Unusual Effect of α-olefins as Chain Transfer Agents in Ethylene Polymerization over the Catalyst with Nonsymmetrical Bis(imino)pyridine Complex of Fe(II) and Modified Methylalumoxane (MMAO) Cocatalyst
Abstract
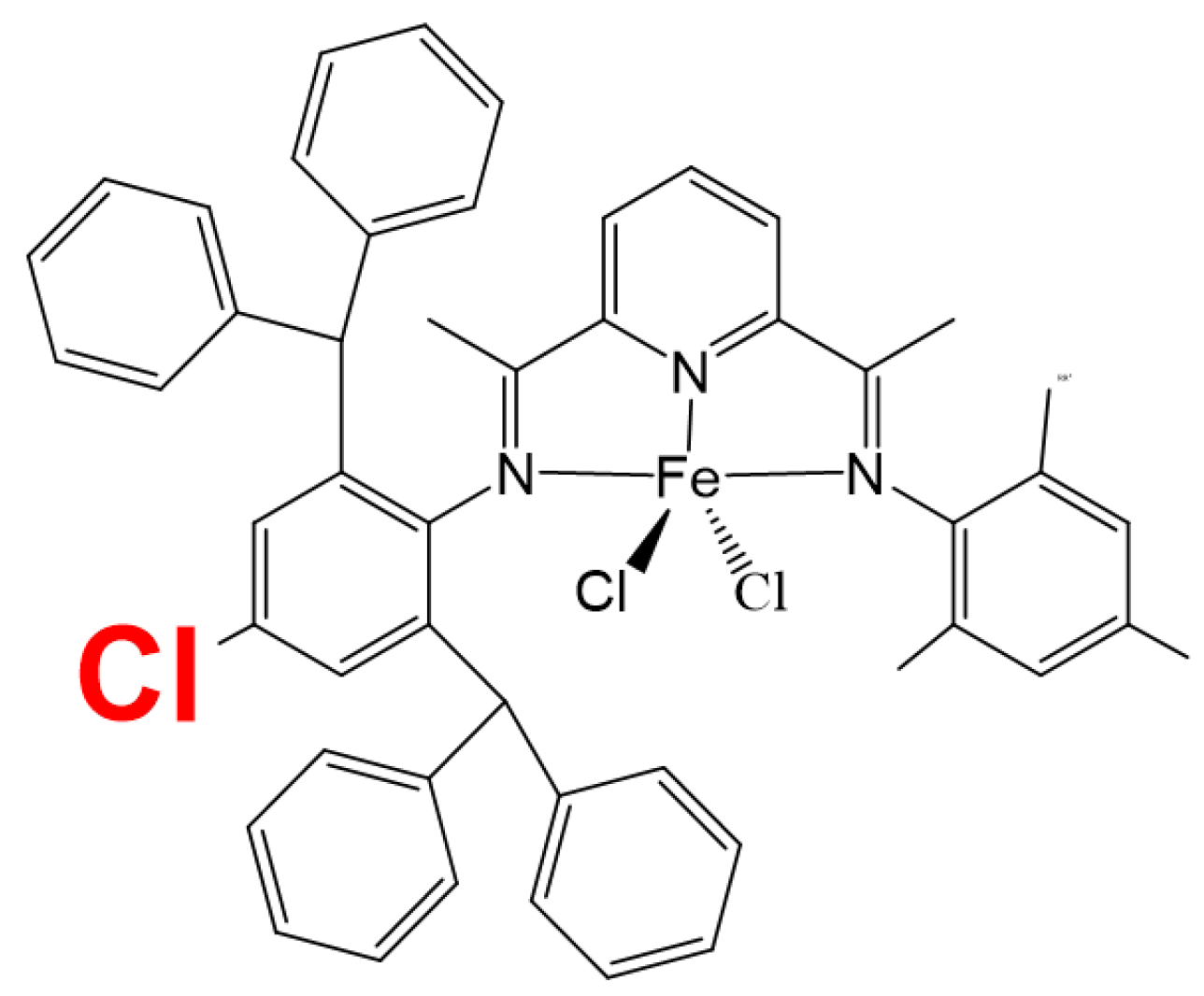
1. Introduction
2. Results and Discussion
- 1.
- The peculiarities of ethylene polymerization kinetics and molecular mass characteristics of polyethylene, produced by the catalytic system composed of nonsymmetrical bis(imino)pyridine Fe(II) complex (*LFeCl2) and cocatalyst MMAO.
- 2.
- Ethylene polymerization over the catalyst *LFeCl2/MMAO in the presence of α-olefins.
3. Materials and Methods
3.1. Polymerization
3.2. Measurements of PE Molecular Weight (MW) and Molecular Weight Distribution (MWD)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Small, B.I.; Brookhart, M.A.; Bennett, M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D. Novel Olefin Polymerization Catalysts based on Iron and Cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Small, B.I.; Brookhart, M. Iron-Based Catalysts with Exceptionally High Activities and Selectivities for Oligomerization of Ethylene to Linear α-Olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. [Google Scholar] [CrossRef]
- Kumar, K.R.; Sivaram, S. Ethylene Polymerization Using Iron(II)bis(imino) Pyridyl and Nickel (diimine) Catalysts: Effect of Cocatalysts and Reaction Parameters. Macromol. Chem. Phys. 2000, 201, 1513–1520. [Google Scholar] [CrossRef]
- Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P.; Babushkin, D.E.; Sobolev, K.P.; Echevskaya, L.G.; Khisniyarov, M.M. Study of the Ethylene Polymerization over Homogeneous and Supported Catalysts Based on 2,6-bis(imino)pyridyl Complexes of Fe(II) and Co(II). J. Mol. Catal. A 2002, 182, 283–284. [Google Scholar] [CrossRef]
- Hoarau, O.D.; Gibson, V.C. Iron and Cobalt Ethylene Polymerization Catalysts bearing 2,6-bis(imino)pyrimidyl Ligands: Synthesis and Polymerization Studies. Polym. Mater. Sci. Eng. 2001, 84, 532–533. [Google Scholar]
- Radhakrishnan, K.; Cramil, H.; Deffiex, A.; Francois, P.; Montaz, A. Influence of Alkylaluminium Activators and Mixtures thereof on Ethylene Polymerization with a Tridentate Bis(imino)pyridinyliron Complex. Macromol. Rapid. Commun. 2003, 24, 251–254. [Google Scholar] [CrossRef]
- Smit, T.M.; Tomov, A.K.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Dramatic Effect of Heteroatom Backbone Substituents on the Ethylene Polymerization Behavior of Bis(imino)pyridine Iron Catalysts. Inorg. Chem. 2004, 43, 6511–6512. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, W.-H.; Xiao, T.; Hao, X. Ferrous and Cobaltous Chlorides Bearing 2,8-bis(imino)quinolines: Highly Active Catalysts for Ethylene Polymerization at High Temperature. Organometallics 2010, 29, 1168–1173. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Cohen, S.A.; Gibson, V.C.; Maddox, P.J.; van Meurs, M. Iron-Catalyzed Polyethylene Chain Growth on Zinc: Linear α-Olefins with a Poisson Distribution. Angew. Chem. Int. Ed. 2002, 41, 489–491. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Mastroianni, S.; Redshow, C.; Solan, G.A.; White, A.J.P.; Williams, D.J. Bis(imino)pyridyl Iron and Cobalt Complexes: Effect of Nitrogen Sybstituents on Ethylene Oligomerization and Polymerization. J. Chem. Soc. Dalton. Trans. 2001, 1639–1644. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, W.-Y.; Li, Z.-L.; Hu, Y.-L.; Li, X.-H. Ethylene Polymerization by Iron Complexes with Symmetrical and Unsymmetrical Ligands. Polym. Int. 2002, 51, 994–997. [Google Scholar] [CrossRef]
- Kim, I.; Han, B.H.; Ha, Y.-S.; Park, D.-W. Effect of Substituent Position on the Ethylene Polymerization by Fe(II) and Co(II) Pyridyl bis-imine Catalysts. Catal. Today 2004, 93, 281–285. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, R.; Qian, C.; Dong, X.; Sun, J. Halogen-Substituted 2,6-Bis(imino)pyridyl Iron and Cobalt Complexes: Highly Active Catalysts for Polymerization and Oligomerization of Ethylene. Organometallics 2003, 22, 4312–4321. [Google Scholar] [CrossRef]
- Tellman, K.P.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Selective Dimerization/Oligomerization of α-Olefins by Cobalt Bis(imino)pyridine Catalysts Stabilized by Trifluoromethyl Substituents: Group 9 Metal Catalysts with Productivities Matching Those of Iron Systems. Organometallics 2005, 24, 280–286. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Guerrero Rios, I.; Meli, A.; Passaglia, E.; Gragnoli, T. Simultaneous Polymerization and Schulz−Flory Oligomerization of Ethylene Made Possible by Activation with MAO of a C1-Symmetric [2,6-Bis(arylimino)pyridyl]iron Dichloride Precursor. Organometallics 2004, 23, 6087–6089. [Google Scholar] [CrossRef]
- Schmidt, R.; Welch, M.B.; Knudsen, R.D.; Goufred, S.; Alt, H.G. N,N,N-Tridentate iron(II) and vanadium(III) complexes: Part I—Synthesis and Characterization. J. Mol. Catal. A 2004, 222, 9–15. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Cohen, S.A.; Gibson, V.C.; Maddox, P.G.; van Meurs, M. Iron Catalyzed Polyethylene Chain Growth on Zinc: A Study of the Factors Delineating Chain Transfer versus Catalyzed Chain Growth in Zinc and Related Metal Alkyl Systems. J. Am. Chem. Soc. 2004, 126, 10701–10712. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Rios, I.G.; Mantovani, G.; Meli, A.; Segarra, A.M. Ethylene Oligomerization, Homopolymerization and Copolymerization by Iron and Cobalt Catalysts with 2,6-(bis-organylimino)pyridyl Ligands. Coord. Chem. Rev. 2006, 250, 1391–1418. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly Reactive Ligands and a Gateway to New Families of Catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P. Frontiers of Mechanistic Studies of Coordination Polymerization and Oligomerization of α-olefins. Coord. Chem Rev. 2012, 256, 2994–3007. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer Ligands as Ring-strain Adjustable Supports for Iron and Cobalt Catalysts in Ethylene Oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Huang, C.; Zakharov, V.A.; Semikolenova, N.V.; Matsko, M.A.; Solan, G.A.; Sun, W.-H. A Comparative Kinetic Study of Ethylene Polymerization Mediated by Iron, Cobalt and Chromium Catalysts Bearing the Same N,N,N-bis(imino)trihydroquinoline. J. Catal. 2019, 369, 1–9. [Google Scholar] [CrossRef]
- Yu, J.; Liu, H.; Zhang, W.; Hao, X.; Sun, W.-H. Access to Highly Active and Thermally Stable Iron Procatalysts Using Bulky 2-[1-(2,6-dibenzhydryl-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridine Ligands. Chem. Commun. 2011, 47, 3257–3259. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Guo, J.; Zhang, W.; Ma, Y.; Liang, T.; Sun, W.-H. Concurrently Improving the Thermal Stability and Activity of Ferrous Precatalysts for the Production of Saturated/Unsaturated Polyethylene. Organometallics 2018, 37, 957–970. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, J.; Song, S.; Liu, H.; Hao, X.; Redshaw, C.; Sun, W.-H. Controlling the Ethylene Polymerization Parameters in Iron Pre-catalysts of the Type 2-[1-(2,4-dibenzhydryl-6-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridyliron dichloride. Polymer 2011, 53, 130–137. [Google Scholar] [CrossRef]
- Sun, W.-H.; Zhao, W.; Yu, J.; Zhang, W.; Hao, X.; Redshaw, C. Enhancing the Activity and Thermal Stability of Iron Precatalysts Using 2-(1-{2,6-bis[bis(4-fluorophenyl)methyl]-4-methylphenylimino}ethyl)-6-[1-(arylimino)ethyl]pyridines. Macromol. Chem. Phys. 2012, 213, 1266–1273. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Guo, C.Y.; Hao, X.; Sun, W.-H. 2-(1-(2,4-Bis((di(4-fluorophenyl)methyl)-6-methylphenylimino)ethyl)-6-(1-(arylimino)ethyl)pyridylmetal (Iron or Cobalt) Complexes: Synthesis, Characterization, and Ethylene Polymerization Behavior. Macromol. Chem. Phys. 2014, 215, 1797–1809. [Google Scholar] [CrossRef]
- Semikolenova, N.V.; Sun, W.-H.; Soshnikov, I.E.; Matsko, M.A.; Kolesova, O.V.; Zakharov, V.A.; Bryliakov, K.P. Origin of “Multisite-like” Ethylene Polymerization Behavior of the Single-Site Nonsymmetrical Bis(imino)pyridine Iron(II) Complex in the Presence of Modified Methylaluminoxane. ACS Catal. 2017, 7, 2868–2877. [Google Scholar] [CrossRef]
- Cao, X.; He, F.; Zhao, W.; Cai, Z.; Hao, X.; Shiono, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-chlorophenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridyliron(II) dichlorides: Synthesis, Characterization and Ethylene Polymerization Behavior. Polymer 2012, 53, 1870–1880. [Google Scholar] [CrossRef]
- Barabanov, A.A.; Bukatov, G.D.; Zakharov, V.A.; Semikolenova, N.V.; Echevskaya, L.G.; Matsko, M.A. Kinetic Peculiarities of Ethylene Polymerization over Homogeneous Bis(imino)pyridine Fe(II) Catalysts with Different Activators. Macromol. Chem. Phys. 2005, 206, 2292–2298. [Google Scholar] [CrossRef]
- Echevskaya, L.G.; Zakharov, V.A.; Golovin, A.V.; Mikenas, T.B. Molecular Structure of Polyethylene Produced with Supported Vanadium-Magnesium Catalyst. Macromol. Chem. Phys. 1999, 200, 1434–1438. [Google Scholar] [CrossRef]




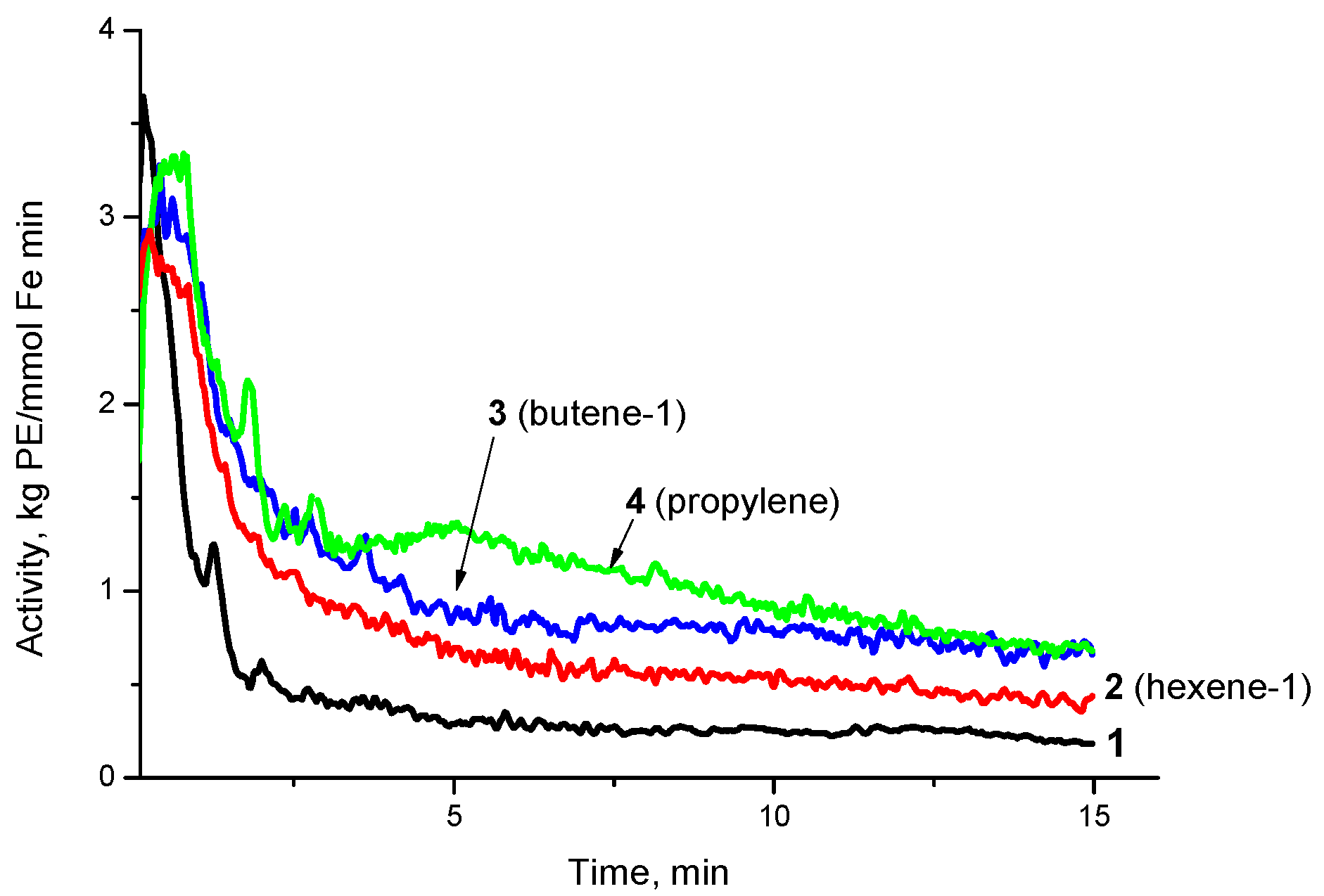

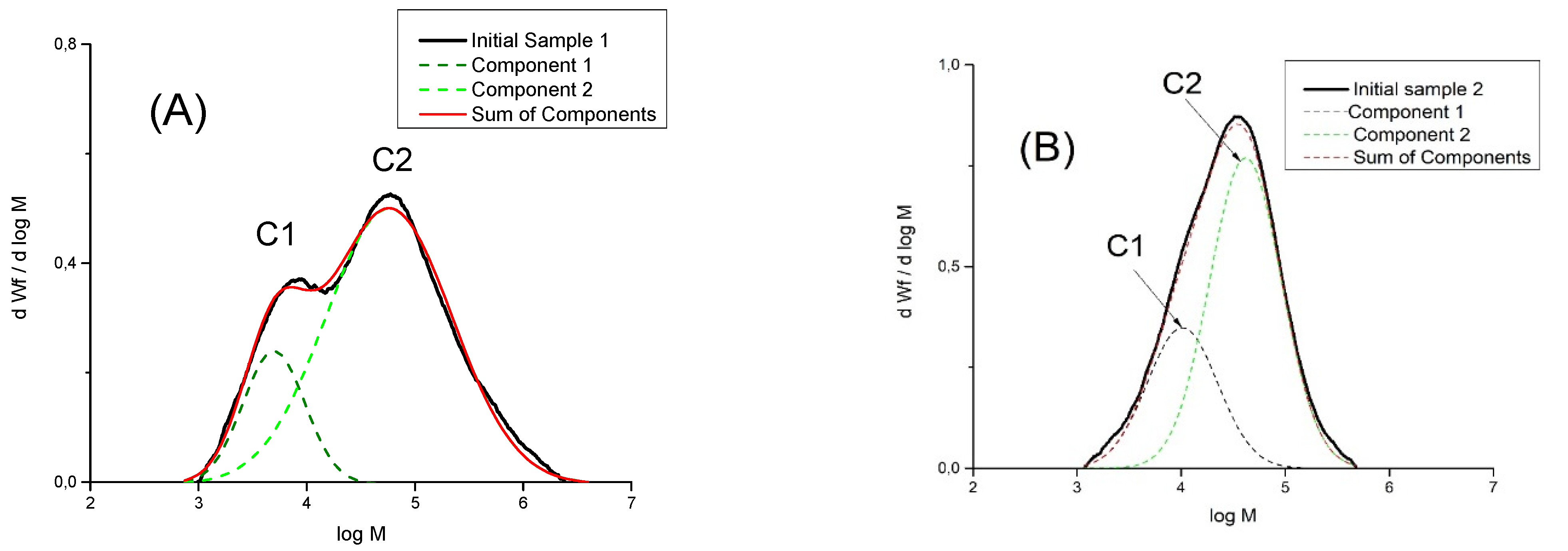
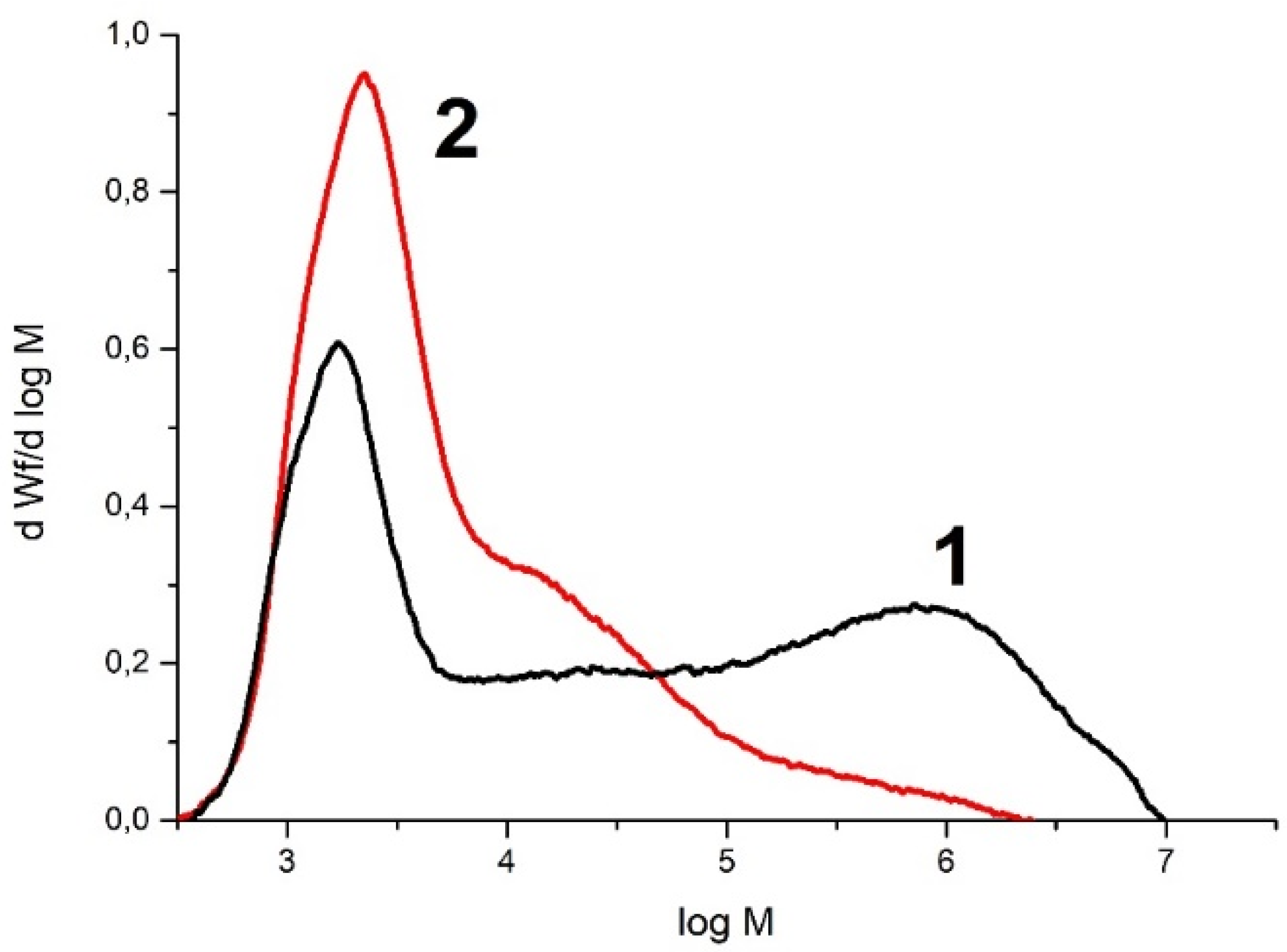
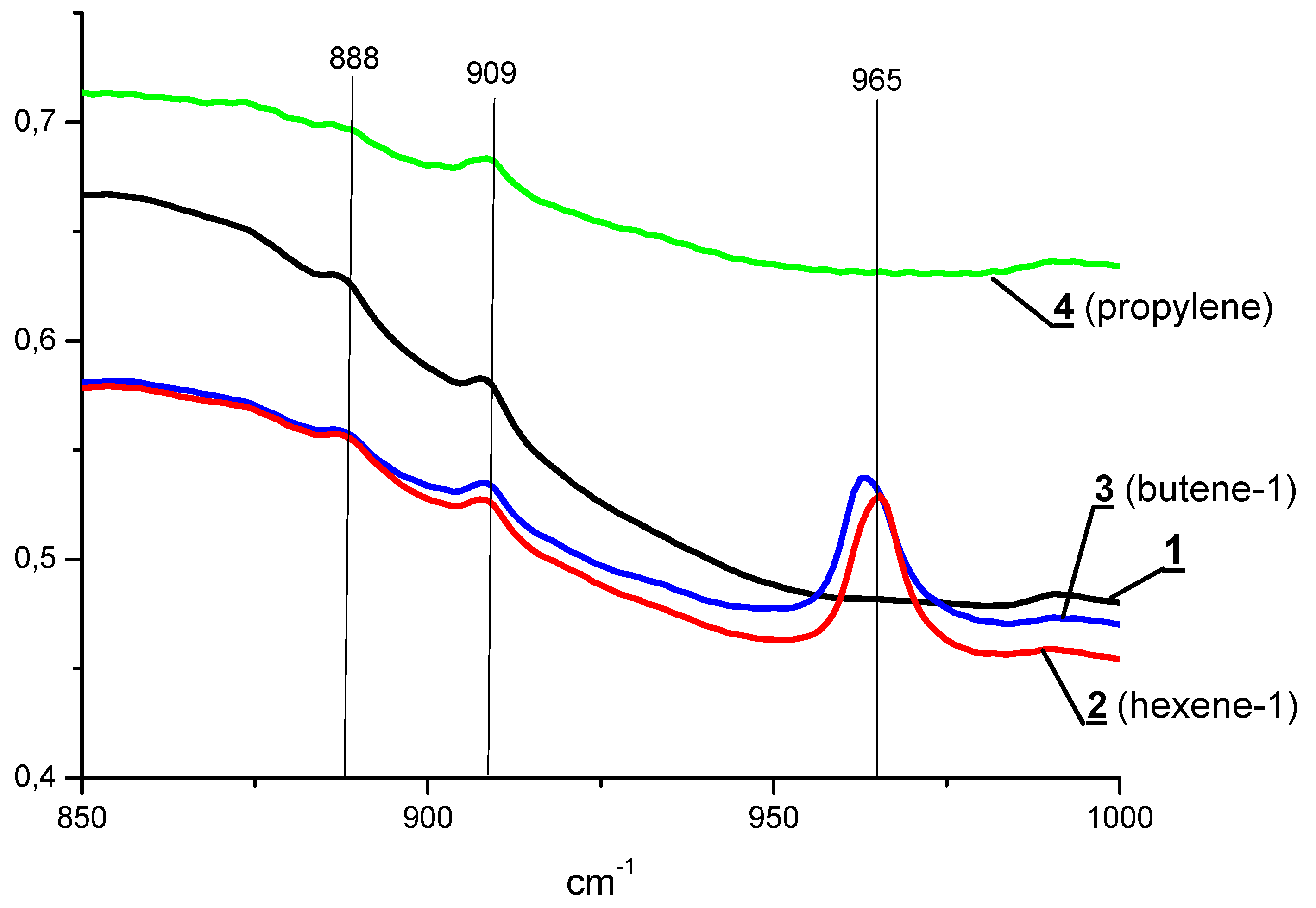

| Exp. No. | AlMe3 | PE Yield 1, kg/mmol Fe | Activity, kg PE/ (mmol Fe×min) | Mn kg/mol | Mw kg/mol | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | - | 10 | 0.67 | 11 | 140 | 13 |
| 2 | 2 AlMe3 | 6.4 | 0.43 | 3.4 | 470 | 138.0 |
| Exp. No. | α-olefin 1 | PE Yield kg PE/mmol Fe | Rp, kg PE/(mmol Fe×min) | |
|---|---|---|---|---|
| 2 Rpmax | 3 Rpst | |||
| 1 | - | 10.0 | 3.5 | 0.2 |
| 2 | hexene-1 | 11.6 | 2.9 | 0.6 |
| 3 | butene-1 | 14.0 | 3.2 | 0.8 |
| 4 | propylene | 18.4 | 3.3 | 1.0 |
| Exp. No. 1 | α-olefin (mol/L) | Mn kg/mol | Pn | Mw kg/mol | Mw/Mn | CH2 = CHR | RCH = CHR’ | -CH3 | Tm 2, °C | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1 *) | (2 *) | (1 *) | (2 *) | (1 *) | (2 *) | |||||||
| 1 | - | 11 | 393 | 140 | 13 | 0.04 | 0.03 | - | - | 1.5 | 1.2 | 134.9 |
| 2 | hexene-1 | 15 | 535 | 45 | 3.0 | 0.05 | 0.06 | 0.3 | 0.32 | 1.1 | 1.2 | 134.3 |
| 3 | butene-1 | 16 | 571 | 54 | 3.4 | 0.06 | 0.07 | 0.3 | 0.34 | 2.0 | 2.3 | 134.9 |
| 4 | propylene | 46 | 1642 | 290 | 6.3 | 0.04 | 0.12 | - | - | 0.6 | 2.0 | 135.5 |
| Sample 1 | Component | Content of Component, % | Mpeak kg/mol | Mn kg/mol | Mw kg/mol | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | C1 | 19 | 4.9 | 4.0 | 6.2 | 1.5 |
| C2 | 81 | 57.5 | 25 | 130 | 5.3 | |
| SUM | 100 | 12.5 | 107 | 8.5 | ||
| 2 | C1 | 30 | 10 | 7.7 | 14 | 1.8 |
| C2 | 70 | 41.5 | 30 | 56 | 1.9 | |
| SUM | 100 | 35 | 16 | 43 | 2.7 |
| Exp. No. | PE Yield, kg/mol | Activity kg PE | Mn, kg/mol | Mw, kg/mol | Mw/Mn |
|---|---|---|---|---|---|
| mmol Fe × min | |||||
| 1 | 6.4 | 0.43 | 3.4 | 470 | 138 |
| 2 1 | 9.6 | 0.64 | 2.6 | 33 | 12.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semikolenova, N.V.; Matsko, M.A.; Zakharov, V.A.; Sun, W.-H. Unusual Effect of α-olefins as Chain Transfer Agents in Ethylene Polymerization over the Catalyst with Nonsymmetrical Bis(imino)pyridine Complex of Fe(II) and Modified Methylalumoxane (MMAO) Cocatalyst. Int. J. Mol. Sci. 2022, 23, 14384. https://doi.org/10.3390/ijms232214384
Semikolenova NV, Matsko MA, Zakharov VA, Sun W-H. Unusual Effect of α-olefins as Chain Transfer Agents in Ethylene Polymerization over the Catalyst with Nonsymmetrical Bis(imino)pyridine Complex of Fe(II) and Modified Methylalumoxane (MMAO) Cocatalyst. International Journal of Molecular Sciences. 2022; 23(22):14384. https://doi.org/10.3390/ijms232214384
Chicago/Turabian StyleSemikolenova, Nina V., Mikhail A. Matsko, Vladimir A. Zakharov, and Wen-Hua Sun. 2022. "Unusual Effect of α-olefins as Chain Transfer Agents in Ethylene Polymerization over the Catalyst with Nonsymmetrical Bis(imino)pyridine Complex of Fe(II) and Modified Methylalumoxane (MMAO) Cocatalyst" International Journal of Molecular Sciences 23, no. 22: 14384. https://doi.org/10.3390/ijms232214384
APA StyleSemikolenova, N. V., Matsko, M. A., Zakharov, V. A., & Sun, W.-H. (2022). Unusual Effect of α-olefins as Chain Transfer Agents in Ethylene Polymerization over the Catalyst with Nonsymmetrical Bis(imino)pyridine Complex of Fe(II) and Modified Methylalumoxane (MMAO) Cocatalyst. International Journal of Molecular Sciences, 23(22), 14384. https://doi.org/10.3390/ijms232214384







