The Role of Exogenous Gibberellic Acid and Methyl Jasmonate against White-Backed Planthopper (Sogatella furcifera) Stress in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Results
2.1. Measurement and Analysis of Agronomic Traits
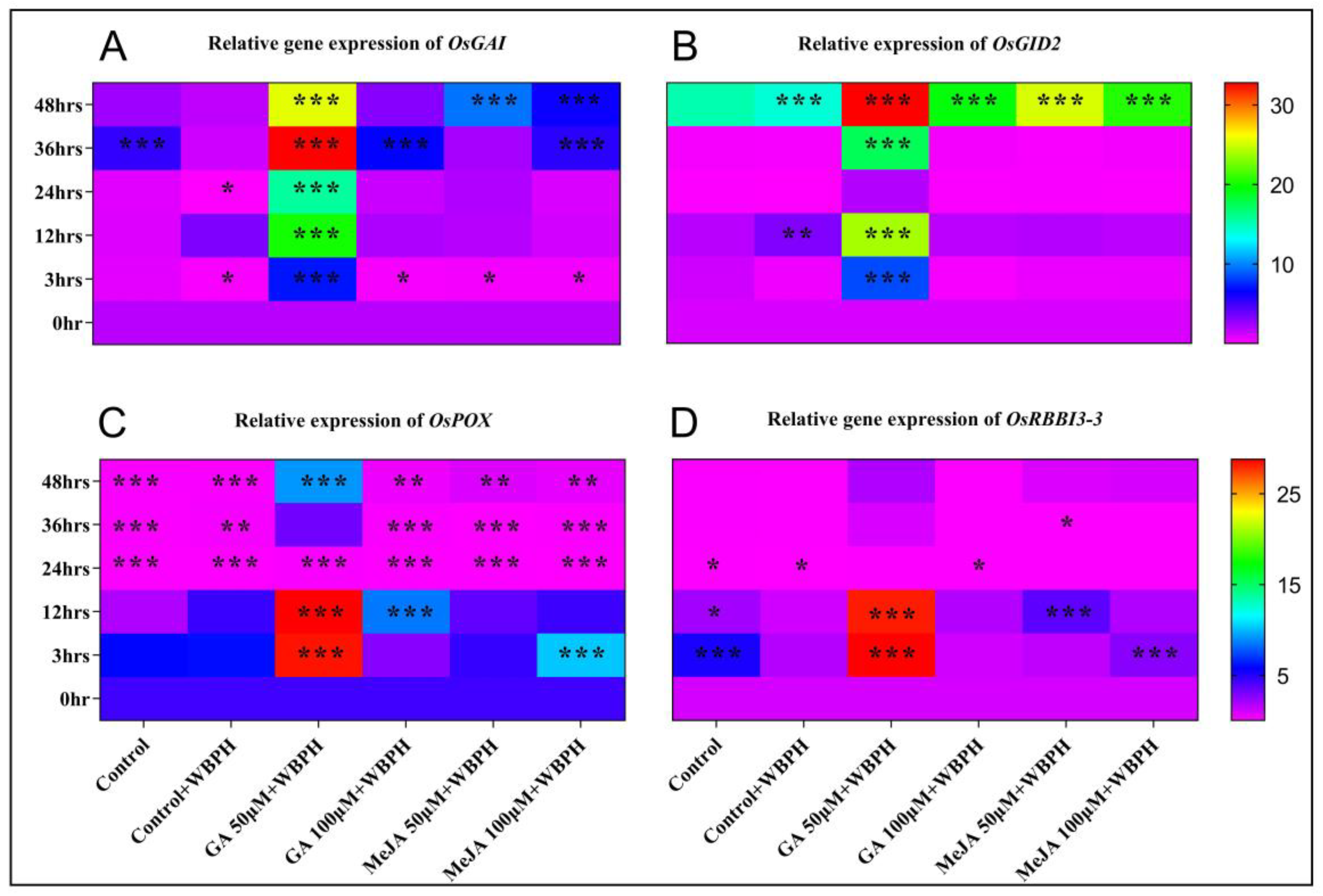
2.2. Relative Gene Expression
2.3. Histochemical Analysis and Antioxidant Accumulation in Response to WBPH Stress and Applying Hormones
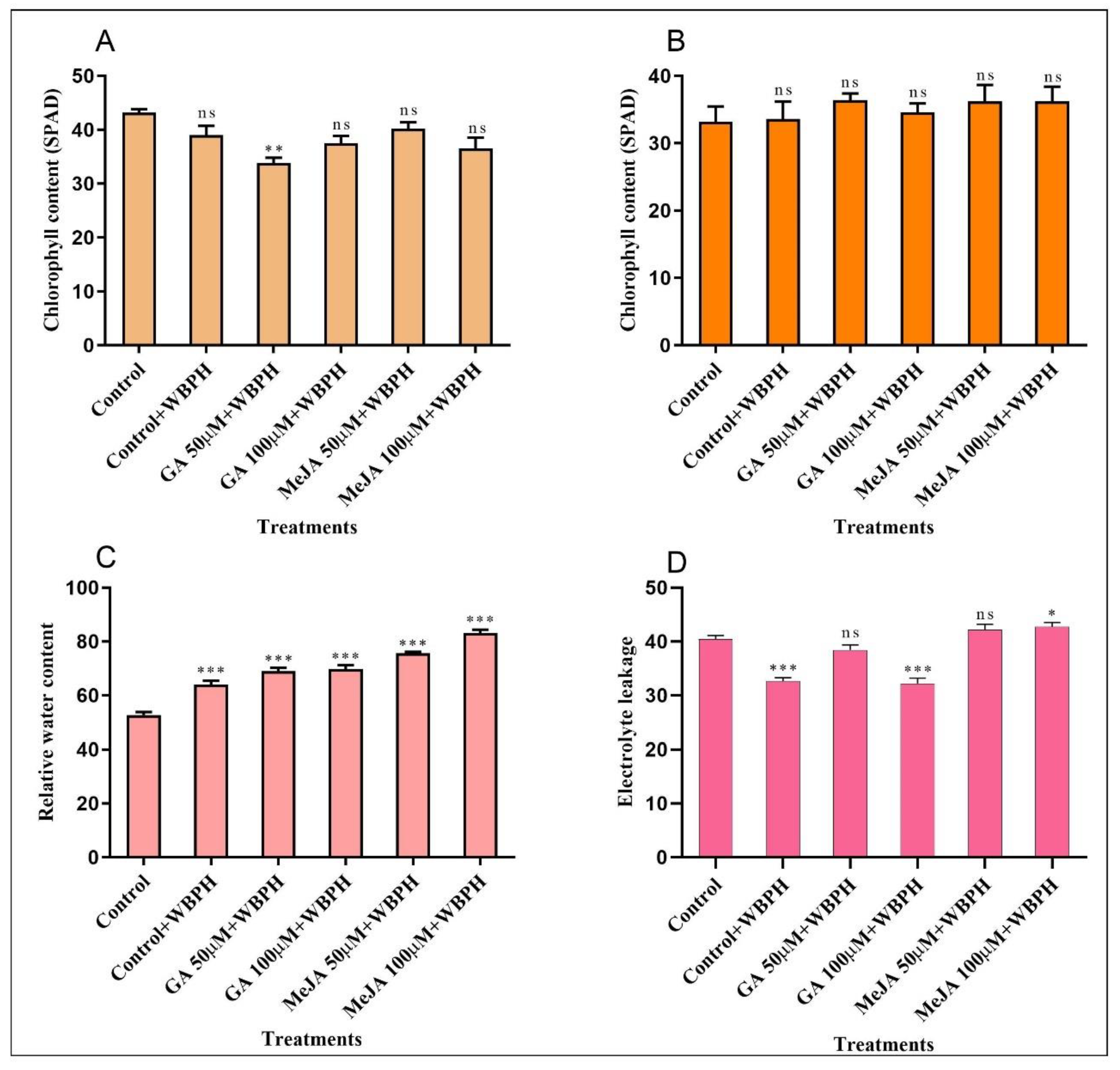
2.4. Measurement of Chlorophyll Content, RWC, and Electrolyte Leakage
2.5. Recovery of Infected Plants after Hormone Treatment
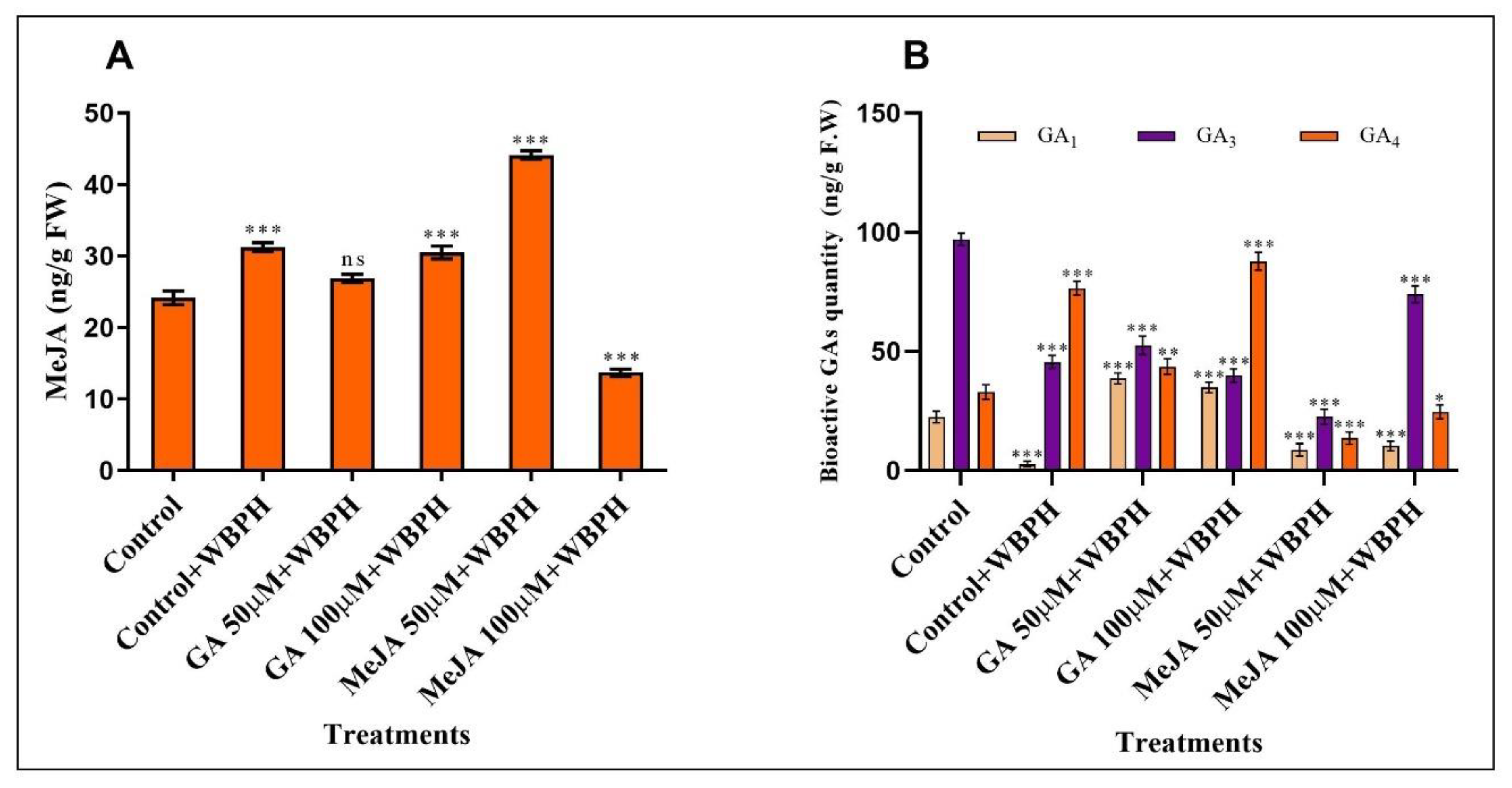
2.6. Quantification of Endogenous GA and MeJA Hormones
3. Discussion
4. Materials and Methods
4.1. Plant Selection and Growth Conditions
4.2. Experimental Design
4.3. Measurement and Analysis of Agronomic Traits
4.4. RNA Isolation and qRT-PCR
4.5. Histochemical Analysis
4.6. Estimation of Antioxidant Activities
4.7. Measurement of Chlorophyll Content
4.8. Relative Water Content and Electrolyte Leakage
4.9. Electrolyte Leakage
4.10. Recovery of Infected Plants
4.11. Quantification of Endogenous GA and MeJA Hormones
4.12. Statistical Analysis
5. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.-J.; Kim, K.-M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. 2020, 10, 14685. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, J.; Norton, G. HOPPER—An expert system for forecasting the risk of white-backed planthopper attack in the first crop season in China. Crop Prot. 1994, 13, 463–473. [Google Scholar] [CrossRef]
- Reissig, W. Illustrated Guide to Integrated Pest Management in Rice in Tropical Asia; International Rice Research Institute: Los Baños, Philippines, 1985. [Google Scholar]
- Zhang, H.-M.; Yang, J.; Chen, J.-P.; Adams, M.J. A black-streaked dwarf disease on rice in China is caused by a novel fijivirus. Arch. Virol. 2008, 153, 1893–1898. [Google Scholar] [CrossRef]
- Pathak, M.D.; Khan, Z.R. Insect Pests of Rice; International Rice Research Institute: Los Baños, Philippines, 1994. [Google Scholar]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.-M. Over-Expression of Chorismate Mutase Enhances the Accumulation of Salicylic Acid, Lignin, and Antioxidants in Response to the White-Backed Planthopper in Rice Plants. Antioxidants 2021, 10, 1680. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Davière, J.-M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Macmillan, J. Occurrence of Gibberellins in Vascular Plants, Fungi, and Bacteria. J. Plant Growth Regul. 2001, 20, 387–442. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Shah, K. Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 2014, 108, 57–66. [Google Scholar] [CrossRef]
- Jannoey, P.; Channei, D.; Kotcharerk, J.; Pongprasert, W.; Nomura, M. Expression Analysis of Genes Related to Rice Resistance against Brown Planthopper, Nilaparvata lugens. Rice Sci. 2017, 24, 163–172. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Xu, J.; Höfte, M. Making sense of hormone-mediated defense networking: From rice to Arabidopsis. Front. Plant Sci. 2014, 5, 611. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. Making Sense of Hormone Crosstalk during Plant Immune Responses. Cell Host Microbe 2008, 3, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Nahar, K.; Kyndt, T.; Hause, B.; Höfte, M.; Gheysen, G. Brassinosteroids Suppress Rice Defense against Root-Knot Nematodes Through Antagonism with the Jasmonate Pathway. Mol. Plant-Microbe Interact. 2013, 26, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Liu, J.H.; Zhao, W.S.; Chen, X.J.; Guo, Z.J.; Peng, Y.L. Gibberellin 20-Oxidase Gene OsGA20ox3 Regulates Plant Stature and Disease Development in Rice. Mol. Plant-Microbe Interact. 2013, 26, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.-L.; Li, Q.; Deng, Y.-W.; Lou, Y.-G.; Wang, M.-Y.; Zhou, G.-X.; Zhang, Y.-Y.; He, Z.-H. Altered Disease Development in the eui Mutants and Eui Overexpressors Indicates that Gibberellins Negatively Regulate Rice Basal Disease Resistance. Mol. Plant 2008, 1, 528–537. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Gheysen, G.; Denil, S.; Lindsey, K.; Topping, J.F.; Nahar, K.; Haegeman, A.; De Vos, W.H.; Trooskens, G.; Van Criekinge, W.; et al. Transcriptional analysis through RNA sequencing of giant cells induced by Meloidogyne graminicola in rice roots. J. Exp. Bot. 2013, 64, 3885–3898. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Kusano, T.; Katsumi, M.; Sano, H. Rice gibberellin-insensitive gene homolog, OsGAI, encodes a nuclear-localized protein capable of gene activation at transcriptional level. Gene 2000, 245, 21–29. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, T.; Wang, W.; Cao, T.; Li, R.; Lou, Y. Silencing OsSLR1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens. Plant Cell Environ. 2017, 40, 2147–2159. [Google Scholar] [CrossRef]
- Gomi, K.; Sasaki, A.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Kitano, H.; Matsuoka, M. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant J. 2004, 37, 626–634. [Google Scholar] [CrossRef]
- Zou, X.; Qin, Z.; Zhang, C.; Liu, B.; Liu, J.; Zhang, C.; Lin, C.; Li, H.; Zhao, T. Over-expression of an S-domain receptor-like kinase extracellular domain improves panicle architecture and grain yield in rice. J. Exp. Bot. 2015, 66, 7197–7209. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, G.K.; Rakwal, R.; Jwa, N.-S. Cloning and characterization of a jasmonate inducible rice (Oryza sativa L.) peroxidase gene, OsPOX, against global signaling molecules and certain inhibitors of kinase-signaling cascade(s). Plant Sci. 2002, 162, 49–58. [Google Scholar] [CrossRef]
- Rakwal, R.; Agrawal, G.K.; Jwa, N.-S. Characterization of a rice (Oryza sativa L.) Bowman–Birk proteinase inhibitor: Tightly light regulated induction in response to cut, jasmonic acid, ethylene and protein phosphatase 2A inhibitors. Gene 2001, 263, 189–198. [Google Scholar] [CrossRef]
- Laura, B.; Silvia, P.; Francesca, F.; Benedetta, S.; Carla, C. Epigenetic control of defense genes following MeJA-induced priming in rice (O. sativa). J. Plant Physiol. 2018, 228, 166–177. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Li, P.; Li, F.; Ali, S.; Hou, M. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Sci. Rep. 2017, 7, 4232. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Corbineau, F.; Gay-Mathieu, C.; Vinel, D.; Côme, D. Decrease in sunflower (Helianthus annuus) seed viability caused by high temperature as related to energy metabolism, membrane damage and lipid composition. Physiol. Plant. 2002, 116, 49–496. [Google Scholar] [CrossRef]
- Wu, G.; Shortt, B.J.; Lawrence, E.B.; Leon, J.; Fitzsimmons, K.C.; Levine, E.B.; Raskin, I.; Shah, D. Activation of Host Defense Mechanisms by Elevated Production of H2O2 in Transgenic Plants. Plant Physiol. 1997, 115, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.; Yu, J.; Bai, J.; Zhu, Z.; Wang, X. Induced defense responses in rice plants against small brown planthopper infestation. Crop J. 2014, 2, 55–62. [Google Scholar] [CrossRef]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Alhaithloul, H.A.S.; Alayafi, A.A.; Soliman, M.H. Effect of gibberellic acid on growth, photosynthesis and antioxidant defense system of wheat under zinc oxide nanoparticle stress. Environ. Pollut. 2019, 254, 113109. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, S.; Singh, V.P.; Srivastava, P.K.; Maurya, J.N. Modification of chromium (VI) phytotoxicity by exogenous gibberellic acid application in Pisum sativum (L.) seedlings. Acta Physiol. Plant. 2010, 33, 1385–1397. [Google Scholar] [CrossRef]
- Wen, F.-P.; Zhang, Z.-H.; Bai, T.; Xu, Q.; Pan, Y.-H. Proteomics reveals the effects of gibberellic acid (GA3) on salt-stressed rice (Oryza sativa L.) shoots. Plant Sci. 2010, 178, 170–175. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, P.; Mo, X.; Hu, L.; Jin, N.; Chen, X.; Yu, Z.; Meng, J.; Erb, M.; Shang, Z.; et al. Induction of defense in cereals by 4-fluorophenoxyacetic acid suppresses insect pest populations and increases crop yields in the field. Proc. Natl. Acad. Sci. USA 2020, 117, 12017–12028. [Google Scholar] [CrossRef]
- Xu, Y.; Li, K.; Zhu, K.; Tian, Y.; Yu, Q.; Zhang, W.; Wang, Z. Effect of exogenous plant hormones on agronomic and physiological performance of a leaf early-senescent rice mutant osled. Plant Growth Regul. 2020, 92, 517–533. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, Y.S.; Park, S.-H.; Koo, Y.J.; Choi, Y.D.; Chung, Y.-Y.; Lee, I.-J.; Kim, J.-K. Methyl Jasmonate Reduces Grain Yield by Mediating Stress Signals to Alter Spikelet Development in Rice. Plant Physiol. 2009, 149, 1751–1760. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Tung, S.A.; Samad, R.A.; Wang, L.; Khan, I.; Rehman, N.U.; Shah, A.N.; Shahzad, B. Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiol. Plant. 2015, 38, 25. [Google Scholar] [CrossRef]
- Siddiqui, M.; Khan, M.; Mohammad, F.; Khan, M. Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008, 194, 214–224. [Google Scholar] [CrossRef]
- Gordy, J.W.; Leonard, B.R.; Blouin, D.; Davis, J.A.; Stout, M.J. Comparative Effectiveness of Potential Elicitors of Plant Resistance against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in Four Crop Plants. PLoS ONE 2015, 10, e0136689. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Li, J.; Han, X.; Li, R.; Wu, J.; Yu, H.; Hu, L.; Xiao, Y.; Lu, J.; Lou, Y. Jasmonic acid carboxyl methyltransferase regulates development and herbivory-induced defense response in rice. J. Integr. Plant Biol. 2016, 58, 564–576. [Google Scholar] [CrossRef]
- Guan, Y.R.; Xue, J.Q.; Xue, Y.Q.; Yang, R.W.; Wang, S.L.; Zhang, X. Effect of exogenous GA3 on flowering quality, endogenous hormones, and hormone-and flowering-associated gene expression in forcing-cultured tree peony (Paeonia suffruticosa). J. Integr. Agric. 2019, 18, 1295–1311. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, J.; Zeng, L.; Goh, M.; Leung, H.; Khush, G.S.; Wang, G.-L. Characterizing Rice Lesion Mimic Mutants and Identifying a Mutant with Broad-Spectrum Resistance to Rice Blast and Bacterial Blight. Mol. Plant-Microbe Interact. 2000, 13, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—Powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Imran, Q.M.; Yun, B.-W.; Lee, I.-J. Osmoprotective functions conferred to soybean plants via inoculation with Sphingomonas sp. LK11 and exogenous trehalose. Microbiol. Res. 2017, 205, 135–145. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.-J. Regulation of salicylic acid, jasmonic acid and fatty acids in cucumber (Cucumis sativus L.) by spermidine promotes plant growth against salt stress. Acta Physiol. Plant. 2013, 35, 3315–3322. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.-M.; Kim, K.-M.; Jan, R.; Lee, I.-J. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of Melatonin on Anti-oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.-J.; Bae, J.-S.; Kim, K.-M. Overexpression of OsCM alleviates BLB stress via phytohormonal accumulation and transcriptional modulation of defense-related genes in Oryza sativa. Sci. Rep. 2020, 10, 19520. [Google Scholar] [CrossRef]
- Lee, I.-J.; Foster, K.R.; Morgan, P.W. Photoperiod Control of Gibberellin Levels and Flowering in Sorghum. Plant Physiol. 1998, 116, 1003–1011. [Google Scholar] [CrossRef]



| Days | Control | 100 µM GA | 100 µM MeJA |
|---|---|---|---|
| 1 | 135 | 84 | 45 |
| 2 | 135 | 48 | 19 |
| 3 | 140 | 20 | 27 |
| 4 | 125 | 21 | 30 |
| 5 | 125 | 22 | 30 |
| 6 | 105 | 25 | 24 |
| 7 | 95 | 26 | 23 |
| No | Name | Stress | Treatment |
|---|---|---|---|
| 1 | Control | No | Only water |
| 2 | Control + WBPH | WBPH | Only water |
| 3 | 50 µM GA + WBPH | WBPH | 50 µM GA |
| 4 | 100 µM GA + WBPH | WBPH | 100 µM GA |
| 5 | 50 µM MeJA + WBPH | WBPH | 50 µM MeJA |
| 6 | 100 µM MeJA + WBPH | WBPH | 100 µM MeJA |
| S/No | Gene | Forward Primers | Reverse Primers | Accession No |
|---|---|---|---|---|
| 1 | OsActin | CTGCGGGTATCCATGAGACT | GGAGCAAGGCAGTGATCTTC | X16280.1 |
| 2 | OsRBBI3-3 | TCGTTCGTTCGATCATTCAG | TTTCCTCATGGTCCACACAA | AK243607 |
| 3 | OsPOX | CCAGAATTTCAGGGACAGGA | TGCTGTAGTAGGCGTTGTCG | AK073202 |
| 4 | OsGAI | CAGGTCATGTCCGAGGTGTA | CTCGCCTGTTTGTAGGCATT | AB030956 |
| 5 | OsGID2 | GGAGCGCAACCTTCTATCAG | AGACCTTGGACTCTGGAGCA | AK068248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, S.; Jang, Y.-H.; Kim, E.-G.; Jan, R.; Asaf, S.; Aaqil Khan, M.; Farooq, M.; Lubna; Kim, N.; Lee, I.-J.; et al. The Role of Exogenous Gibberellic Acid and Methyl Jasmonate against White-Backed Planthopper (Sogatella furcifera) Stress in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 14737. https://doi.org/10.3390/ijms232314737
Asif S, Jang Y-H, Kim E-G, Jan R, Asaf S, Aaqil Khan M, Farooq M, Lubna, Kim N, Lee I-J, et al. The Role of Exogenous Gibberellic Acid and Methyl Jasmonate against White-Backed Planthopper (Sogatella furcifera) Stress in Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2022; 23(23):14737. https://doi.org/10.3390/ijms232314737
Chicago/Turabian StyleAsif, Saleem, Yoon-Hee Jang, Eun-Gyeong Kim, Rahmatullah Jan, Sajjad Asaf, Muhammad Aaqil Khan, Muhammad Farooq, Lubna, Nari Kim, In-Jung Lee, and et al. 2022. "The Role of Exogenous Gibberellic Acid and Methyl Jasmonate against White-Backed Planthopper (Sogatella furcifera) Stress in Rice (Oryza sativa L.)" International Journal of Molecular Sciences 23, no. 23: 14737. https://doi.org/10.3390/ijms232314737







