Human Milk Oligosaccharide 2′-Fucosyllactose Inhibits Ligand Binding to C-Type Lectin DC-SIGN but Not to Langerin
Abstract
:1. Introduction
2. Results
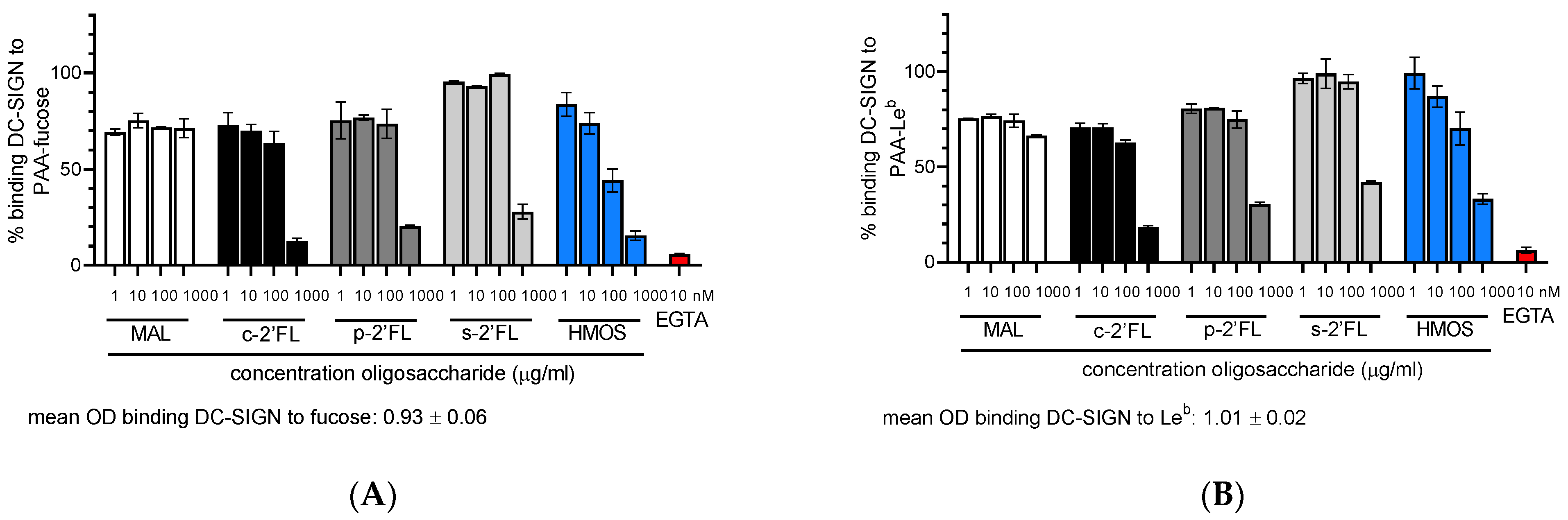
2.1. c2′-FL, p2′-FL, s2′-FL and HMOs Inhibit the Ligand Binding to DC-SIGN, but Not to Langerin
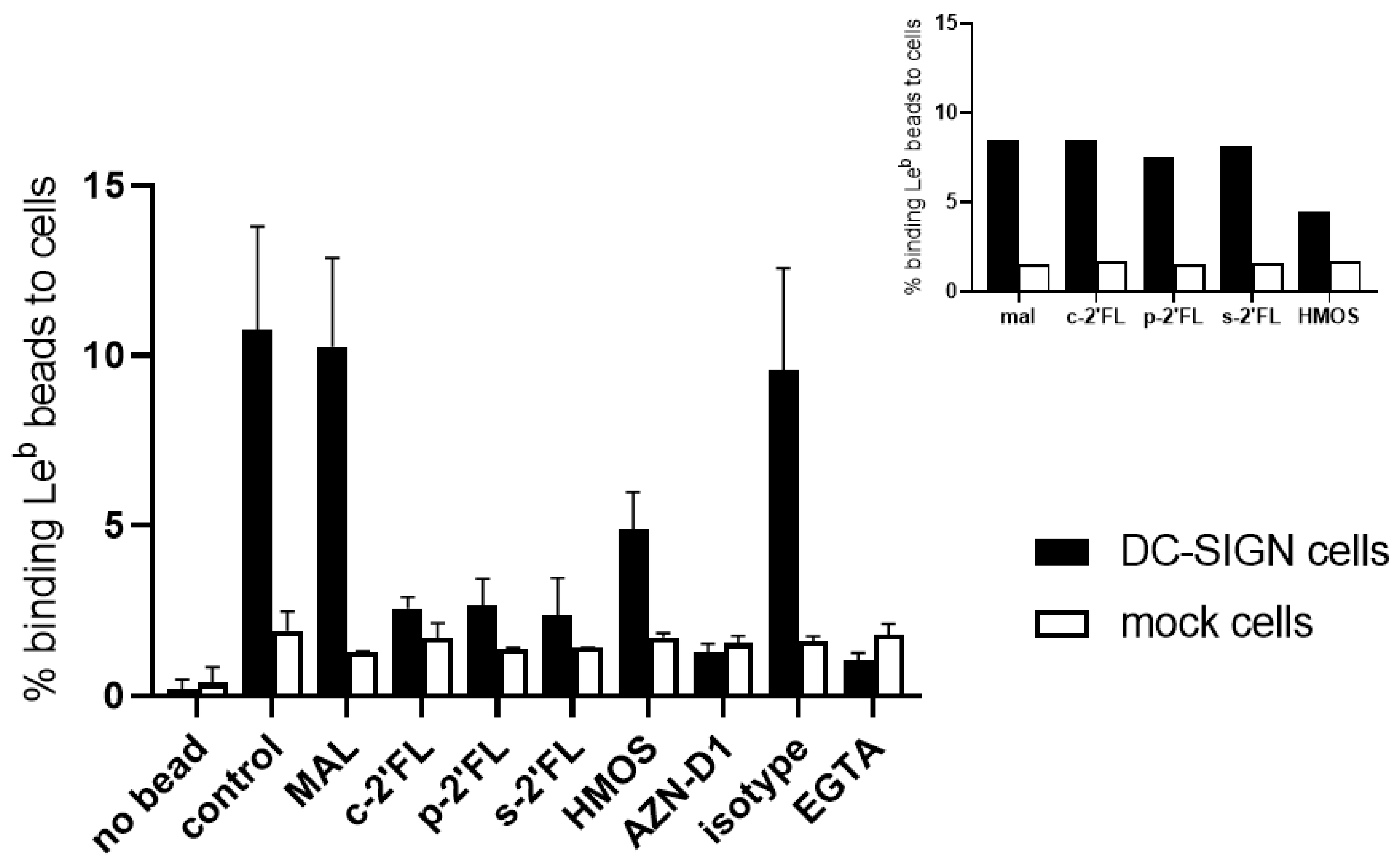
2.2. c2′-FL, p2′-FL, and s2′-FL Inhibit the Binding of Leb to the DC-SIGN Expressing Cells
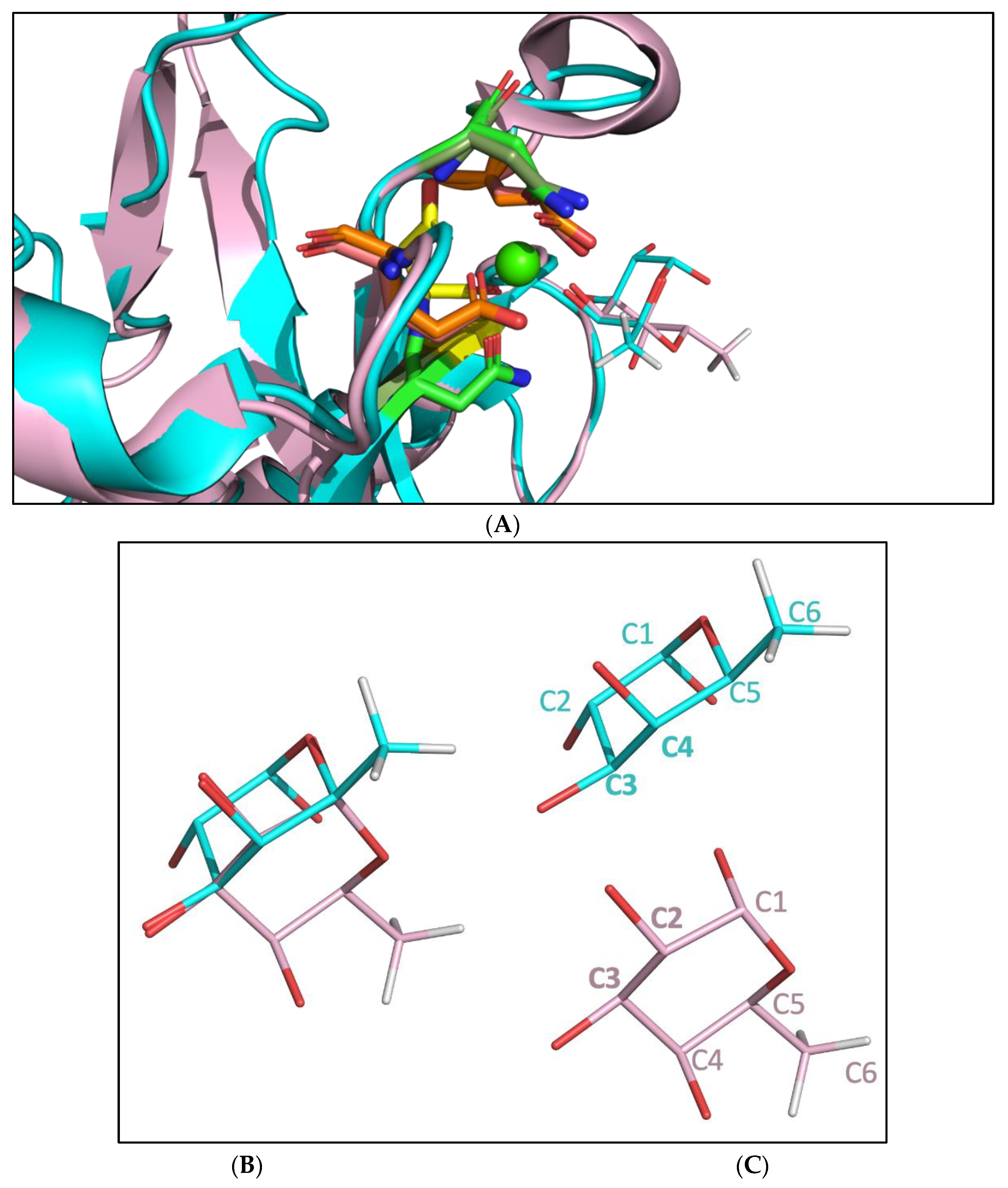
2.3. Molecular Dynamic Simulation Suggested That 2′-FL Exists in Bioactive Conformation to Bind DC-SIGN but Not Langerin
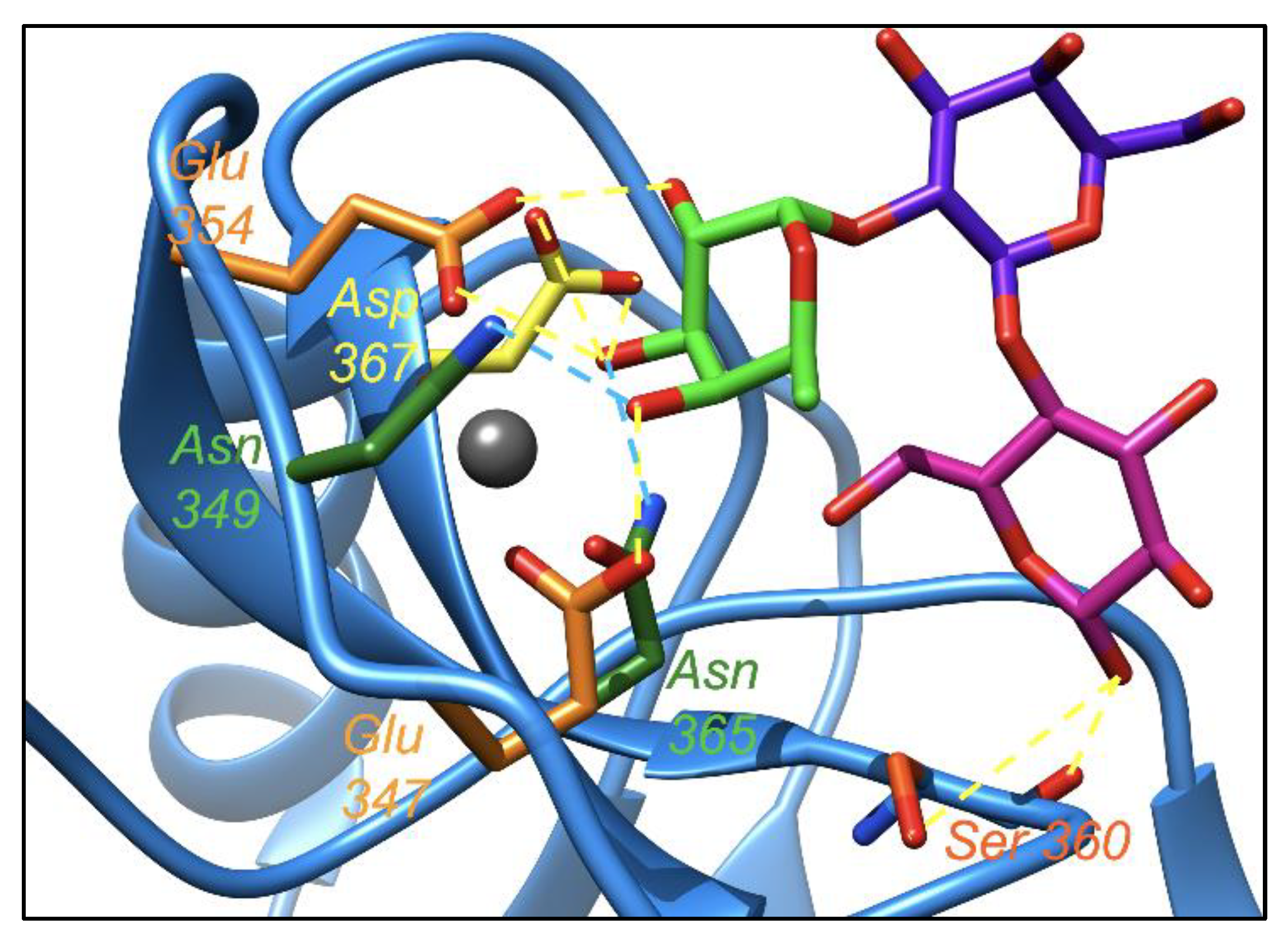
2.4. Leb Interacts with DC-SIGN with Two Fucoses Operating One at a Time: Consequences for the Displacement by 2′-FL
3. Discussion
4. Materials and Methods
4.1. Antibodies and Beads
4.2. Cells
4.3. HMOs
4.4. Purification of c2′-FL to Obtain p2′-FL
4.5. Ligand-Receptor Competition Assay
4.6. Fluorescent Bead Adhesion Cell-Ligand Competition Assay
4.7. Molecular Dynamics Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chiodo, F.; de Haas, A.; van Vliet, S.J.; van Kooyk, Y. Human C-Type Lectins, MGL, DC-SIGN and Langerin, Their Interactions with Endogenous and Exogenous Ligand Patterns. In Comprehensive Glycoscience, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 3, pp. 425–441. [Google Scholar]
- Weis, W.I.; Taylor, M.; Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998, 163, 19–34. [Google Scholar] [CrossRef]
- Foster, A.J.; Bird, J.H.; Timmer, M.S.M.; Stocker, B. The Ligands of C-Type Lectins. In C-Type Lectin Receptors in Immunity; Yamasaki, S., Ed.; Springer: Tokyo, Japan, 2016; pp. 191–215. [Google Scholar]
- Valverde, P.; Martínez, J.D.; Cañada, F.J.; Ardá, A.; Jiménez-Barbero, J. Molecular Recognition in C-Type Lectins: The Cases of DC-SIGN, Langerin, MGL, and L-Sectin. ChemBioChem 2020, 21, 2999–3025. [Google Scholar] [CrossRef]
- van Vliet, S.J.; García-Vallejo, J.J.; van Kooyk, Y.Y. Dendritic cells and C-type lectin receptors: Coupling innate to adaptive immune responses. Immunol. Cell Biol. 2008, 86, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Ginhoux, F.; Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008, 8, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Valverde, P.; Delgado, S.; Martínez, J.D.; Vendeville, J.-B.; Malassis, J.; Linclau, B.; Reichardt, N.-C.; Cañada, F.J.; Jiménez-Barbero, J.; Ardá, A. Molecular Insights into DC-SIGN Binding to Self-Antigens: The Interaction with the Blood Group A/B Antigens. ACS Chem. Biol. 2019, 14, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Pederson, K.; Mitchell, D.A.; Prestegard, J.H. Structural Characterization of the DC-SIGN–LewisX Complex. Biochemistry 2014, 53, 5700–5709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.T.; Hsu, T.-L.; Huang, S.K.; Hsieh, S.-L.; Wong, C.-H.; Lee, Y.C. Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology 2011, 21, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The Predominance of Type I Oligosaccharides Is a Feature Specific to Human Breast Milk. Adv. Nutr. Int. Rev. J. 2012, 3, 473S–482S. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.S. Glycobiology of human milk. Biochemistry 2013, 78, 771–785. [Google Scholar] [CrossRef]
- Asadpoor, M.; Peeters, C.; Henricks, P.A.J.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Anti-Pathogenic Functions of Non-Digestible Oligosaccharides In Vitro. Nutrients 2020, 12, 1789. [Google Scholar] [CrossRef]
- Holscher, H.; Bode, L.; Tappenden, K. Human Milk Oligosaccharides Influence Intestinal Epithelial Cell Maturation In Vitro. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 296–301. [Google Scholar] [CrossRef]
- Holscher, H.D.; Davis, S.R.; Tappenden, K.A. Human milk oligosaccharides influence maturation of human intestinal Caco-2Bbe and HT-29 cell lines. J. Nutr. 2014, 144, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Kiewiet, M.B.; Groeneveld, A.; Nauta, A.; de Vos, P. Human milk oligosaccharides and its acid hydrolysate LNT2 show immunomodulatory effects via TLRs in a dose and structure-dependent way. J. Funct. Foods 2019, 59, 174–184. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The human milk oligosaccharides 2′-Fucosyllactose and 6′-Sialyllactose protect against the development of necrotizing enterocolitis by inhibiting Toll-Like Receptor 4 signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J. Nutr. Immunol. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni Binds Intestinal H(O) Antigen (Fucα1, 2Galβ1, 4GlcNAc), and Fucosyloligosaccharides of Human Milk Inhibit Its Binding and Infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [Green Version]
- Weichert, S.; Jennewein, S.; Hüfner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef]
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk Oligosaccharides Inhibit Human Rotavirus Infectivity in MA104 Cells. J. Nutr. 2017, 147, 1709–1714. [Google Scholar] [CrossRef]
- Koromyslova, A.; Tripathi, S.; Morozov, V.; Schroten, H.; Hansman, G.S. Human norovirus inhibition by a human milk oligosaccharide. Virology 2017, 508, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Bych, K.; Mikš, M.H.; Johanson, T.; Hederos, M.J.; Vigsnæs, L.K.; Becker, P. Production of HMOs using microbial hosts—from cell engineering to large scale production. Curr. Opin. Biotechnol. 2018, 56, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Noll, A.J.; Yu, Y.; Lasanajak, Y.; Duska-McEwen, G.; Buck, R.H.; Smith, D.F.; Cummings, R.D. Human DC-SIGN binds specific human milk glycans. Biochem. J. 2016, 473, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Zhou, Y.; Neelamegham, S. DrawGlycan-SNFG: A robust tool to render glycans and glycopeptides with fragmentation information. Glycobiology 2017, 27, 200–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdijk, O.; van Neerven, R.; Meijer, B.; Savelkoul, H.F.J.; Brugman, S. Induction of human tolerogenic dendritic cells by 3′-sialyllactose via TLR4 is explained by LPS contamination. Glycobiology 2017, 28, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erridge, C. Endogenous ligands of TLR2 and TLR4: Agonists or assistants? J. Leukoc. Biol. 2010, 87, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Perdijk, O.; van Neerven, J.; van Den Brink, E.; Savelkoul, H.F.J.; Brugman, S. The oligosaccharides 6′-sialyllactose, 2′-fucosyllactose or galactooligosaccharides do not directly modulate human dendritic cell differentiation or maturation. PLoS ONE 2018, 13, e0200356. [Google Scholar] [CrossRef] [PubMed]
- van Liempt, E.; Bank, C.M.C.; Mehta, P.; Garciá-Vallejo, J.J.; Kawar, Z.S.; Geyer, R.; Alvarez, R.A.; Cummings, R.D.; van Kooyk, Y.; van Die, I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006, 580, 6123–6131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewald, D.R.; Summer, S.C.J. Human Microbiota, Blood Group Antigens, and Disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1413. [Google Scholar] [CrossRef] [PubMed]
- Triantis, V.; Bode, L.; van Neerven, R.J. Immunological Effects of Human Milk Oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.; Adema, G.J.; van Kooyk, Y.; Figdor, C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 2000, 100, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Marzi, A.; Möller, P.; Hanna, S.L.; Harrer, T.; Eisemann, J.; Steinkasserer, A.; Becker, S.; Baribaud, F.; Pöhlmann, S. Analysis of the Interaction of Ebola Virus Glycoprotein with DC-SIGN (Dendritic Cell–Specific Intercellular Adhesion Molecule 3–Grabbing Nonintegrin) and Its Homologue DC-SIGNR. J. Infect. Dis. 2007, 196, S237–S246. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a Dendritic Cell–Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Bloem, K.; Garcia-Vallejo, J.J.; Vuist, I.M.; Cobb, B.A.; Van Vliet, S.J.; Van Kooyk, Y. Interaction of the Capsular Polysaccharide A from Bacteroides fragilis with DC-SIGN on Human Dendritic Cells is Necessary for Its Processing and Presentation to T Cells. Front. Immunol. 2013, 4, 103. [Google Scholar] [CrossRef] [Green Version]
- Fehres, C.M.; Kalay, H.; Bruijns, S.C.M.; Musaafir, S.A.M.; Ambrosini, M.; van Bloois, L.; van Vliet, S.J.; Storm, G.; Garcia-Vallejo, J.J.; van Kooyk, Y. Cross-presentation through langerin and DC-SIGN targeting requires different formulations of glycan-modified antigens. J. Control. Release 2015, 203, 67–76. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.H.; van Duijnhoven, G.C.; van Vliet, S.J.; Krieger, E.; Vriend, G.; Figdor, C.G.; van Kooyk, Y. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 2002, 277, 11314–11320. [Google Scholar] [CrossRef] [Green Version]
- Geijtenbeek, T.B.H.; van Kooyk, Y.; van Vliet, S.J.; Renes, M.H.; Raymakers, R.A.P.; Figdor, C.J. High Frequency of Adhesion Defects in B-Lineage Acute Lymphoblastic Leukemia. Blood 1999, 94, 754–764. [Google Scholar] [CrossRef]
- Woods Group. (2005–2022) GLYCAM Web. Complex Carbohydrate Research Center, University of Georgia, Athens, GA. Available online: http://legacy.glycam.org (accessed on 13 September 2022).
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutt, D.S.; Cheatham, T.E.; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. AMBER 2018, University of California, San Francisco. 2018. Available online: https://ambermd.org/ (accessed on 13 September 2022).
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters From ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Darden, T.; Darrin, Y.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, R.; Somovilla, V.J.; Chiodo, F.; Bruijns, S.; Pieters, R.J.; Garssen, J.; van Kooyk, Y.; Kraneveld, A.D.; van Bergenhenegouwen, J. Human Milk Oligosaccharide 2′-Fucosyllactose Inhibits Ligand Binding to C-Type Lectin DC-SIGN but Not to Langerin. Int. J. Mol. Sci. 2022, 23, 14745. https://doi.org/10.3390/ijms232314745
Mukherjee R, Somovilla VJ, Chiodo F, Bruijns S, Pieters RJ, Garssen J, van Kooyk Y, Kraneveld AD, van Bergenhenegouwen J. Human Milk Oligosaccharide 2′-Fucosyllactose Inhibits Ligand Binding to C-Type Lectin DC-SIGN but Not to Langerin. International Journal of Molecular Sciences. 2022; 23(23):14745. https://doi.org/10.3390/ijms232314745
Chicago/Turabian StyleMukherjee, Reshmi, Victor J. Somovilla, Fabrizio Chiodo, Sven Bruijns, Roland J. Pieters, Johan Garssen, Yvette van Kooyk, Aletta D. Kraneveld, and Jeroen van Bergenhenegouwen. 2022. "Human Milk Oligosaccharide 2′-Fucosyllactose Inhibits Ligand Binding to C-Type Lectin DC-SIGN but Not to Langerin" International Journal of Molecular Sciences 23, no. 23: 14745. https://doi.org/10.3390/ijms232314745
APA StyleMukherjee, R., Somovilla, V. J., Chiodo, F., Bruijns, S., Pieters, R. J., Garssen, J., van Kooyk, Y., Kraneveld, A. D., & van Bergenhenegouwen, J. (2022). Human Milk Oligosaccharide 2′-Fucosyllactose Inhibits Ligand Binding to C-Type Lectin DC-SIGN but Not to Langerin. International Journal of Molecular Sciences, 23(23), 14745. https://doi.org/10.3390/ijms232314745








