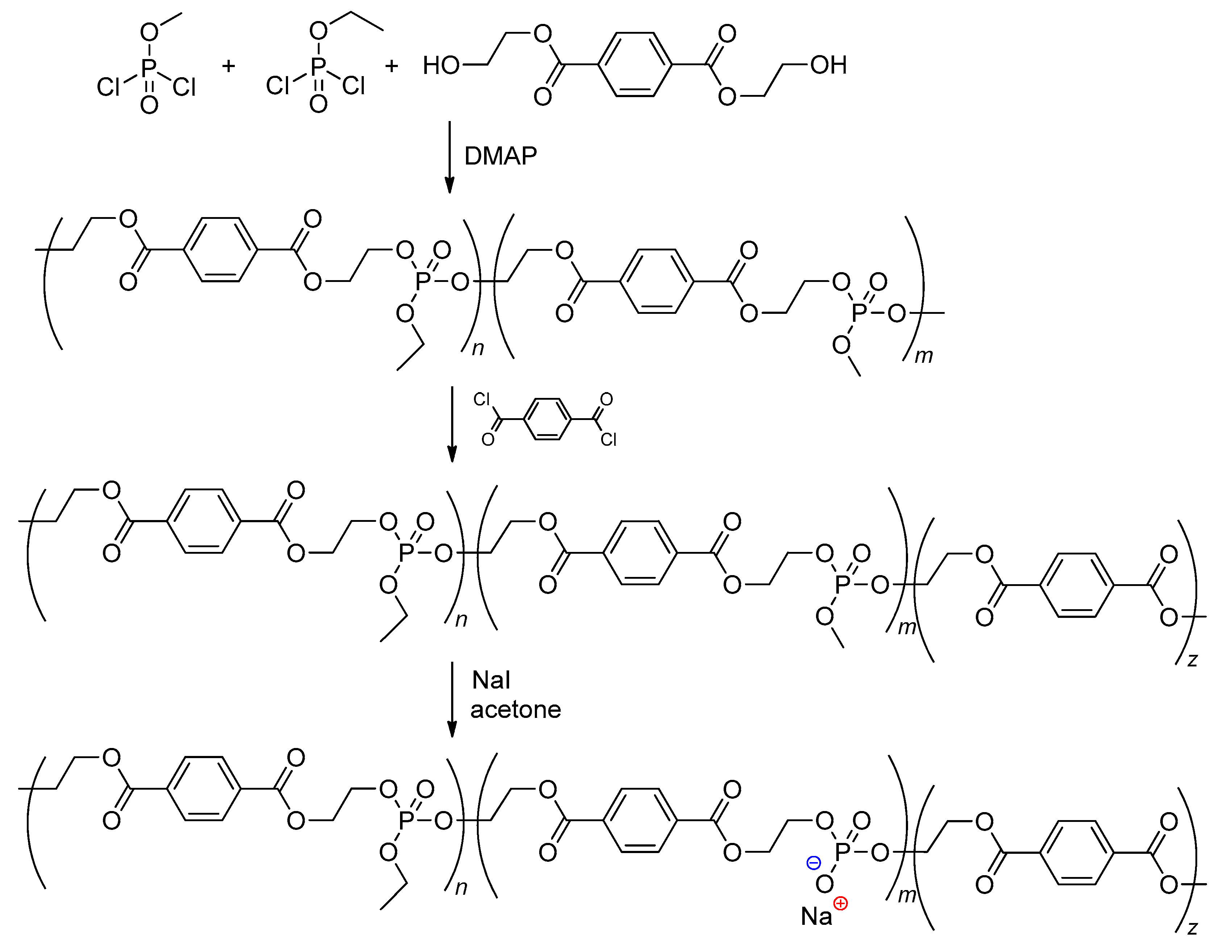
2.5.7. Post-Modification of Polyphosphodiesters
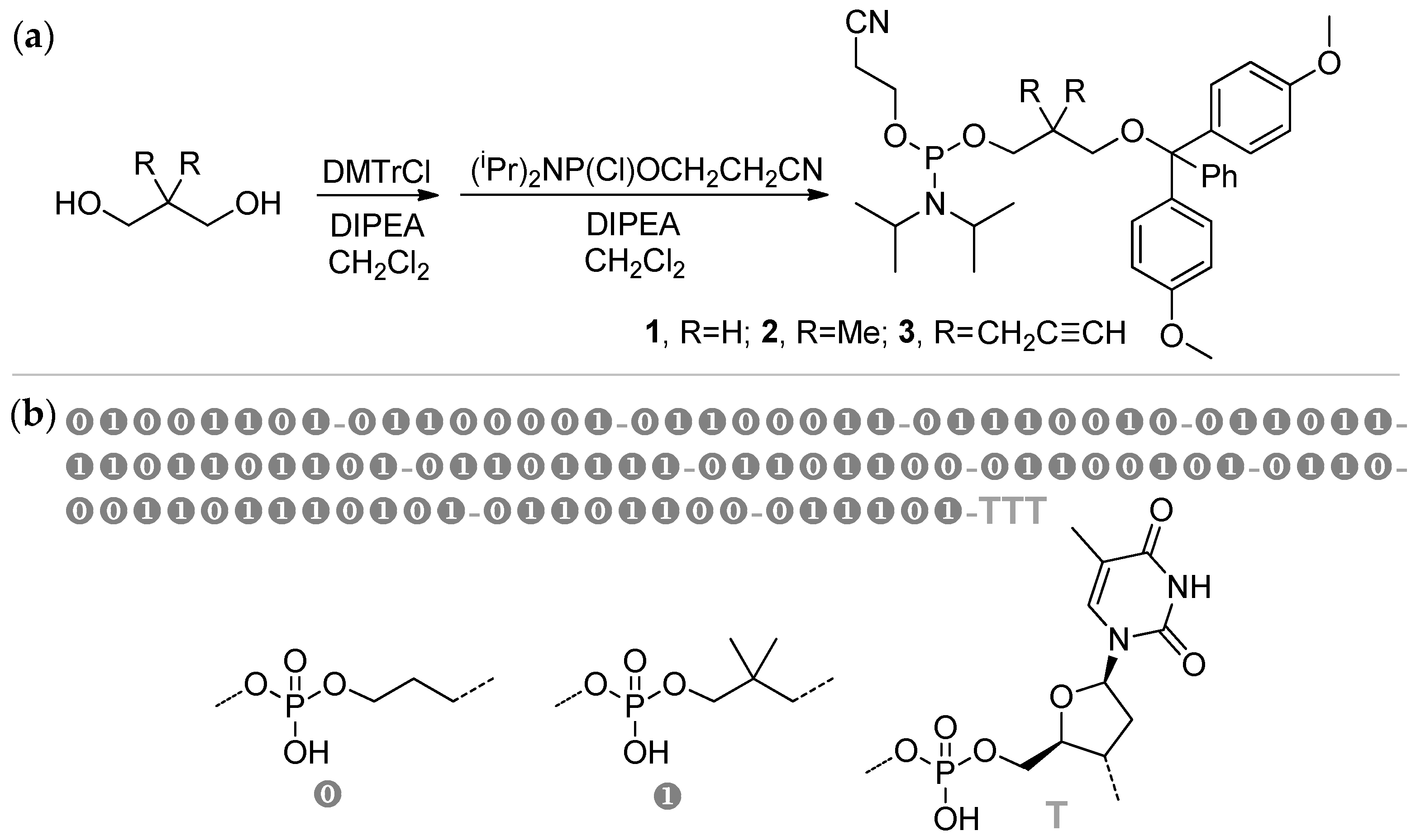
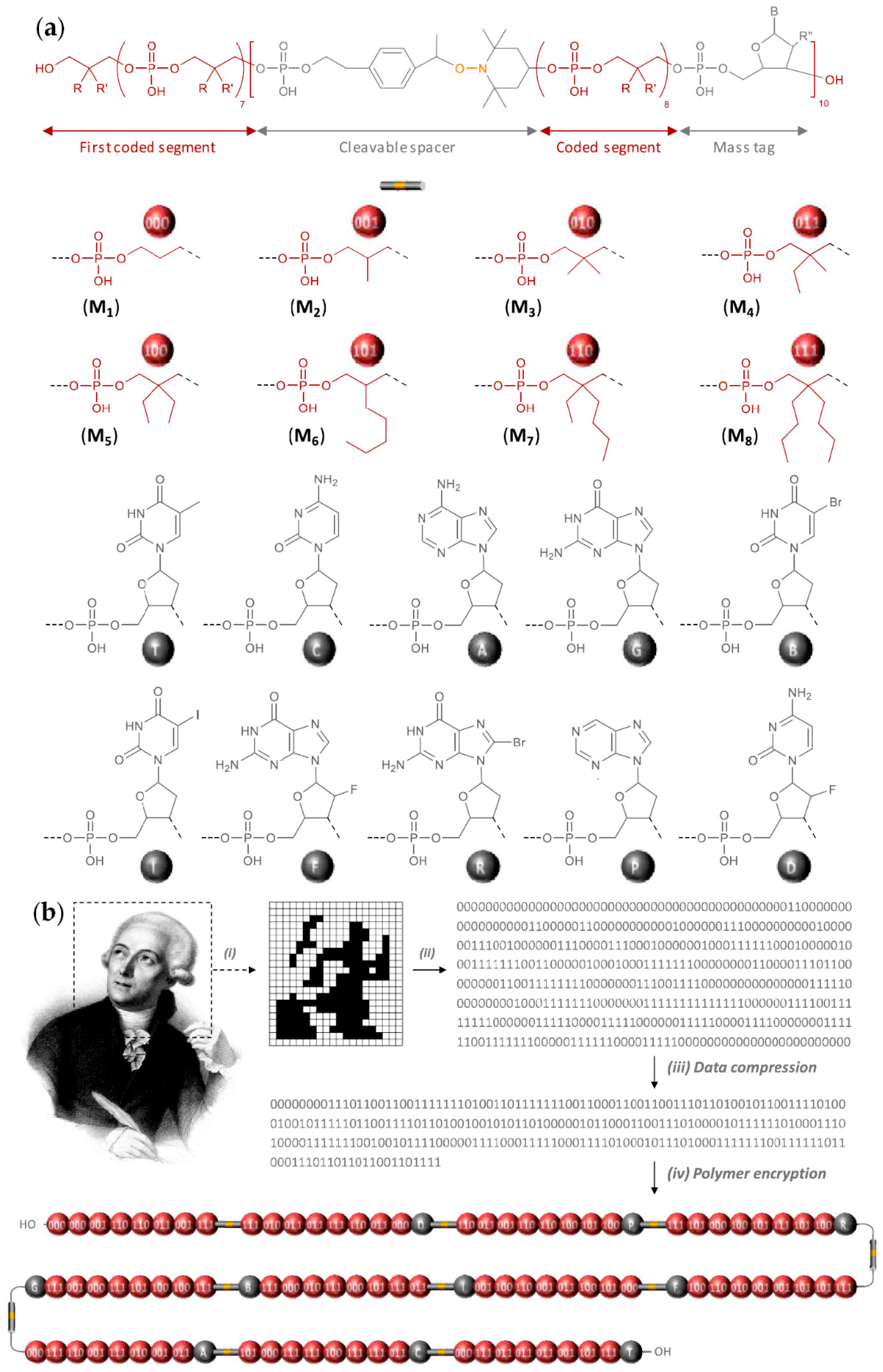
2.6. Sequence-Defined Oligophosphodiesters
3. Properties and Applications of Polyphosphodiesters
3.1. Physico-Chemical Characteristics of Polyphosphodiesters
3.1.1. Physical State and Mechanical Properties of Polyphosphodiesters
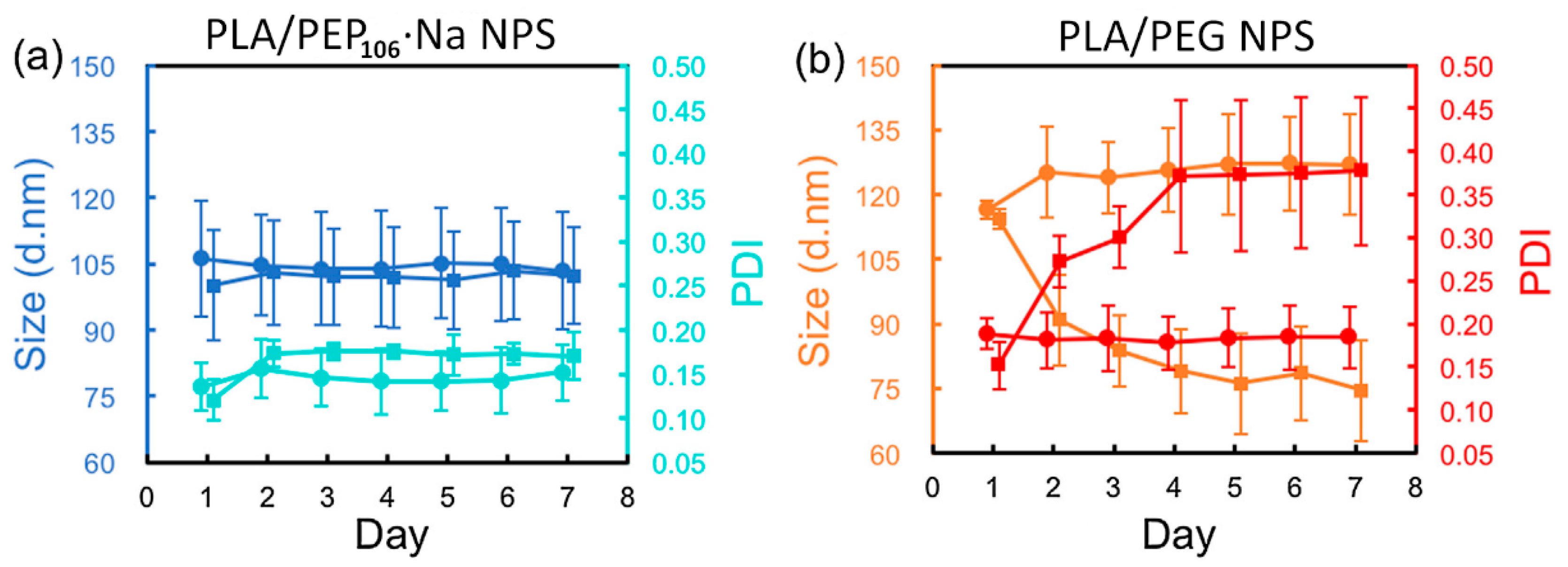
3.1.2. Solution and Colloidal Behavior
3.1.3. Chemical Stability of Polyphosphodiesters
3.2. Metal Complexation of the Polyphosphodiesters and Polymer-Inorganic Hybrids
3.2.1. Complexation of Polyphosphodiesters with Metal Ions
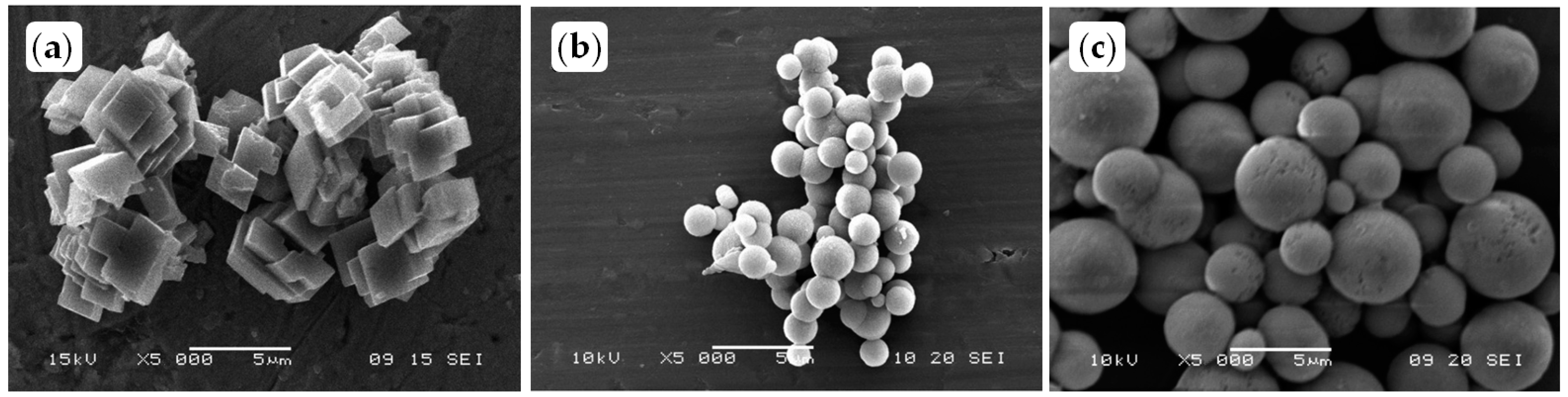
3.2.2. Effects of the Polyphosphodiesters on Crystal Growth and Morphology
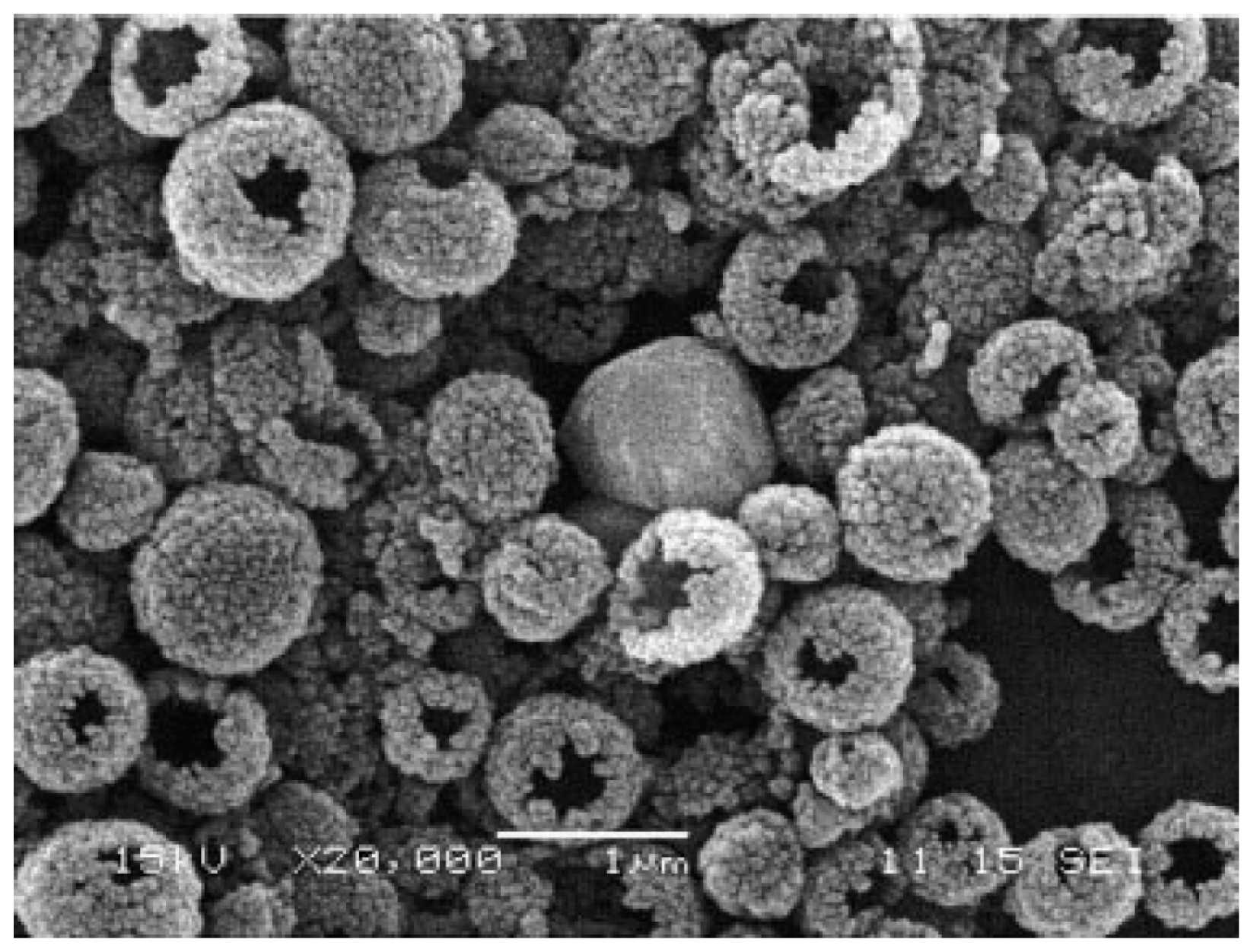
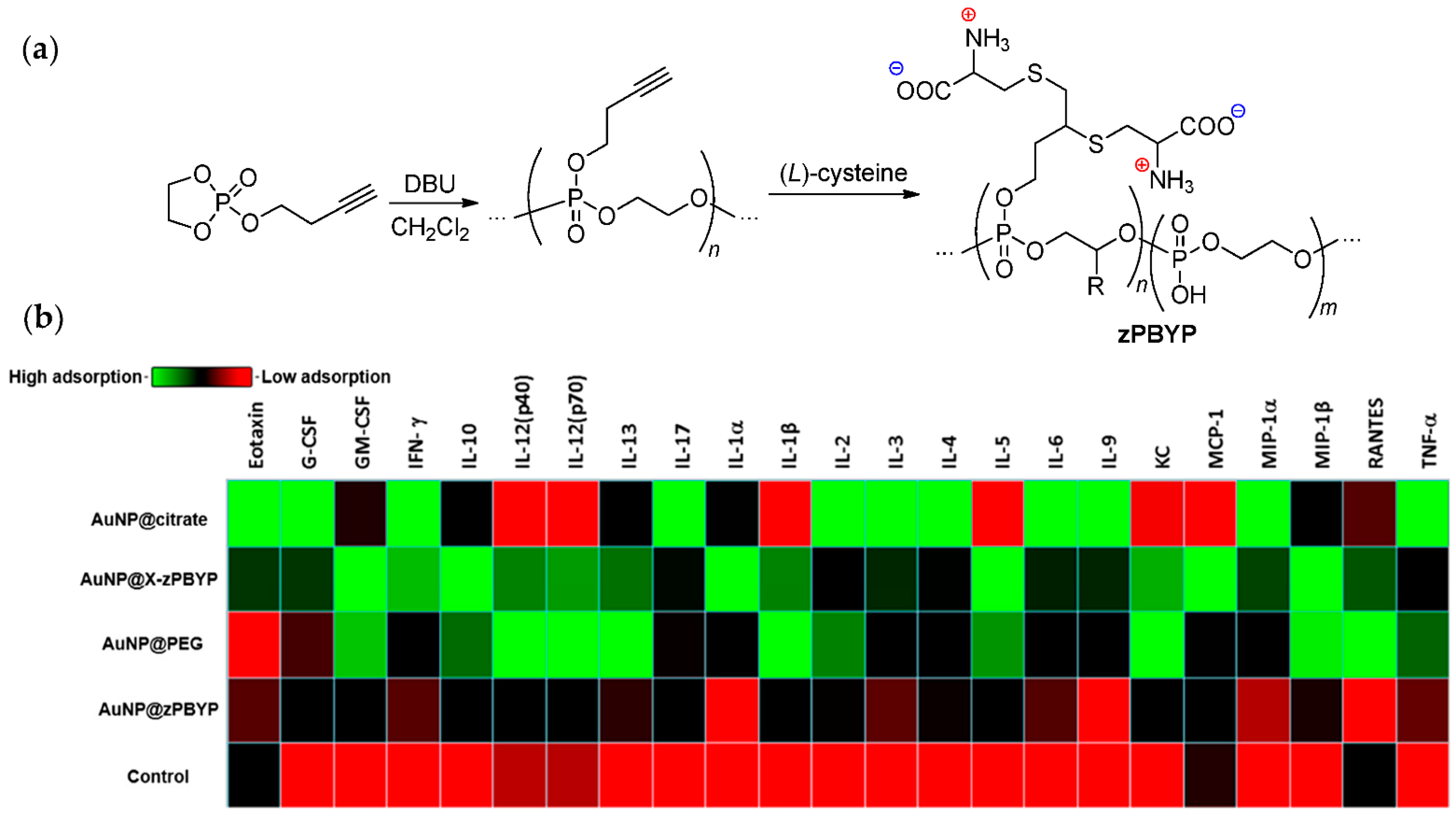
3.2.3. Hybrid Nanoparticle Formation by Polyphosphodiesters
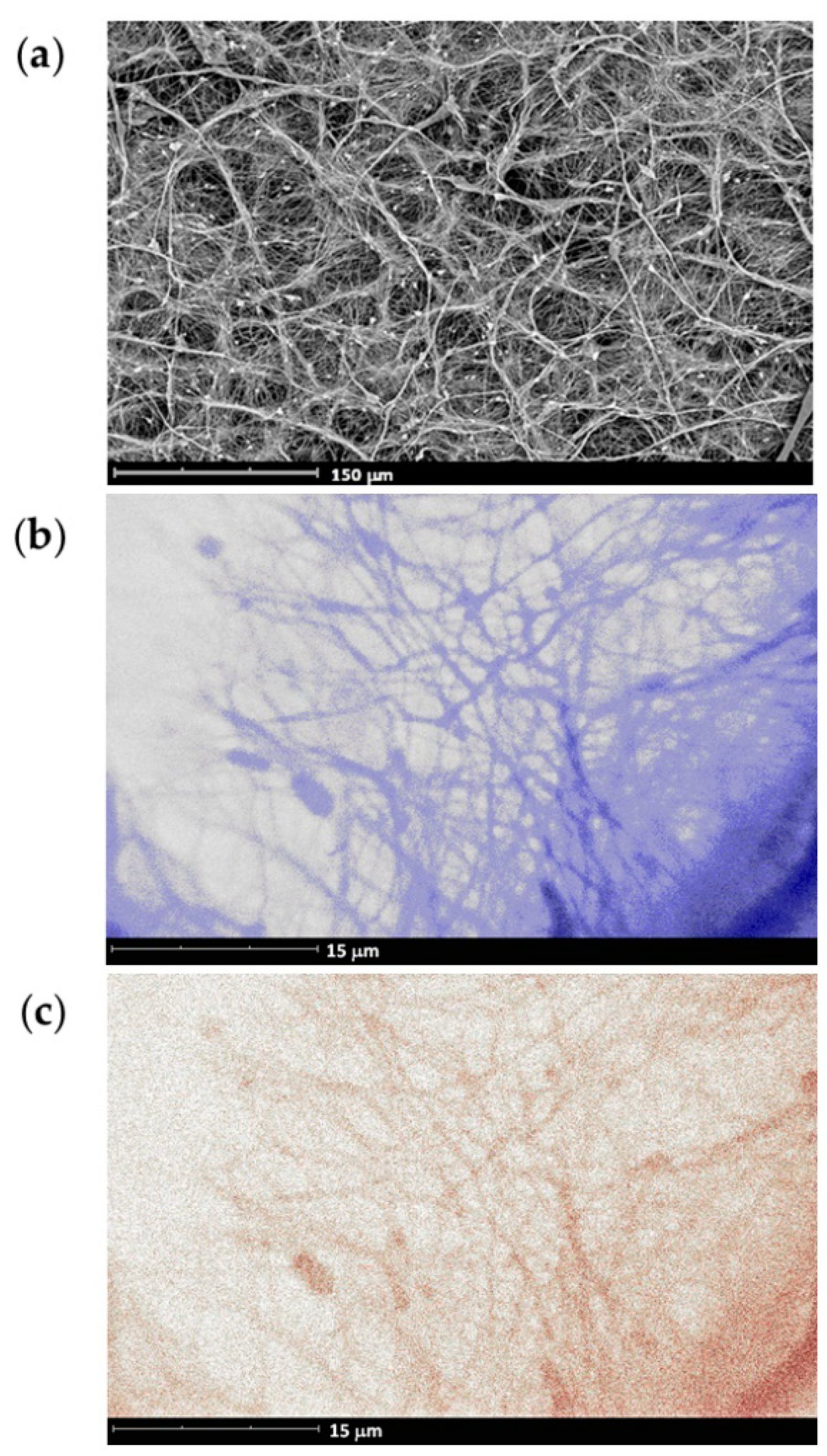
3.3. Biomedical Applications of Polyphosphodiesters
3.3.1. Polyphosphodiesters and Cell Viability/Metabolism
3.3.2. Polyphosphodiesters and Cell Differentiation
3.3.3. Polyphosphodiesters and Nucleic Acids, Proteins and Other Substances in the Body
3.3.4. Biocompatibility and Inflammatory Effect of Polyphosphodiesters
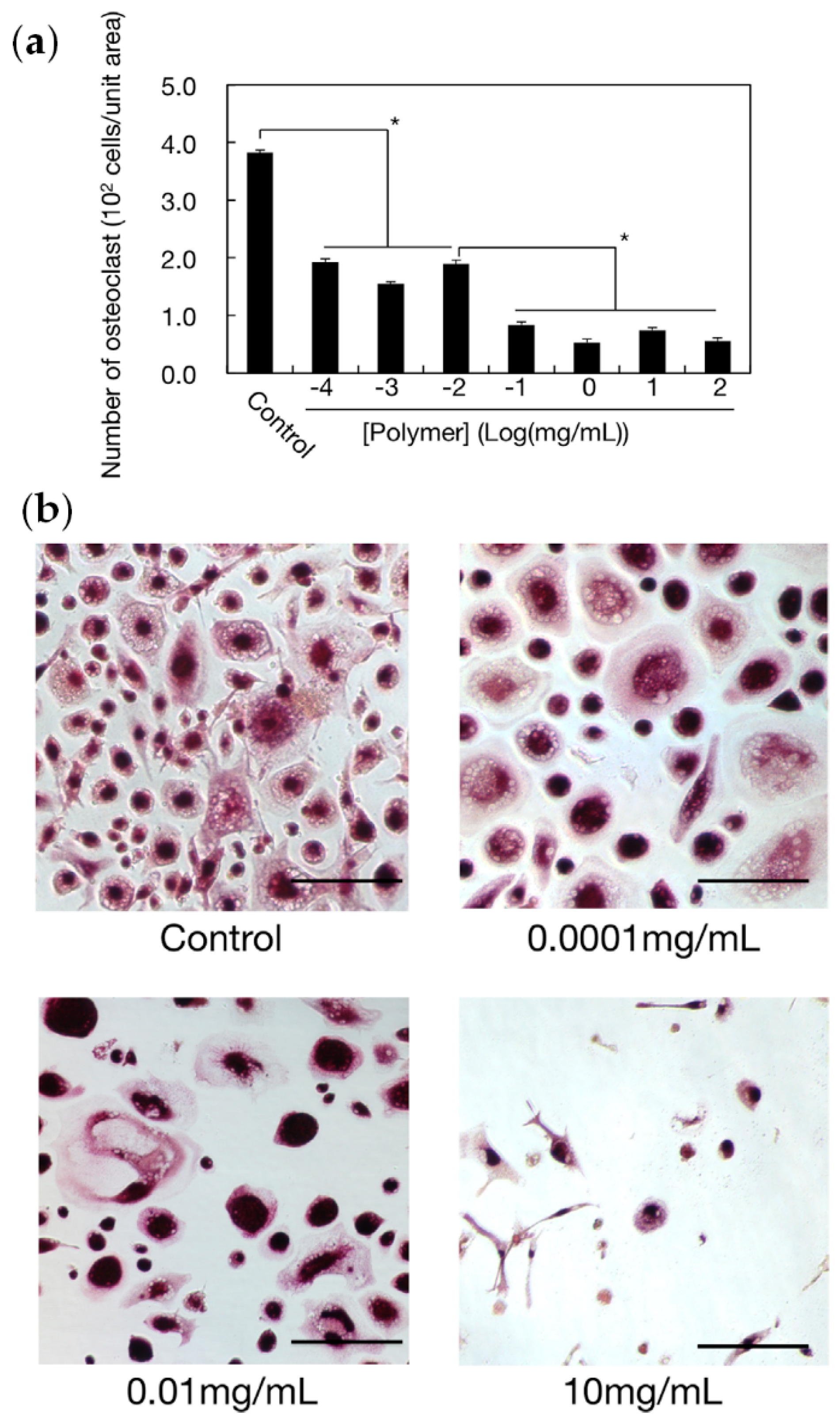
3.3.5. Bone Affinity of Polyphosphodiesters and Their Prospects for Bone Surgery
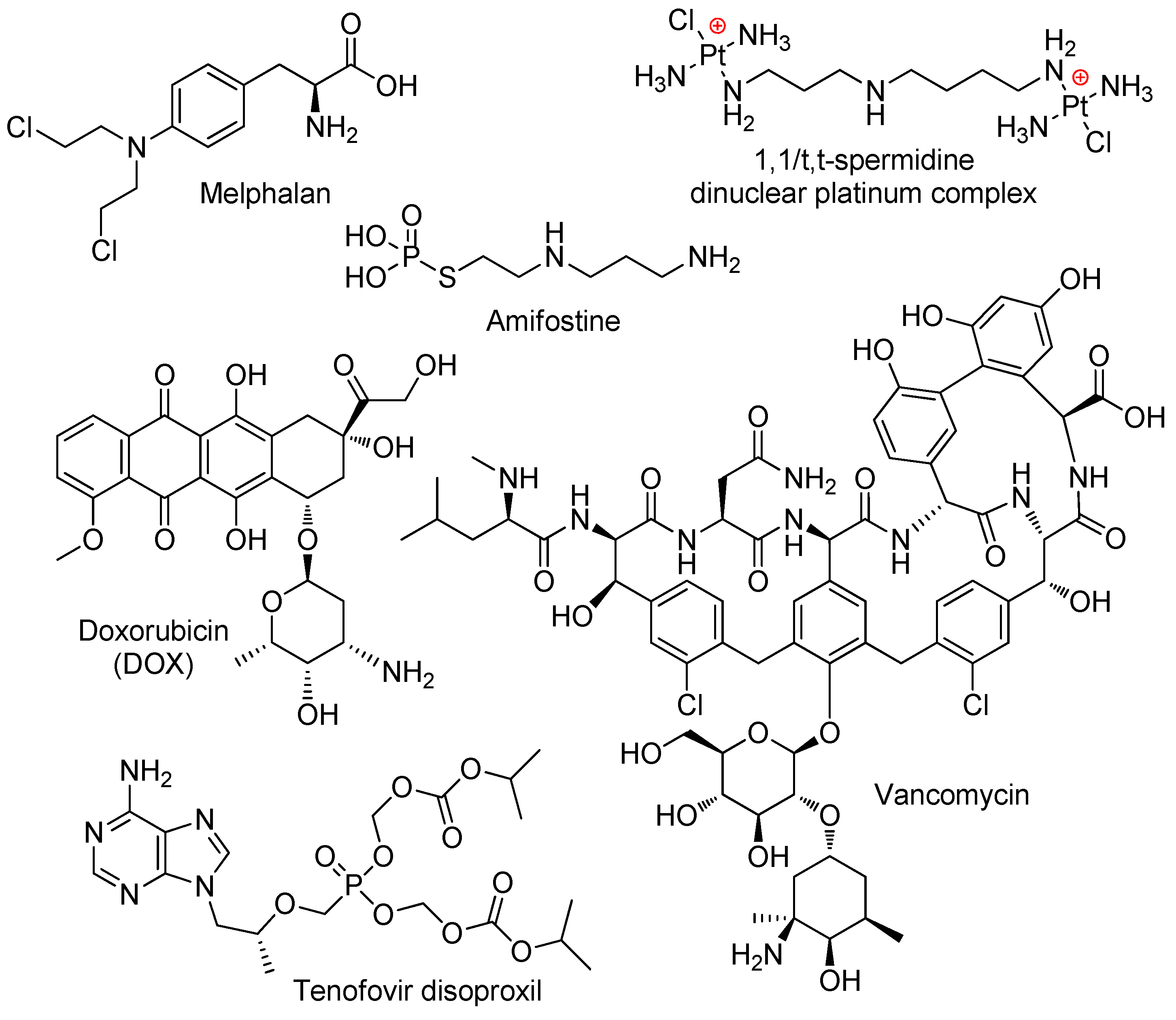
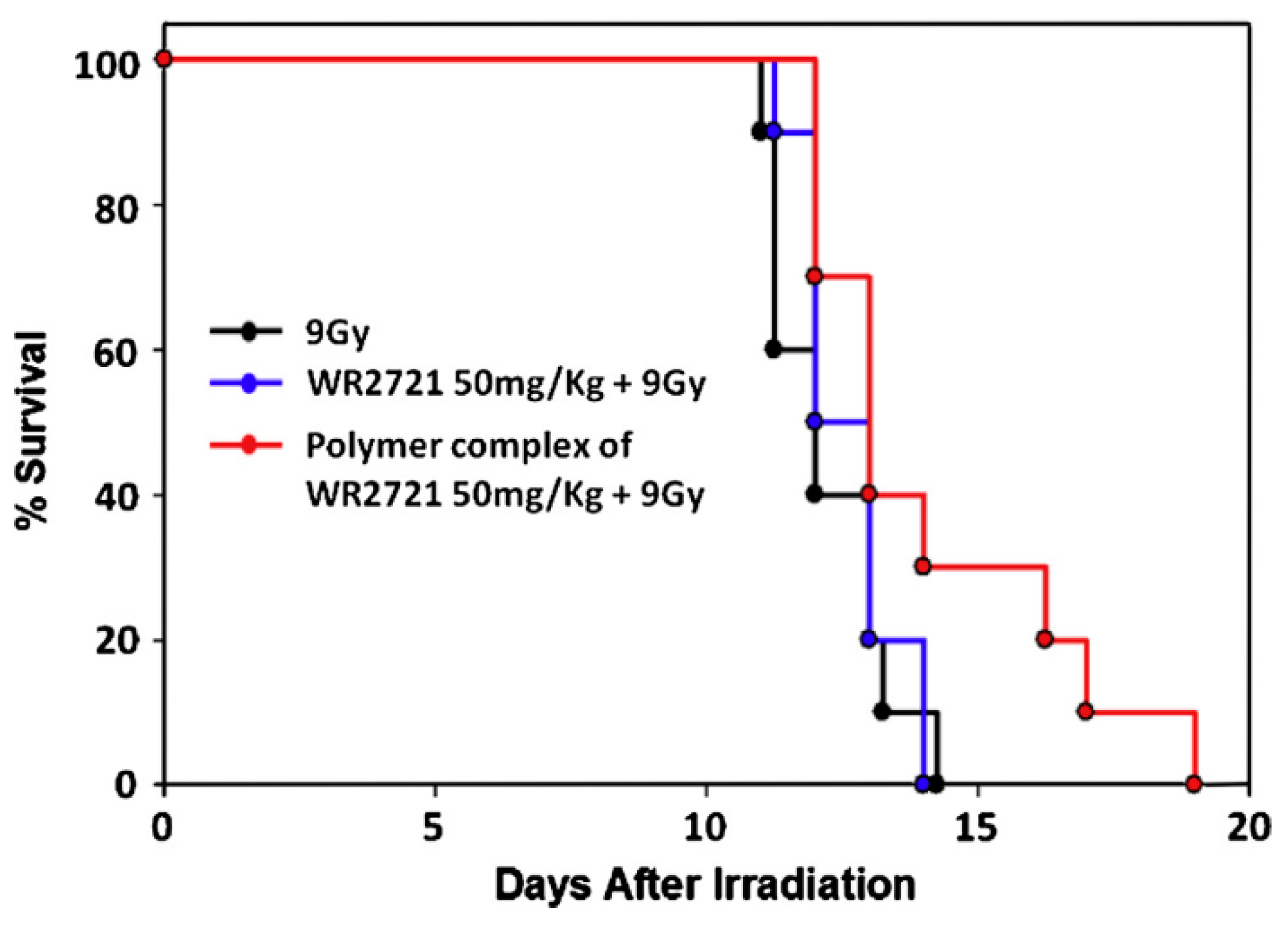
3.3.6. Drug Delivery and Drug Release with the Use of Polyphosphodiesters
- compatibilization effect of copolymers, containing polyester and PEPA block, on formation and properties of polyester/HAp composites.
- influence of PEPA on drug absorption and release by polymer/HAp composite.
3.4. Other Applications of Polyphosphodiesters
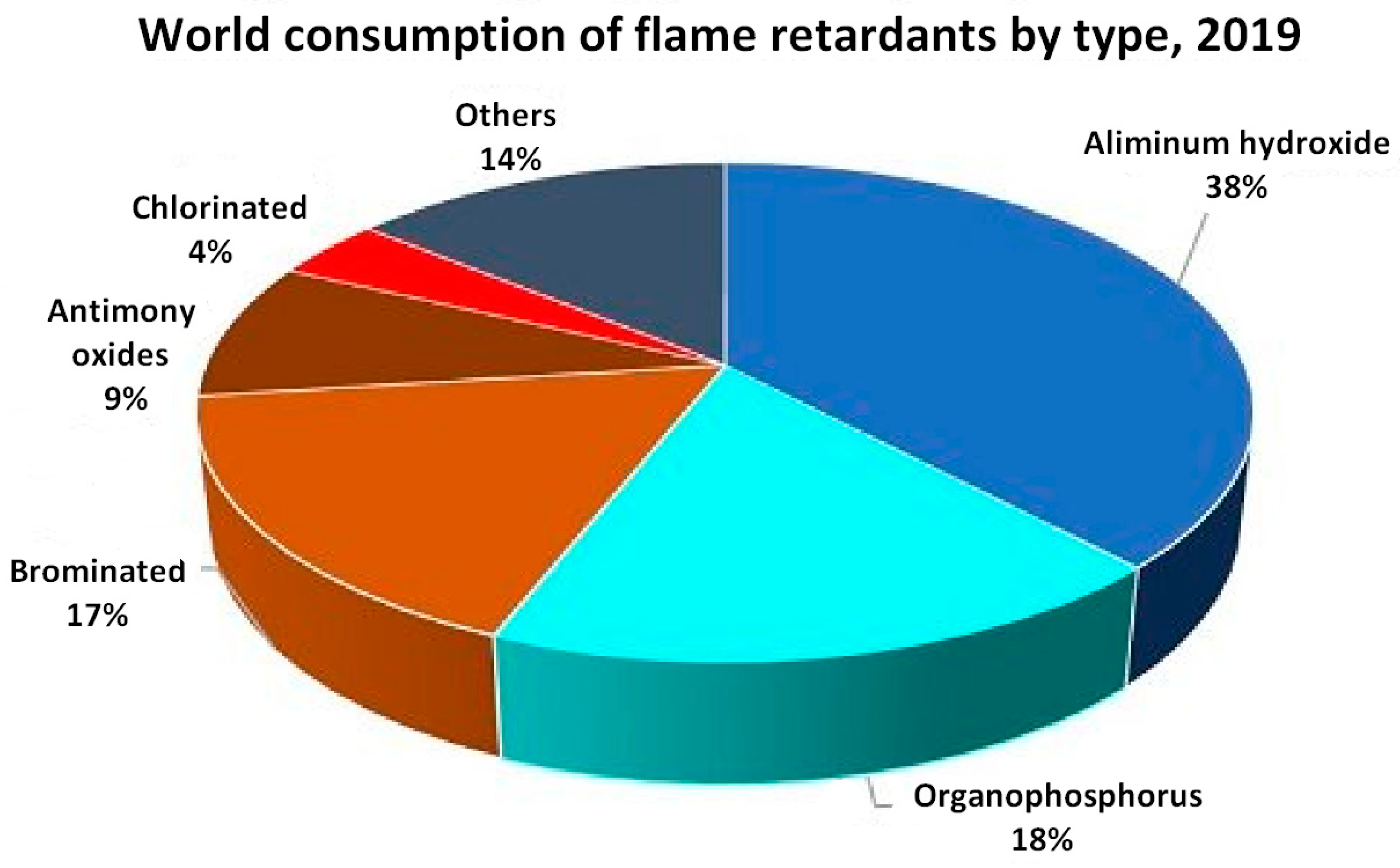
Polyphosphodiesters as Flame Retardants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penczek, S.; Pretula, J.; Kubisa, P.; Kaluzynski, K.; Szymanski, R. Reactions of H3PO4 forming polymers. Apparently simple reactions leading to sophisticated structures and applications. Prog. Polym. Sci. 2015, 45, 44–70. [Google Scholar] [CrossRef]
- Appukutti, N.; Serpell, C.J. High definition polyphosphoesters: Between nucleic acids and plastics. Polym. Chem. 2018, 9, 2210–2226. [Google Scholar] [CrossRef]
- Yilmaz, Z.E.; Jérôme, C. Polyphosphoesters: New trends in synthesis and drug delivery applications. Macromol. Biosci. 2016, 16, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio- and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Iwasaki, Y. Bone Mineral Affinity of Polyphosphodiesters. Molecules 2020, 25, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penczek, S.; Pretula, J.; Kaluzynski, K. Poly(alkylene phosphates): From Synthetic Models of Biomacromolecules and Biomembranes toward Polymer−Inorganic Hybrids (Mimicking Biomineralization). Biomacromolecules 2005, 6, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Penczek, S.; Pretula, J.B.; Kaluzynski, K.; Lapienis, G. Polymers with Esters of Phosphoric Acid Units: From Synthesis, Models of Biopolymers to Polymer—Inorganic Hybrids. Isr. J. Chem. 2012, 52, 306–319. [Google Scholar] [CrossRef]
- Xie, L.; Jakob, U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J. Biol. Chem. 2019, 294, 2180–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, W.E.G.; Schröder, H.C.; Wang, X. Inorganic Polyphosphates As Storage for and Generator of Metabolic Energy in the Extracellular Matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef]
- Mao, C. The Emergence of Complexity: Lessons from DNA. PLoS Biol. 2004, 2, e431. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall Teichoic Acid Function, Biosynthesis, and Inhibition. ChemBioChem 2010, 11, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbach, T.; Wurm, F.R. Poly(phosphoester)s: A new platform for degradable polymers. Angew. Chem. Int. Ed. 2015, 54, 6098–6108. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-Containing Polymers: A Great Opportunity for the Biomedical Field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Teasdale, I. Main-Chain Phosphorus-Containing Polymers for Therapeutic Applications. Molecules 2020, 25, 1716. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, C.; Tinè, M.R.; Wurm, F.R. Main-chain water-soluble polyphosphoesters: Multi-functional polymers as degradable PEG-alternatives for biomedical applications. Eur. Polym. J. 2020, 141, 110079. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Cai, X.; Huang, Q.; Xiao, J.; Cheng, Y. Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials 2021, 265, 120404. [Google Scholar] [CrossRef]
- Hiranphinyophat, S.; Iwasaki, Y. Controlled biointerfaces with biomimetic phosphorus-containing polymers. Sci. Technol. Adv. Biomater. 2021, 22, 301–316. [Google Scholar] [CrossRef]
- Wurm, F.R. Binding matters: Binding patterns control the degradation of phosphorus-containing polymers. Green Mater. 2016, 4, 135–139. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Jones, A.S. Synthetic analogues of nucleic acids—a review. Int. J. Biol. Macromol. 1979, 1, 194–207. [Google Scholar] [CrossRef]
- Kashida, H.; Murayama, K.; Asanuma, H. Acyclic artificial nucleic acids with phosphodiester bonds exhibit unique functions. Polym. J. 2016, 48, 781–786. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Libisowski, J.; Penczek, S. A New Class of Synthetic Polyelectrolytes. Acidic Polyesters of Phosphoric Acid (Poly(hydroxyalkylene phosphates)). Macromolecules 1976, 9, 365–367. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J. High-molecular-weight poly(alkylene phosphates) and preparation of amphiphilic polymers thereof. Macromolecules 1993, 26, 2228–2233. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. Poly(ethylene glycol) ionomers with phosphate diester linkages. Makromol. Chem. Rapid. Commun. 1988, 9, 731–737. [Google Scholar] [CrossRef]
- Mitova, V.; Slavcheva, S.; Shestakova, P.; Momekova, D.; Stoyanov, N.; Momekov, G.; Troev, K.; Koseva, N. Polyphosphoester conjugates of dinuclear platinum complex: Synthesis and evaluation of cytotoxic and the proapoptotic activity. Eur. J. Med. Chem. 2014, 72, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Troev, K.; Naruoka, A.; Terada, H.; Kikuchi, A.; Makino, K. New Efficient Method of Oxidation of Poly(alkylene-H-phosphonate)s: A Promising Route to Novel co-Polyphosphoesters. Macromolecules 2012, 45, 5698–5703. [Google Scholar] [CrossRef]
- Troev, K.; Tsatcheva, I.; Koseva, N.; Georgieva, R.; Gitsov, I. Immobilization of aminothiols on poly(oxyethylene H-phosphonate)s and poly(oxyethylene phosphate)s—An approach to polymeric protective agents for radiotherapy of cancer. J. Polym. Sci. A Polym. Chem. 2007, 45, 1349–1363. [Google Scholar] [CrossRef]
- Wódzki, R.; Świa̧tkowski, M.; Pretula, J.; Kałużyñski, K. Poly[poly(oxypropylene) phosphate] macroionophores for transport and separation of cations in a hybrid: Cation-exchange polymer and liquid membrane system. J. Appl. Polym. Sci. 2004, 93, 1436–1445. [Google Scholar] [CrossRef]
- Ilia, G.; Simulescu, V.; Mak, C.A.; Crasmareanu, E. The Use of Transesterification Method for Obtaining Phosphorus-Containing Polymers. Adv. Polym. Technol. 2014, 33, 21437. [Google Scholar] [CrossRef]
- Penczek, S.; Cypryk, M.; Duda, A.; Kubisa, P.; Slomkowski, S. Living ring-opening polymerizations of heterocyclic monomers. Prog. Polym. Sci. 2007, 32, 247–282. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmann, M.; Markwart, J.; Wurm, F.R. Poly(alkylidene chlorophosphate)s via Acyclic Diene Metathesis Polymerization: A General Platform for the Postpolymerization Modification of Poly(phosphoester)s. Macromolecules 2014, 47, 8506–8513. [Google Scholar] [CrossRef]
- Becker, G.; Ackermann, L.-M.; Schechtel, E.; Klapper, M.; Tremel, W.; Wurm, F.R. Joining Two Natural Motifs: Catechol-Containing Poly(phosphoester)s. Biomacromolecules 2017, 18, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.T.; Koynov, K.; Reichel, T.; Wurm, F.R. Noncovalent Hydrogen Bonds Tune the Mechanical Properties of Phosphoester Polyethylene Mimics. ACS Omega 2019, 4, 9324–9332. [Google Scholar] [CrossRef] [Green Version]
- Markwart, J.C.; Wurm, F.R. The 2-acetylthioethyl ester group: A versatile protective group for P-OH-groups. Tetrahedron 2018, 74, 7426–7430. [Google Scholar] [CrossRef]
- Markwart, J.C.; Suraeva, O.; Haider, T.; Lieberwirth, I.; Graf, R.; Wurm, F.R. Defect engineering of polyethylene-like polyphosphoesters: Solid-state NMR characterization and surface chemistry of anisotropic polymer nanoplatelets. Polym. Chem. 2020, 11, 7235–7243. [Google Scholar] [CrossRef]
- Schulz, M.D.; Wagener, K.B. Precision Polymers through ADMET Polymerization. Macromol. Chem. Phys. 2014, 215, 1936–1945. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Wisniewski, B.; Szymanski, R.; Loontjens, T.; Penczek, S. Formation of poly(ethylene phosphates) in polycondensation of H3PO4 with ethylene glycol. Kinetic and mechanistic study. J. Polym. Sci. A Polym. Chem. 2008, 46, 830–843. [Google Scholar] [CrossRef] [Green Version]
- Pretula, J.; Kaluzynski, K.; Wisniewski, B.; Szymanski, R.; Loontjens, T.; Penczek, S. H3PO4 in a direct synthesis of oligo–poly(ethylene phosphate)from ethylene glycol. J. Polym. Sci. Part A. Polym. Chem. 2006, 44, 2358–2362. [Google Scholar] [CrossRef] [Green Version]
- Penczek, S.; Kaluzynski, K.; Pretula, J. Phosphorylation of Polyols with H3PO4: Towards Simple Synthesis of Poly(alkylene phosphate)s. Phosph. Sulfur Silikon Rel. Elem. 2009, 184, 1935–1945. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Polycondensation of H3PO4 with glycerol: From branched structures to hydrolytically reversible gels. J. Polym. Sci. A Polym. Chem. 2014, 52, 3533–3542. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Penczek, S. Polycondensation of diglycerol with H3PO4. Reversibly degradable gels giving multireactive, highly branched macromolecules. J. Polym. Sci. A Polym. Chem. 2016, 54, 3303–3317. [Google Scholar] [CrossRef]
- Munoz, A.; Vives, J.P.; Petit, J. Sur la polycindensation le l'acide phosphorique et du carbonate cyclique d'éthylène-glycol. C. R. Acad. Sci. 1963, 257(pt6), 1863–1866. [Google Scholar]
- Imoto, M.; Ouchi, T.; Sakae, M.; Yamamoto, H. Vinyl polymerization, 367. Polymerization of methyl methacrylate initiated with sodium polyethylenephosphate in the presence of an aqueous solution of copper(II) chloride. Macromol. Chem. Phys. 1980, 181, 341–349. [Google Scholar] [CrossRef]
- Biela, T.; Szymanski, R.; Kubisa, P. Oligomerization of oxiranes in the presence of phosphorus acids, 2. Kinetics of addition of ethylene oxide to phosphoric and phosphorous acid. Makromol. Chem. 1992, 193, 285–301. [Google Scholar] [CrossRef]
- Biela, T.; Nyk, A.; Kubisa, P. Polyphosphate chains by addition of oxiranes to phosphoric acid. Makromol. Chem. Macromol. Symp. 1992, 60, 155–163. [Google Scholar] [CrossRef]
- Imran, M.; Kim, B.-K.; Han, M.; Cho, B.G.; Kim, D.H. Sub- and supercritical glycolysis of polyethylene terephthalate (PET) into the monomer bis(2-hydroxyethyl) terephthalate (BHET). Polym. Degrad. Stab. 2010, 95, 1686–1693. [Google Scholar] [CrossRef]
- Ghasemi, M.H.; Neekzad, N.; Ajdari, F.B.; Kowsari, E.; Ramakrishna, S. Mechanistic aspects of poly(ethylene terephthalate) recycling–toward enabling high quality sustainability decisions in waste management. Environ. Sci. Poll. Res. 2021, 28, 43074–43101. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Mao, H.-Q.; Wang, S.; Phua, S.H.; Lee, G.P.; Pan, J.; Lu, S.; Wang, J.; Leong, K.W. Poly(phosphoester) ionomers as tissue-engineering scaffolds. J. Biomed. Mater. Res. B: Appl. Biomat. 2004, 70, 91–102. [Google Scholar] [CrossRef]
- Klosinski, P.; Penczek, S. Synthesis of models of teichoic acids by ring-opening polymerization. Macromolecules 1983, 16, 316–320. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. High-molecular-weight poly(alkylene phosphonate)s by condensation of dialkylphosphonates with diols. Makromol. Chem. 1990, 191, 671–680. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Transesterification of oligomeric dialkyl phosphonates, leading to the high-molecular-weight poly-H-phosphonates. J. Polym. Sci. A Polym. Chem. 1999, 37, 1365–1381. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Kaluzynski, K. Synthesis of a triblock copolymer: Poly(ethylene glycol)-poly(alkylene phosphate)-poly(ethylene glycol) as a modifier of CaCO3 crystallization. J. Polym. Sci. A Polym. Chem. 2005, 43, 650–657. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Preparation of Poly(alkylene H-phosphonate)s and Their Derivatives by Polycondensation of Diphenyl H-Phosphonate with Diols and Subsequent Transformations. Macromolecules 1997, 30, 8172–8176. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Penczek, S. Amino acids attached to poly(alkylene phosphate)s, 1. Synthesis. Macromol. Chem. Phys. 1994, 195, 3855–3862. [Google Scholar] [CrossRef]
- Penczek, S.; Kaluzynski, K.; Baran, J. Amino acid couples to poly(alkylene phosphates). In Macromolecules 1992: Invited lectures of the 34th IUPAC. Kahovec J, Ed.; VSP International Science Publishers: Utrecht, The Netherlands, 1993; pp. 231–240. [Google Scholar]
- Baran, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Hydrolysis of Poly(alkylene amidophosphate)s Containing Amino Acid or Peptide Residues in the Side Groups. Kinetics and Selectivity of Hydrolysis. Biomacromolecules 2004, 5, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Busch, H.; Schiebel, E.; Sickinger, A.; Mecking, S. Ultralong-Chain-Spaced Crystalline Poly(H-phosphonate)s and Poly(phenylphosphonate)s. Macromolecules 2017, 50, 7901–7910. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Lewinski, P.; Kaźmierski, S.; Penczek, S. Catalysis in polymerization of cyclic esters. Catalyst and initiator in one molecule. Polymerization of ε-caprolactone. J. Catal. 2020, 392, 97–107. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Lewinski, P.; Kaźmierski, S.; Penczek, S. Synthesis and Properties of Functionalized Poly(ε-caprolactone); Chain Polymerization Followed by Polycondensation in One Pot with Initiator and Catalyst in One Molecule. Synthesis and Molecular Structures. Macromolecules 2022, 55, 2210–2221. [Google Scholar] [CrossRef]
- Lucas, H.J.; Mitchell Jr., F. W.; Scully, C.N. Cyclic Phosphites of Some Aliphatic Glycols. J. Am. Chem. Soc. 1950, 72, 5491–5497. [Google Scholar] [CrossRef]
- Nifant'ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidates catalysed by hereroleptic BHT-alkoxy magnesium complexes. Polym. Chem. 2017, 8, 6806–6816. [Google Scholar] [CrossRef]
- Oussadi, K.; Montembault, V.; Belbachir, M.; Fontaine, L. Ring-opening bulk polymerization of five- and six-membered cyclic phosphonates using maghnite, a nontoxic proton exchanged montmorillonite clay. J. Appl. Polym. Sci. 2011, 122, 891–897. [Google Scholar] [CrossRef]
- Maffei, M.; Buono, G. A two step synthesis of 2-oxo-2-vinyl 1,3,2-dioxaphospholanes and -dioxaphosphorinanes. Tetrahedron 2003, 59, 8821–8825. [Google Scholar] [CrossRef]
- Biela, T.; Penczek, S.; Slomkowski, S.; Vogl, O. Racemic and optically active poly(4-methyl-2-oxo-2-hydro-1,3,2-dioxaphospholane). Synthesis and oxidation to the polyacids. Makromol. Chem. Rapid Commun. 1982, 3, 667–671. [Google Scholar] [CrossRef]
- Lapienis, G.; Penczek, S. Cationic Polymerization of 2-Alkoxy-2-oxo-1,3,2-dioxaphosphorinanes (1,3-Propylene Alkyl Phosphates). Macromolecules 1977, 10, 1301–1306. [Google Scholar] [CrossRef]
- Libiszowski, J.; Kałużynski, K.; Penczek, S. Polymerization of cyclic esters of phosphoric acid. VI. Poly(alkyl ethylene phosphates). Polymerization of 2-alkoxy-2-oxo-1,3,2-dioxaphospholans and structure of polymers. J. Polym. Sci. Polym. Chem. Ed. 1978, 16, 1275–1283. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Breathing air as oxidant: Optimization of 2-chloro-2-oxo-1,3,2-dioxaphospholane synthesis as a precursor for phosphoryl choline derivatives and cyclic phosphate monomers. Tetrahedron 2017, 73, 3536–3540. [Google Scholar] [CrossRef]
- Morodo, R.; Riva, R.; van den Akker, N.M.S.; Molin, D.G.M.; Jérôme, C.; Monbaliu, J.-C.M. Accelerating the end-to-end production of cyclic phosphate monomers with modular flow chemistry. Chem. Sci. 2022, 13, 10699–10706. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kawakita, T.; Yusa, S. Thermoresponsive Polyphosphoesters Bearing Enzyme-cleavable Side Chains. Chem. Lett. 2009, 38, 1054–1055. [Google Scholar] [CrossRef]
- Yasuda, H.; Sumitani, M.; Nakamura, A. Novel Synthesis of Acidic Polyesters of Phosphoric Acid by Thermal Elimination of Isobutylene from Poly(alkylene tert-butyl phosphates). Macromolecules 1981, 14, 458–460. [Google Scholar] [CrossRef]
- Lapienis, G.; Penczek, S.; Pretula, J. Poly (dialkylphosphates) based on deoxyribose. Macromolecules 1983, 16, 153–158. [Google Scholar] [CrossRef]
- Olsén, P.; Odelius, K.; Albertsson, A.-C. Thermodynamic Presynthetic Considerations for Ring-Opening Polymerization. Biomacromolecules 2016, 17, 699–709. [Google Scholar] [CrossRef] [Green Version]
- Sosnowski, S.; Libiszowski, J.; Słomkowski, S.; Penczek, S. Thermodynamics of the polymerization of ethylene methyl phosphate. Makromol. Chem. Rapid Commun. 1984, 5, 239–244. [Google Scholar] [CrossRef]
- Penczek, S. Mechanism of ionic polymerization of cyclic esters of phosphoric acid (a new route to models of biopolymers). J. Polym. Sci. Polym. Symp. 1980, 67, 149–168. [Google Scholar] [CrossRef]
- Penczek, S.; Biela, T.; Klosinski, P.; Lapienis, G. Polymerization of phosphorus containing cyclic monomers: Synthesis of polymers related to biopolymers. Makromol. Chem., Macromol. Symp. 1986, 6, 123–153. [Google Scholar] [CrossRef]
- Pretula, J.; Kałużyṅski, K.; Penczek, S. Living reversible anonic polymerization of N,N--diethylamine-1,3,2-dioxaphosphorinan. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 1251–1258. [Google Scholar] [CrossRef]
- Bauer, K.N.; Liu, L.; Wagner, M.; Andrienko, D.; Wurm, F.R. Mechanistic study on the hydrolytic degradation of polyphosphates. Eur. Polym. J. 2018, 108, 286–294. [Google Scholar] [CrossRef]
- Nifant'ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Kosarev, M.A.; Gavrilov, D.E.; Komarov, P.D.; Ilyin, S.O.; Karchevsky, S.G.; Ivchenko, P.V. Mechanistic study of transesterification in TBD-catalyzed ring-opening polymerization of methyl ethylene phosphate. Eur. Polym. J. 2019, 118, 393–403. [Google Scholar] [CrossRef]
- Hirano, Y.; Iwasaki, Y. Bone-specific poly(ethylene sodium phosphate)-bearing biodegradable nanoparticles. Coll. Surf. B: Biointerfaces 2017, 153, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Noree, S.; Iwasaki, Y. Thermally Assisted Generation of Protein–Poly(ethylene sodium phosphate) Conjugates with High Mineral Affinity. ACS Omega 2019, 4, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Otaka, A.; Iwasaki, Y. Endocytosis of poly(ethylene sodium phosphate) by macrophages and the effect of polymer length on cellular uptake. J. Ind. Eng. Chem. 2019, 75, 115–122. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yokota, A.; Otaka, A.; Inoue, N.; Yamaguchi, A.; Yoshitomi, T.; Yoshimotode, K.; Neo, M. Bone-targeting poly(ethylene sodium phosphate). Biomater. Sci. 2018, 6, 91–95. [Google Scholar] [CrossRef]
- Clément, B.; Molin, D.G.; Jérôme, C.; Lecomte, P. Synthesis of polyphosphodiesters by ring-opening polymerization of cyclic phosphates bearing allyl phosphoester protecting groups. J. Polym. Sci. A Polym. Chem. 2015, 53, 2642–2648. [Google Scholar] [CrossRef]
- Ergul Yilmaz, Z.; Debuigne, A.; Calvignac, B.; Boury, F.; Jérôme, C. Double hydrophilic polyphosphoester containing copolymers as efficient templating agents for calcium carbonate microparticles. J. Mater. Chem. B 2015, 3, 7227–7236. [Google Scholar] [CrossRef]
- Nifant'ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Tavtorkin, A.N.; Minyaev, M.E.; Kosarev, M.A.; Ivchenko, P.V. Synthesis in aqueous media of poly(ethylene phosphoric acids) by mild thermolysis of homopolymers and block copolymers based on tert-butyl ethylene phosphate. Eur. Polym. J. 2018, 106, 249–256. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Siniavin, A.; Karamov, E.; Kosarev, M.; Kovalchuk, S.; Turgiev, A.; Nametkin, S.; Bagrov, V.; Tavtorkin, A.; Ivchenko, P. A New Approach to Developing Long-Acting Injectable Formulations of Anti-HIV Drugs: Poly(Ethylene Phosphoric Acid) Block Copolymers Increase the Efficiency of Tenofovir against HIV-1 in MT-4 Cells. Int. J. Mol. Sci. 2021, 22, 340. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Katayama, K.; Yoshida, M.; Yamamoto, M.; Tabata, Y. Comparative physicochemical properties and cytotoxicity of polyphosphoester ionomers with bisphosphonates. J. Biomater. Sci. Polym. Ed. 2012, 24, 882–895. [Google Scholar] [CrossRef]
- Ikeuchi, R.; Iwasaki, Y. High mineral affinity of polyphosphoester ionomer–phospholipid vesicles. J. Biomed. Mater. Res. 2013, 101, 318–325. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Shen, Y.; Zhang, F.; Seetho, K.; Zou, J.; Taylor, J.-S.A.; Dove, A.P.; Wooley, K.L. A simple and efficient synthesis of an acid-labile polyphosphoramidate by organobase-catalyzed ring-opening polymerization and transformation to polyphosphoester ionomers by acid treatment. Macromolecules 2013, 46, 5141–5149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Karchevsky, S.; Ivchenko, P. Mechanistic Insights of BHT-Mg-Catalyzed Ethylene Phosphate’s Coordination Ring-Opening Polymerization: DFT Modeling and Experimental Data. Polymers 2018, 10, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nifant’ev, I.; Gavrilov, D.; Tavtorkin, A.; Chinova, M.; Besprozvannykh, V.; Komarov, P.; Zaitsev, V.; Podoprigora, I.; Ivchenko, P. Antibacterial Poly(ε-CL)/Hydroxyapatite Electrospun Fibers Reinforced by Poly(ε-CL)-b-poly(ethylene phosphoric acid). Int. J. Mol. Sci. 2021, 22, 7690. [Google Scholar] [CrossRef] [PubMed]
- Lapienis, G. Ring-Opening Polymerization of Cyclic Phosphorus Monomers. In Polymer Science: A Comprehensive Reference; Elsevier B.V.: Amsterdam, Netherlands, 2012; Volume 4, pp. 477–505. [Google Scholar] [CrossRef]
- Kluger, R.; Taylor, S.D. Endocyclic cleavage in the alkaline hydrolysis of the cyclic phosphonate methyl propylphostonate: Dianionic intermediates and barriers to pseudorotation. J. Am. Chem. Soc. 1991, 113, 5714–5719. [Google Scholar] [CrossRef]
- Wang, J.; Sun, D.D.N.; Shin-ya, Y.; Leong, K.W. Stimuli-Responsive Hydrogel Based on Poly(propylene phosphate). Macromolecules 2004, 37, 670–672. [Google Scholar] [CrossRef]
- Gehrmann, T.; Vogt, W. Polymer ester von sären des phosphors, 7. Polymerisation des 1-oxo-2,6,7-trioxa-1-phosphabicyclo [2.2.1]heptans. Makromol. Chem. 1981, 182, 3069–3076. [Google Scholar] [CrossRef]
- Baran, J.; Penczek, S. Hydrolysis of Polyesters of Phosphoric Acid. 1. Kinetics and the pH Profile. Macromolecules 1995, 28, 5167–5176. [Google Scholar] [CrossRef]
- Yasuda, H.; Sumitani, M.; Lee, K.; Araki, T.; Nakamura, A. High molecular weight poly(2-methoxy-1,3,2-dioxaphospholane 2-oxide) by ring-opening catalysis of tertiary amines. Initiation and stepwise propagation mechanisms as studied by the stoichiometric reaction with triethylamine. Macromolecules 1982, 15, 1231–1237. [Google Scholar] [CrossRef]
- Kootala, S.; Tokunaga, M.; Hilborn, J.; Iwasaki, Y. Anti-Resorptive Functions of Poly(ethylene sodium phosphate) on Human Osteoclasts. Macromol. Biosci. 2015, 15, 1634–1640. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Takahata, Y.; Fujii, S. Self-setting particle-stabilized emulsion for hard-tissue engineering. Colloids Surfaces B: Biointerfaces 2015, 126, 394–400. [Google Scholar] [CrossRef]
- Kunomura, S.; Iwasaki, Y. Immobilization of polyphosphoesters on poly(ether ether ketone) (PEEK) for facilitating mineral coating. J. Biomater. Sci. Polym. Ed. 2019, 30, 861–876. [Google Scholar] [CrossRef]
- Li, R.; Elsabahy, M.; Song, Y.; Wang, H.; Su, L.; Letteri, R.A.; Khan, S.; Heo, G.S.; Sun, G.; Liu, Y.; et al. Functional, Degradable Zwitterionic Polyphosphoesters as Biocompatible Coating Materials for Metal Nanostructures. Langmuir 2019, 35, 1503–1512. [Google Scholar] [CrossRef]
- Caruthers, M.H. Chemical synthesis of DNA and DNA analogs. Acc. Chem. Res. 1991, 24, 278–284. [Google Scholar] [CrossRef]
- Dera, R.; Diliën, H.; Billen, B.; Gagliardi, M.; Rahimi, N.; Van Den Akker, N.M.S.; Molin, D.G.M.; Grandfils, C.; Adriaensens, P.; Guedens, W.; et al. Phosphodiester Hydrogels for Cell Scaffolding and Drug Release Applications. Macromol. Biosci. 2019, 19, 1900090. [Google Scholar] [CrossRef] [PubMed]
- Dera, R.; Diliën, H.; Adriaensens, P.; Guedens, W.; Cleij, T.J. An Efficient Thermal Elimination Pathway toward Phosphodiester Hydrogels via a Precursor Approach. Macromol. Chem. Phys. 2020, 221, 1900466. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Narukawa, Y.; Saegusa, T. Hydrolysis polymerization of spiro(acylpentaoxy)phosphoranes to polyphosphates. Macromolecules 1984, 17, 134–138. [Google Scholar] [CrossRef]
- Steinmann, M.; Wurm, F.R. Water-soluble and degradable polyphosphorodiamidates via thiol-ene polyaddition. Polym. Degrad. Stab. 2020, 179, 109224. [Google Scholar] [CrossRef]
- Yuan, Y.-Y.; Du, J.-Z.; Song, W.-J.; Wang, F.; Yang, X.-Z.; Xiong, M.-H.; Wang, J. Biocompatible and functionalizable polyphosphate nanogel with a branched structure. J. Mater. Chem. 2012, 22, 9322–9329. [Google Scholar] [CrossRef]
- Al Ouahabi, A.; Kotera, M.; Charles, L.; Lutz, J.-F. Synthesis of Monodisperse Sequence-Coded Polymers with Chain Lengths above DP100. ACS Macro Lett. 2015, 4, 1077–1080. [Google Scholar] [CrossRef]
- Vybornyi, M.; Vyborna, Y.; Häner, R. DNA-inspired oligomers: From oligophosphates to functional materials. Chem. Soc. Rev. 2019, 48, 4347–4360. [Google Scholar] [CrossRef] [Green Version]
- Charles, L.; Lutz, J.-F. Design of Abiological Digital Poly(phosphodiester)s. Acc. Chem. Res. 2021, 54, 1791–1800. [Google Scholar] [CrossRef]
- Meiser, L.C.; Nguyen, B.H.; Chen, Y.-J.; Nivala, J.; Strauss, K.; Ceze, L.; Grass, R.N. Synthetic DNA applications in information technology. Nat. Commun. 2022, 13, 352. [Google Scholar] [CrossRef]
- Al Ouahabi, A.; Charles, L.; Lutz, J.-F. Synthesis of Non-Natural Sequence-Encoded Polymers Using Phosphoramidite Chemistry. J. Am. Chem. Soc. 2015, 137, 5629–5635. [Google Scholar] [CrossRef] [PubMed]
- Roszak, I.; Oswald, L.; Al Ouahabi, A.; Bertin, A.; Laurent, E.; Felix, O.; Carvin-Sergent, I.; Charles, L.; Lutz, J.-F. Synthesis and sequencing of informational poly(amino phosphodiester)s. Polym. Chem. 2021, 12, 5279–5282. [Google Scholar] [CrossRef]
- Loth, C.; Charles, L.; Lutz, J.-F.; Nerantzaki, M. Precisely Defined Aptamer-b-Poly(phosphodiester) Conjugates Prepared by Phosphoramidite Polymer Chemistry. ACS Macro Lett. 2021, 10, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Laurent, E.; Amalian, J.-A.; Parmentier, M.; Oswald, L.; Al Ouahabi, A.; Dufour, F.; Launay, K.; Clément, J.-L.; Gigmes, D.; Delsuc, M.-A.; et al. High-Capacity Digital Polymers: Storing Images in Single Molecules. Macromolecules 2020, 53, 4022–4029. [Google Scholar] [CrossRef]
- de Rochambeau, D.; Sun, Y.; Barlog, M.; Bazzi, H.S.; Sleiman, H.F. Modular Strategy To Expand the Chemical Diversity of DNA and Sequence-Controlled Polymers. J. Org. Chem. 2018, 83, 9774–9786. [Google Scholar] [CrossRef]
- Launay, K.; Amalian, J.-A.; Laurent, E.; Oswald, L.; Al Ouahabi, A.; Burel, A.; Dufour, F.; Carapito, C.; Clément, J.-L.; Lutz, J.-F.; et al. Precise Alkoxyamine Design to Enable Automated Tandem Mass Spectrometry Sequencing of Digital Poly(phosphodiester)s. Angew. Chem. Int. Ed. 2021, 60, 917–926. [Google Scholar] [CrossRef] [PubMed]
- König, N.F.; Al Ouahabi, A.; Oswald, L.; Szweda, R.; Charles, L.; Lutz, J.-F. Photo-editable macromolecular information. Nat. Commun. 2019, 10, 3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appukutti, N.; Jones, J.R.; Serpell, C.J. Sequence isomerism in uniform polyphosphoesters programmes self-assembly and folding. Chem. Commun. 2020, 56, 5307–5310. [Google Scholar] [CrossRef]
- Amalian, J.-A.; Mondal, T.; Konishcheva, E.; Cavallo, G.; Petit, B.E.; Lutz, J.-F.; Charles, L. Desorption Electrospray Ionization (DESI) of Digital Polymers: Direct Tandem Mass Spectrometry Decoding and Imaging from Materials Surfaces. Adv. Mater. Technol. 2021, 6, 2001088. [Google Scholar] [CrossRef]
- Amalian, J.-A.; Al Ouahabi, A.; Cavallo, G.; König, N.F.; Poyer, S.; Lutz, J.-F.; Charles, L. Controlling the structure of sequence-defined poly(phosphodiester)s for optimal MS/MS reading of digital information. J. Mass Spectr. 2017, 52, 788–798. [Google Scholar] [CrossRef]
- Laurent, E.; Amalian, J.-A.; Schutz, T.; Launay, K.; Clément, J.-L.; Gigmes, D.; Burel, A.; Carapito, C.; Charles, L.; Delsuc, M.-A.; et al. Storing the portrait of Antoine de Lavoisier in a single macromolecule. Compt. Rend. Chim. 2021, 24, 69–76. [Google Scholar] [CrossRef]
- Wódzki, R.; Kłosiński, P. Magnesium and calcium ions transport by synthetic analogues of teichoic acids with 1,2- and 1,3-linked phosphodiester units. Makromol. Chem. 1990, 191, 921–931. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Bukharova, T.; Dyakonov, A.; Goldshtein, D.; Galitsyna, E.; Kosarev, M.; Shlyakhtin, A.; Gavrilov, D.; Ivchenko, P. Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s. Int. J. Mol. Sci. 2019, 20, 6242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otaka, A.; Kiyono, K.; Iwasaki, Y. Enhancement of osteoblast differentiation using poly(ethylene sodium phosphate). Materialia 2021, 15, 100977. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nature Nanotech. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Moriyama, R.; Iwasaki, Y.; Miyoshi, D. Stabilization of DNA Structures with Poly(ethylene sodium phosphate). J. Phys. Chem. B 2014, 2014, 11969–11977. [Google Scholar] [CrossRef] [PubMed]
- Noree, S.; Thongthai, P.; Kitagawa, H.; Imazato, S.; Iwasaki, Y. Reduction of Acidic Erosion and Oral Bacterial Adhesion through the Immobilization of Zwitterionic Polyphosphoesters on Mineral Substrates. Chem. Lett. 2019, 48, 1529–1532. [Google Scholar] [CrossRef]
- Koseva, N.; Tsacheva, I.; Mitova, V.; Vodenicharova, E.; Molkentine, J.; Mason, K.; Troev, K. Polymer complex of WR 2721. Synthesis and radioprotective efficiency. Eur. J. Pharm. Sci. 2014, 65, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bogomilova, A.; Höhn, M.; Günther, M.; Herrmann, A.; Troev, K.; Wagner, E.; Schreiner, L. A polyphosphoester conjugate of melphalan as antitumoral agent. Eur. J. Pharm. Sci. 2013, 50, 410–419. [Google Scholar] [CrossRef]
- Vahabi, H.; Laoutid, F.; Mehrpouya, M.; Saeb, M.R.; Dubois, P. Flame retardant polymer materials: An update and the future for 3D printing developments. Mater. Sci. Eng. R Rep. 2021, 144, 100604. [Google Scholar] [CrossRef]
- Özer, M.S.; Gaan, S. Recent developments in phosphorus based flame retardant coatings for textiles: Synthesis, applications and performance. Progr. Org. Coatings 2022, 171, 107027. [Google Scholar] [CrossRef]
- Flameretardants-Online. The Flame Retardants Market. Available online: https://www.flameretardants-online.com/flame-retardants/market (accessed on 22 October 2022).
- Balzade, Z.; Sharif, F.; Anbaran, S.R.G. Tailor-Made Functional Polyolefins of Complex Architectures: Recent Advances, Applications, and Prospects. Macromolecules 2022, 55, 6938–6972. [Google Scholar] [CrossRef]




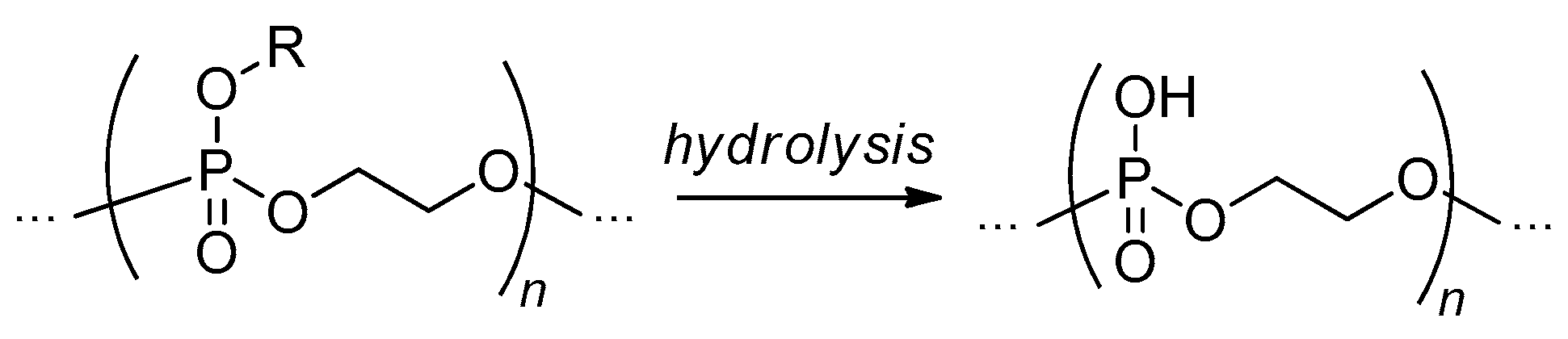

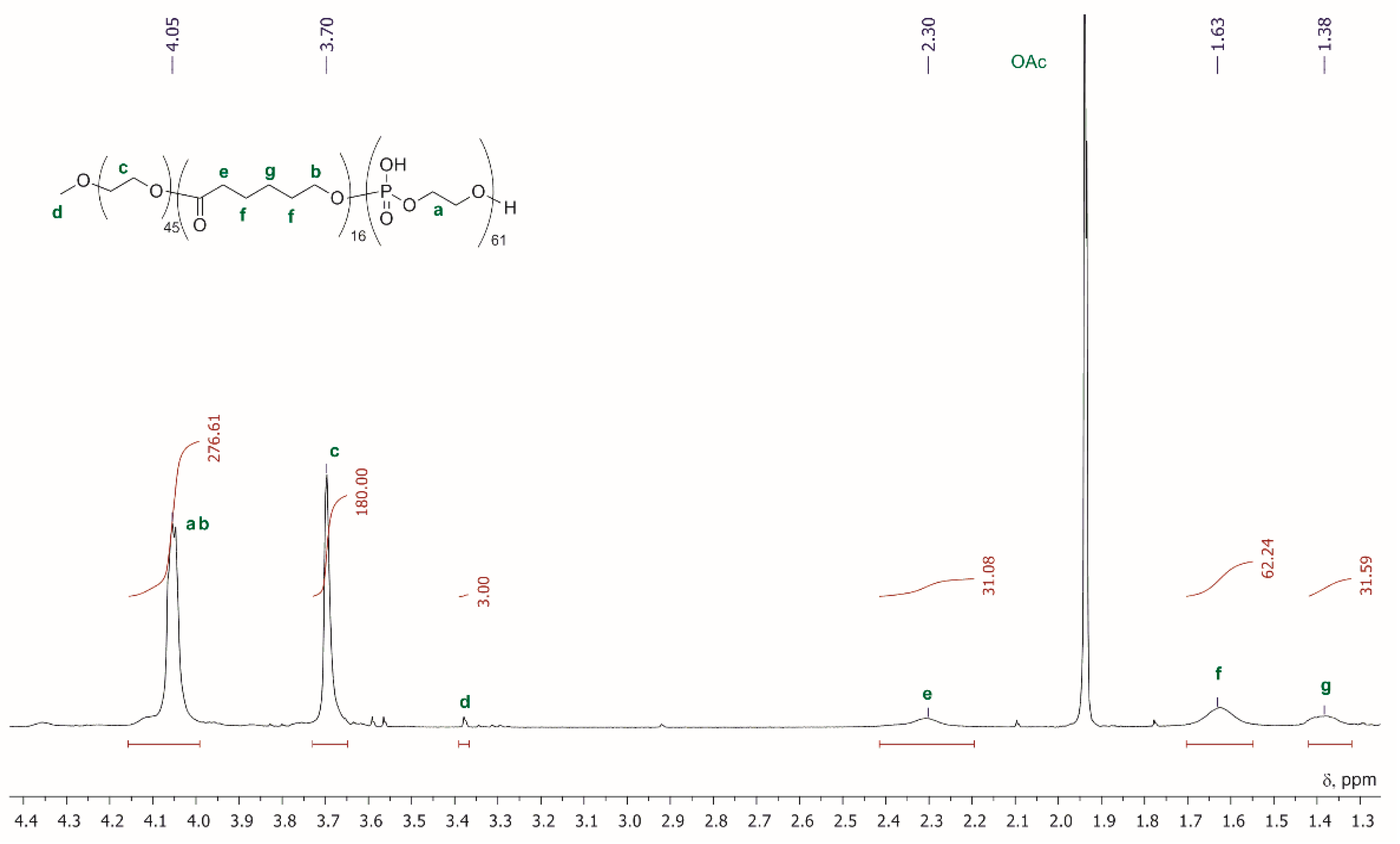









| Entry | Monomer | Catalyst | Reaction Conditions/Conversion, % | Mn, kDa | DPn a | ÐM | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 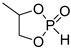 | iBu3Al | CH2Cl2, 20 °C | - | - | - | [65] |
| 2 | 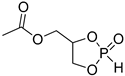 | iBu3Al | CH2Cl2, –20 °C, 6 h/80 | [50] | |||
| 3 | 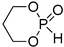 | iBu3Al | CH2Cl2, from 0 to 20 °C | 90 | 740 | - | [22,76] |
| 4 | 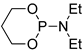 | tBuOK | THF, 20 °C, several days/99 | - | - | - | [77] |
| 5 | 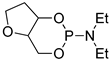 | tBuOK | C6H6, 20 °C, several days/99 | - | - | - | [72] |
| 6 | 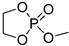 | iBu3Al | CH2Cl2, from –20 to 20 °C | 30–100 | - | - | [22] |
| Et2Mg | CH2Cl2, from –20 to 20 °C | 30–100 | - | - | [22,67] | ||
| DBU/TU | CH2Cl2, 20 °C, 15 min/83 | 9.2 | 68 | 1.17 | [62] | ||
| DBU/TU | CH2Cl2, 0 °C, 1.4 h/92 | - | 97 | - | [78] | ||
| Mg1 | CH2Cl2, –20 °C, 5 min/99 | 9.5 | 70 | 1.35 | [62] | ||
| TBD/BnOH | CH2Cl2, –20 °C, 5 min/99 | 9.3 | 68 | 1.24 | [62] | ||
| TBD/BnOH | CH2Cl2, 1 eqiv. TMP, –20 °C, 5 min/99 | 6.4 | 47 | 1.13 | [79] | ||
| DBU/Cholesterol | CH2Cl2, 20 °C, 5 h/ | [80,81] | |||||
| 7 | 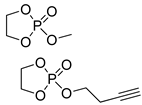 | DBU/EtOH DBU/MeOH | 9:1 comonomer ratio, CH2Cl2/– - | - - | 38, 85, 127 73 | - - | [82] [83] |
| 8 | 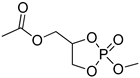 | iBu3Al | CH2Cl2, 0 °C | 25 | 119 | - | [50] |
| 9 | 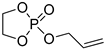 | DBU/TU BnOH | toluene, 0 °C, 10 min/80 | - | - | - | [84] |
| DBU/TU mPEG5000 | toluene, 0 °C, 10 min/80 | 7.5 | 16 | <1.2 | [85] | ||
| 10 | 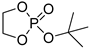 | Et2Mg | C6H6, 40 °C, 10 h/80 | 25 | 139 | - | [71] |
| Mg1 | CH2Cl2, 20 °C, 18 h | 6.4 | 36 | 1.19 | [62] | ||
| Mg1 | CH2Cl2, 20 °C, 18 h | – | 63 | – | [86] | ||
| Mg2/mPEG5000 | CH2Cl2, 20 °C, 30 h | 3.6 | 13 | 1.45 | [87] | ||
| CH2Cl2, 20 °C, 30 h | 8.1 | 49 | 1.48 | [87] | |||
| 11 | 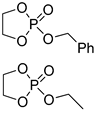 | iBu3Al | 1:10 comonomer ratio, bulk/69.3 | 6.0–7.0 | - | - | [70] |
| TBD/BnOH TBD/ Cholesterol | 5:95–20:80 comonomer ratio, toluene 4:96 and 17:83 comonomer ratios, CH2Cl2 | 9.5–11.9 4.6; 6.4 | - - | 1.45–1.62 1.3; 1.2 | [88] [89] | ||
| 12 | 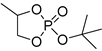 | Et2Mg | C6H6, 40 °C, 10 h/90 | 25 | 139 | n.d. | [71] |
| 13 | 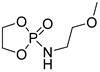 | TBD/BnOH | CH2Cl2, 0 °C, 1 min/99 | 13 | 72 | 1.17 | [90] |
| 14 |  | Et3Al/H2O | C6H6, 40 °C/50 | - | - | - | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.E.; Ivchenko, P.V. Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 1. Polyphosphodiesters. Int. J. Mol. Sci. 2022, 23, 14857. https://doi.org/10.3390/ijms232314857
Nifant’ev IE, Ivchenko PV. Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 1. Polyphosphodiesters. International Journal of Molecular Sciences. 2022; 23(23):14857. https://doi.org/10.3390/ijms232314857
Chicago/Turabian StyleNifant’ev, Ilya E., and Pavel V. Ivchenko. 2022. "Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 1. Polyphosphodiesters" International Journal of Molecular Sciences 23, no. 23: 14857. https://doi.org/10.3390/ijms232314857
APA StyleNifant’ev, I. E., & Ivchenko, P. V. (2022). Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 1. Polyphosphodiesters. International Journal of Molecular Sciences, 23(23), 14857. https://doi.org/10.3390/ijms232314857










