Galectin-3 and Blood Group: Binding Properties, Effects on Plasma Levels, and Consequences for Prognostic Performance
Abstract
1. Introduction
2. Results
2.1. Study Population
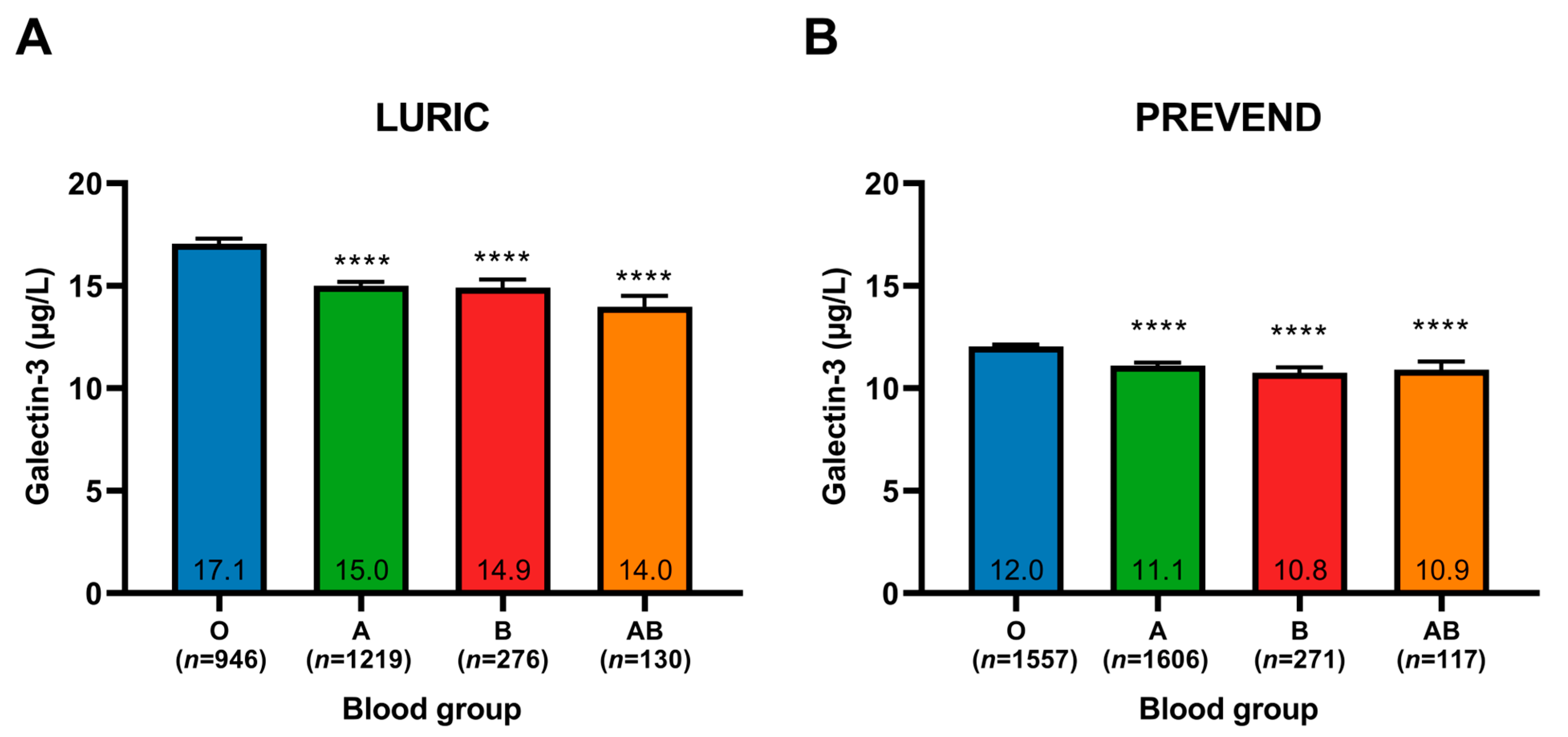
2.2. Galectin-3 Plasma Levels Stratified by Blood Group
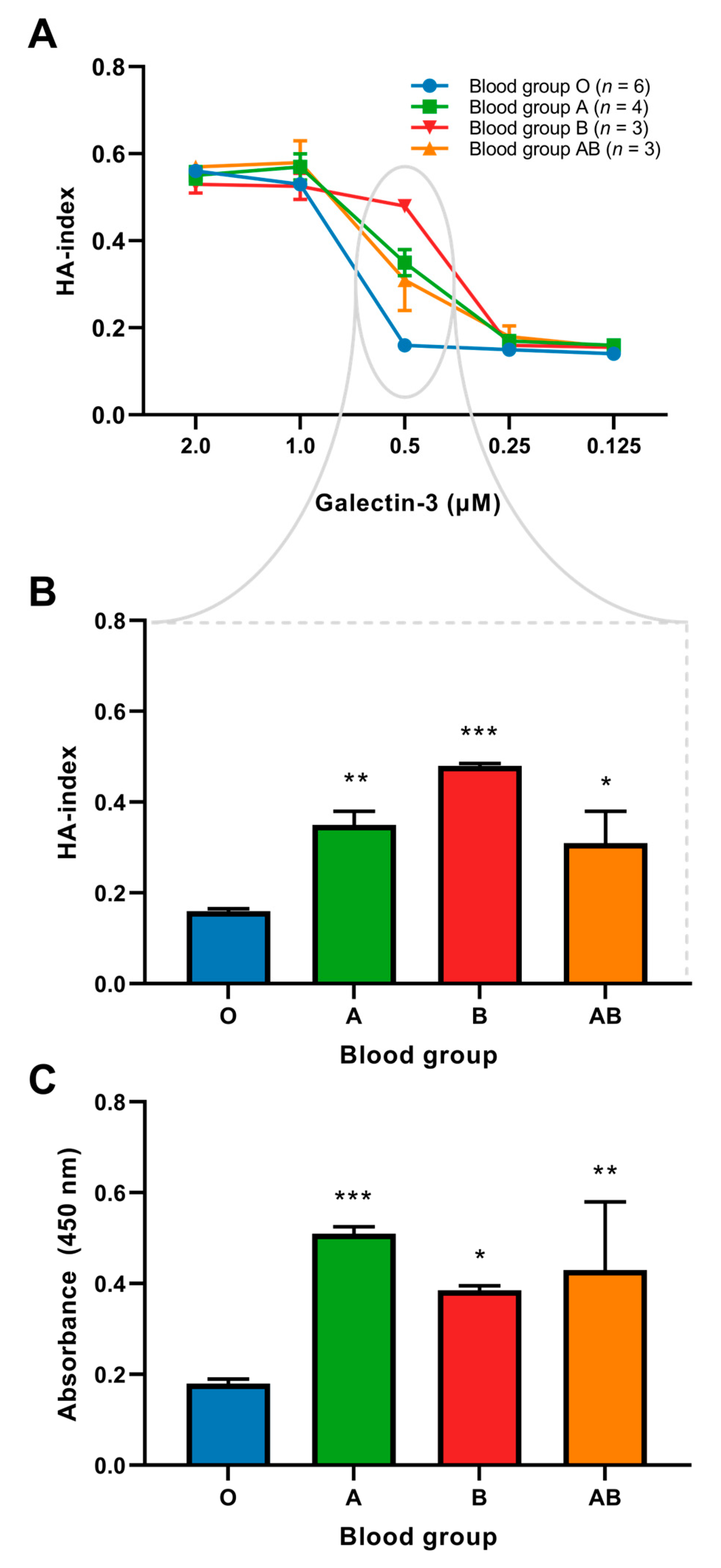
2.3. Binding of Galectin-3 and Red Blood Cells
2.4. Interaction between VWF and Galectin-3
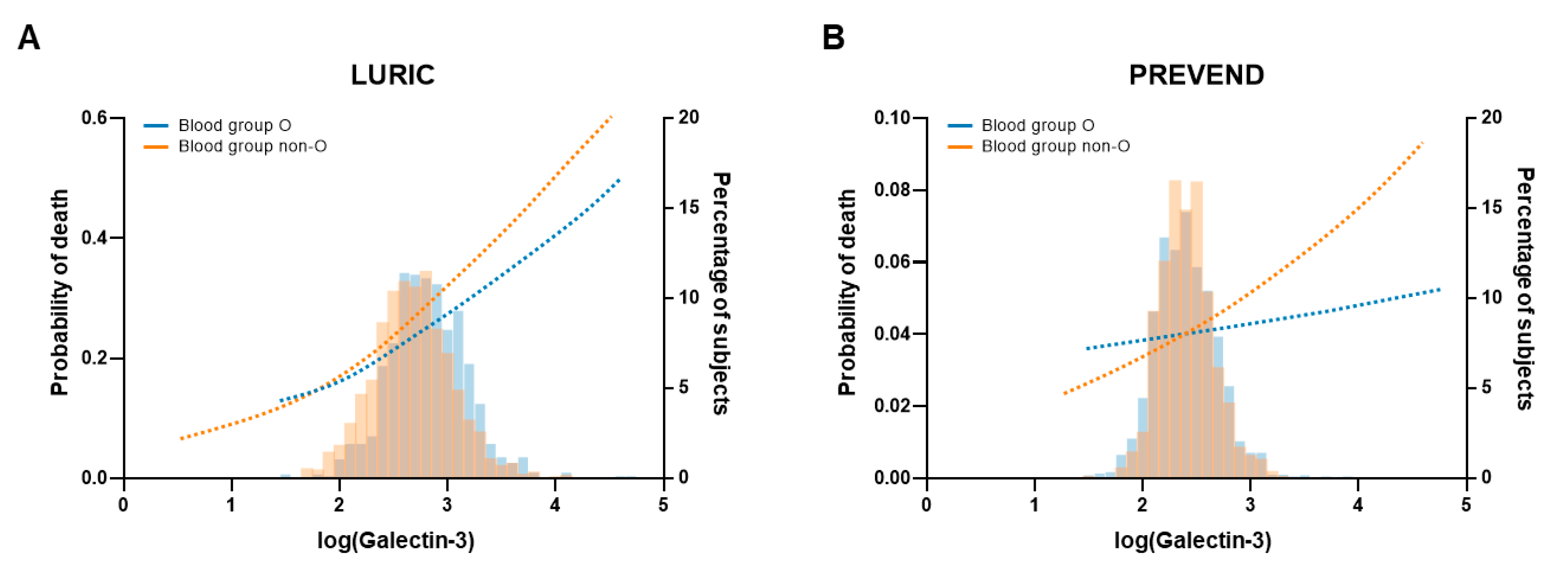
2.5. Prognostic Value of Galectin-3
3. Discussion
4. Materials and Methods
4.1. Study Population
4.1.1. LURIC
4.1.2. PREVEND
4.2. Galectin-3 Measurements
4.3. Blood Group Determination
4.4. Clinical Endpoints
4.5. In Vitro Studies
4.5.1. Isolation of Red Blood Cells
4.5.2. Hemagglutination Assay
4.5.3. Von Willebrand Factor ELISA
4.5.4. Galectin-3—von Willebrand Factor Binding Study
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 4 December 2022).
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef]
- Vasan, R.S.; Sullivan, L.M.; Wilson, P.W.; Sempos, C.T.; Sundström, J.; Kannel, W.B.; Levy, D.; D’Agostino, R.B. Relative importance of borderline and elevated levels of coronary heart disease risk factors. Ann. Intern. Med. 2005, 142, 393–402. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Groot, H.E.; Sierra, L.E.V.; Said, M.A.; Lipsic, E.; Karper, J.C.; van der Harst, P. Genetically determined ABO blood group and its associations with health and disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 830–838. [Google Scholar] [CrossRef]
- He, M.; Wolpin, B.; Rexrode, K.; Manson, J.E.; Rimm, E.; Hu, F.B.; Qi, L. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2314–2320. [Google Scholar] [CrossRef]
- Ketch, T.R.; Turner, S.J.; Sacrinty, M.T.; Lingle, K.C.; Applegate, R.J.; Kutcher, M.A.; Sane, D.C. ABO blood types: Influence on infarct size, procedural characteristics and prognosis. Thromb. Res. 2008, 123, 200–205. [Google Scholar] [CrossRef]
- Carpeggiani, C.; Coceani, M.; Landi, P.; Michelassi, C.; L’abbate, A. ABO blood group alleles: A risk factor for coronary artery disease. An angiographic study. Atherosclerosis 2010, 211, 461–466. [Google Scholar] [CrossRef]
- Franchini, M.; Favaloro, E.J.; Targher, G.; Lippi, G. ABO blood group, hypercoagulability, and cardiovascular and cancer risk. Crit. Rev. Clin. Lab. Sci. 2012, 49, 137–149. [Google Scholar] [CrossRef]
- Vischer, U.M. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J. Thromb. Haemost. 2006, 4, 1186–1193. [Google Scholar] [CrossRef]
- Spiel, A.O.; Gilbert, J.C.; Jilma, B. von Willebrand factor in cardiovascular disease: Focus on acute coronary syndromes. Circulation 2008, 117, 1449–1459. [Google Scholar] [CrossRef]
- Saint-Lu, N.; Oortwijn, B.D.; Pegon, J.N.; Odouard, S.; Christophe, O.D.; de Groot, P.G.; Denis, C.V.; Lenting, P.J. Identification of galectin-1 and galectin-3 as novel partners for von Willebrand factor. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 894–901. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Calvier, L.; Fernández-Celis, A.; Rousseau, E.; Jurado-López, R.; Rossoni, L.V.; Jaisser, F.; Zannad, F.; Rossignol, P.; Cachofeiro, V.; et al. Galectin-3 Blockade Inhibits Cardiac Inflammation and Fibrosis in Experimental Hyperaldosteronism and Hypertension. Hypertension 2015, 66, 767–775. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef]
- De Boer, R.A.; Voors, A.A.; Muntendam, P.; Van Gilst, W.H.; Van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; de Boer, R.A. Galectin-3 in Heart Failure: An Update of the Last 3 Years. Heart Fail. Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart failure stimulates tumor growth by circulating factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- De Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015, 13, 7. [Google Scholar] [CrossRef]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef]
- Gotsman, I.; Keren, A.; Zwas, D.R.; Lotan, C.; Admon, D. Clinical Impact of ABO and Rhesus D Blood Type Groups in Patients with Chronic Heart Failure. Am. J. Cardiol. 2018, 122, 413–419. [Google Scholar] [CrossRef]
- Franchini, M.; Capra, F.; Targher, G.; Montagnana, M.; Lippi, G. Relationship between ABO blood group and von Willebrand factor levels: From biology to clinical implications. Thromb. J. 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Chan, A.T.; Hartge, P.; Chanock, S.J.; Kraft, P.; Hunter, D.J.; Giovannucci, E.L.; Fuchs, C.S. ABO blood group and the risk of pancreatic cancer. J. Natl. Cancer Inst. 2009, 101, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Kamangar, F.; Islami, F.; Poustchi, H.; Pourshams, A.; Brennan, P.; Boffetta, P.; Malekzadeh, R.; Dawsey, S.M.; Abnet, C.C.; et al. Mortality and cancer in relation to ABO blood group phenotypes in the Golestan Cohort Study. BMC Med. 2015, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mengoli, C.; Bonfanti, C.; Rossi, C.; Lippi, G. Genetic determinants of extreme longevity: The role of ABO blood group. Thromb. Haemost. 2015, 115, 458–460. [Google Scholar] [PubMed]
- Chen, Z.; Yang, S.H.; Xu, H.; Li, J.J. ABO blood group system and the coronary artery disease: An updated systematic review and meta-analysis. Sci. Rep. 2016, 6, 23250. [Google Scholar] [CrossRef] [PubMed]
- Parente, E.B.; Harjutsalo, V.; Lehto, M.; Forsblom, C.; Sandholm, N.; Groop, P.H. Relationship between ABO blood groups and cardiovascular disease in type 1 diabetes according to diabetic nephropathy status. Cardiovasc. Diabetol. 2020, 19, 68. [Google Scholar] [CrossRef]
- Blais, C.; Germain, M.; Delage, G.; Grégoire, Y. The association between blood group and the risk of vascular disease in Quebec blood donors. Blood Transfus. 2016, 14, 455–459. [Google Scholar]
- Biswas, S.; Ghoshal, P.K.; Halder, B.; Mandal, N. Distribution of ABO blood group and major cardiovascular risk factors with coronary heart disease. Biomed Res. Int. 2013, 2013, 782941. [Google Scholar] [CrossRef]
- Yamamoto, F.; Clausen, H.; White, T.; Marken, J.; Hakomori, S. Molecular genetic basis of the histo-blood group ABO system. Nature 1990, 345, 229–233. [Google Scholar] [CrossRef]
- Lowe, J.B. The blood group-specific human glycosyltransferases. Baillieres. Clin. Haematol. 1993, 6, 465–492. [Google Scholar] [CrossRef]
- Franchini, M.; Liumbruno, G.M. ABO blood group: Old dogma, new perspectives. Clin. Chem. Lab. Med. 2013, 51, 1545–1553. [Google Scholar] [CrossRef]
- Ewald, D.R.; Sumner, S.C.J. Blood type biochemistry and human disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 517–535. [Google Scholar] [CrossRef]
- Reddy, V.M.; Daniel, M.; Bright, E.; Broad, S.R.; Moir, A.A. Is there an association between blood group O and epistaxis? J. Laryngol. Otol. 2008, 122, 366–368. [Google Scholar] [CrossRef]
- Zhang, H.; Mooney, C.J.; Reilly, M.P. ABO blood groups and cardiovascular diseases. Int. J. Vasc. Med. 2012, 2012, 641917. [Google Scholar] [CrossRef]
- Souto, J.C.; Almasy, L.; Muñiz-Diaz, E.; Soria, J.M.; Borrell, M.; Bayén, L.; Mateo, J.; Madoz, P.; Stone, W.; Blangero, J.; et al. Functional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial thromboplastin time. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2024–2028. [Google Scholar] [CrossRef]
- Matsui, T.; Titani, K.; Mizuochi, T. Structures of the asparagine-linked oligosaccharide chains of human von Willebrand factor. Occurrence of blood group A, B, and H(O) structures. J. Biol. Chem. 1992, 267, 8723–8731. [Google Scholar] [CrossRef]
- Sarode, R.; Goldstein, J.; Sussman, I.I.; Nagel, R.L.; Tsai, H.M. Role of A and B blood group antigens in the expression of adhesive activity of von Willebrand factor. Br. J. Haematol. 2000, 109, 857–864. [Google Scholar] [CrossRef]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef]
- Chen, A.; Hou, W.; Zhang, Y.; Chen, Y.; He, B. Prognostic value of serum galectin-3 in patients with heart failure: A meta-analysis. Int. J. Cardiol. 2015, 182, 168–170. [Google Scholar] [CrossRef]
- Meijers, W.C.; Januzzi, J.L.; Defilippi, C.; Adourian, A.S.; Shah, S.J.; van Veldhuisen, D.J.; de Boer, R.A. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am. Heart J. 2014, 167, 853–860.e4. [Google Scholar] [CrossRef]
- Meijers, W.C.; de Boer, R.A.; van Veldhuisen, D.J.; Jaarsma, T.; Hillege, H.L.; Maisel, A.S.; Di Somma, S.; Voors, A.A.; Peacock, W.F. Biomarkers and low risk in heart failure. Data from COACH and TRIUMPH. Eur. J. Heart Fail. 2015, 17, 1271–1282. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef]
- Feizi, T.; Solomon, J.C.; Yuen, C.T.; Jeng, K.C.; Frigeri, L.G.; Hsu, D.K.; Liu, F.T. The adhesive specificity of the soluble human lectin, IgE-binding protein, toward lipid-linked oligosaccharides. Presence of the blood group A, B, B-like, and H monosaccharides confers a binding activity to tetrasaccharide (lacto-N-tetraose and lacto-N-ne. Biochemistry 1994, 33, 6342–6349. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta. 2002, 1572, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Ho, A.D.; Kamili, N.A.; Wang, J.; Murdock, K.L.; Cummings, R.D.; Arthur, C.M.; Stowell, S.R. Full-length galectin-3 is required for high affinity microbial interactions and antimicrobial activity. Front. Microbiol. 2021, 12, 731026. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; Dias-Baruffi, M.; Rodrigues, L.C.; Gourdine, J.-P.; Heimburg-Molinaro, J.; Ju, T.; Molinaro, R.J.; Rivera-Marrero, C.; Xia, B.; et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010, 16, 295–301. [Google Scholar] [CrossRef]
- Paterson, A.D.; Lopes-Virella, M.F.; Waggott, D.; Boright, A.P.; Hosseini, S.M.; Carter, R.E.; Shen, E.; Mirea, L.; Bharaj, B.; Sun, L.; et al. Genome-Wide Association Identifies the ABO Blood Group as a Major Locus Associated with Serum Levels of Soluble E-Selectin. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1958. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Bermejo, B.; Pascual-Figal, D.A.; Hurtado-Martínez, J.A.; Montserrat-Coll, J.; Peñafiel-Verdú, P.; Pastor-Pérez, F.; Giner-Caro, J.A.; Valdés-Chávar, M. Clinical determinants and prognostic value of hemoglobin in hospitalized patients with systolic heart failure. Rev. Española Cardiol. 2007, 60, 597–606. [Google Scholar] [CrossRef]
- Meijers, W.C.; van der Velde, A.R.; Ruifrok, W.P.; Schroten, N.F.; Dokter, M.M.; Damman, K.; Assa, S.; Franssen, C.F.; Gansevoort, R.T.; van Gilst, W.H.; et al. Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis. J. Am. Heart Assoc. 2014, 3, e000962. [Google Scholar] [CrossRef]
- Mueller, C.; Laule-Kilian, K.; Scholer, A.; Nusbaumer, C.; Zeller, T.; Staub, D.; Perruchoud, A.P. B-type natriuretic peptide for acute dyspnea in patients with kidney disease: Insights from a randomized comparison. Kidney Int. 2005, 67, 278–284. [Google Scholar] [CrossRef]
- Das, S.R.; Drazner, M.H.; Dries, D.L.; Vega, G.L.; Stanek, H.G.; Abdullah, S.M.; Canham, R.M.; Chung, A.K.; Leonard, D.; Wians, F.H.; et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: Results from the Dallas Heart Study. Circulation 2005, 112, 2163–2168. [Google Scholar] [CrossRef]
- Winkelmann, B.R.; März, W.; Boehm, B.O.; Zotz, R.; Hager, J.; Hellstern, P.; Senges, J. Rationale and design of the LURIC study--a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics 2001, 2, S1–S73. [Google Scholar] [CrossRef]
- De Boer, R.A.; Schroten, N.F.; Bakker, S.J.L.; Mahmud, H.; Szymanski, M.K.; van der Harst, P.; Gansevoort, R.T.; van Veldhuisen, D.J.; van Gilst, W.H.; Hillege, H.L. Plasma renin and outcome in the community: Data from PREVEND. Eur. Heart J. 2012, 33, 2351–2359. [Google Scholar] [CrossRef]
- Diercks, G.F.; Janssen, W.M.; van Boven, A.J.; Bak, A.A.; de Jong, P.E.; Crijns, H.J.; van Gilst, W. Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular E. Am. J. Cardiol. 2000, 86, 635–638. [Google Scholar] [CrossRef]
- Diercks, G.F.H.; van Boven, A.; Hillege, H.; Janssen, W.; Kors, J.; De Jong, P.; Grobbee, D.; Crijns, H.; van Gilst, W. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur. Heart J. 2000, 21, 1922–1927. [Google Scholar] [CrossRef]
- Meijers, W.C.; Van Der Velde, A.R.; De Boer, R.A. The ARCHITECT galectin-3 assay: Comparison with other automated and manual assays for the measurement of circulating galectin-3 levels in heart failure. Expert Rev. Mol. Diagn. 2014, 14, 257–266. [Google Scholar] [CrossRef]
- Christenson, R.H.; Duh, S.-H.; Wu, A.H.; Smith, A.; Abel, G.; Defilippi, C.R.; Wang, S.; Adourian, A.; Adiletto, C.; Gardiner, P. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin. Biochem. 2010, 43, 683–690. [Google Scholar] [CrossRef]
- Bedu-Addo, G.; Gai, P.P.; Meese, S.; Eggelte, T.A.; Thangaraj, K.; Mockenhaupt, F.P. Reduced prevalence of placental malaria in primiparae with blood group O. Malar. J. 2014, 13, 289. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Ritz, E.; Boehm, B.O.; Grammer, T.B.; März, W. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur. Heart J. 2010, 31, 1591–1598. [Google Scholar] [CrossRef]
| Clinical Characteristics | Total | Blood Group O | Blood Group A | Blood Group B | Blood Group AB | p-Value |
|---|---|---|---|---|---|---|
| (n = 2571) | (n = 946) | (n = 1219) | (n = 276) | (n = 130) | ||
| Age (y), mean (SD) | 63 (10) | 63 (10) | 63 (11) | 63 (11) | 62 (11) | 0.68 |
| Male sex, n (%) | 1756 (68) | 642 (68) | 842 (69) | 187 (68) | 85 (65) | 0.81 |
| Current smoker, n (%) | 495 (19) | 201 (21) | 211 (17) | 57 (21) | 26 (20) | 0.12 |
| BMI (kg/m2), mean (SD) | 278 (4) | 28 (4) | 27 (4) | 27 (4) | 27 (3) | 0.08 |
| Heart rate (bpm), median [IQR] | 68 [61–75] | 68 [61–75] | 68 [61–76] | 66 [60–74] | 66 [60–76] | 0.40 |
| Systolic blood pressure, median [IQR] | 140 [123–157] | 142 [123–158] | 140 [124–156] | 140 [123–158] | 136 [121–152] | 0.30 |
| Diastolic blood pressure, median [IQR] | 81 [73–88] | 81 [73–89] | 80 [73–88] | 81 [73–90] | 80 [72–89] | 0.89 |
| Medical history, n (%) | ||||||
| Type 2 diabetes mellitus | 1032 (40) | 372 (39) | 507 (42) | 104 (38) | 49 (38) | 0.50 |
| (History of) hypertension | 1865 (73) | 691 (73) | 880 (72) | 200 (72) | 91 (70) | 0.90 |
| (History of) coronary artery disease | 1986 (77) | 720 (76) | 949 (78) | 216 (78) | 96 (74) | 0.59 |
| Laboratory measurements | ||||||
| eGFR (MDRD), median [IQR] | 81 [69–92] | 80 [68–91] | 81 [70–93] | 80 [70–92] | 81 [70–92] | 0.11 |
| Glucose (mmol/L), median [IQR] | 5.7 [5.2–6.6] | 5.7 [5.2–6.6] | 5.7 [5.2–6.6] | 5.7 [5.2–6.6] | 5.6 [5.2–6.3] | 0.62 |
| Cholesterol (mmol/L), median [IQR] | 4.9 [4.3–5.6] | 4.9 [4.2–5.5] | 4.9 [4.3–5.7] | 4.9 [4.4–5.6] | 4.8 [4.2–5.4] | 0.25 |
| LDL (mmol/L), median [IQR] | 2.9 [2.4–3.6] | 2.9 [2.4–3.5] | 3.0 [2.4–3.6] | 3.0 [2.5–3.6] | 2.9 [2.3–3.5] | 0.16 |
| HDL (mmol/L), median [IQR] | 1.0 [0.8–1.2] | 1.0 [0.8–1.2] | 1.0 [0.8–1.2] | 1.0 [0.8–1.2] | 1.0 [0.8–1.2] | 0.39 |
| Triglycerides (mmol/L), median [IQR] | 3.8 [2.8–5.2] | 3.9 [2.8–5.3] | 3.7 [2.7–5.1] | 3.7 [2.8–5.2] | 3.6 [2.6–5.0] | 0.28 |
| NT-proBNP (ng/L), median [IQR] | 296 [108–884] | 286 [104–895] | 299 [109–907] | 345 [112–873] | 292 [104–733] | 0.70 |
| Galectin-3 (µg/L), mean (SD) | 15.7 (7.0) | 17.1 (7.4) | 15.0 (6.7) | 14.9 (6.6) | 14.0 (6.1) | <0.001 |
| VWF (U/dL), median [IQR] | 156 [120–198] | 132 [100–176] | 165 [130–206] | 176 [136–214] | 189 [145–228] | <0.001 |
| LURIC Study | Blood Group O | p-Value | Blood Group Non-O | p-Value | p-Value for Interaction | |
|---|---|---|---|---|---|---|
| All-cause mortality | HR [95% CI] | HR [95% CI] | ||||
| Galectin-3 | 2.73 [2.03–3.67] | <0.001 | 3.06 [2.45–3.83] | <0.001 | 0.53 | |
| Galectin-3 | 2.31 [1.65–3.24] | <0.001 | 2.20 [1.73–2.80] | <0.001 | 0.78 | |
| +sex & age | ||||||
| Galectin-3 | 1.89 [1.28–2.79] | 0.001 | 2.19 [1.67–2.86] | <0.001 | 0.89 | |
| +fully adjusted * | ||||||
| PREVEND Study | Blood Group O | p-Value | Blood Group Non-O | p-Value | p-Value for Interaction | |
| All-cause mortality | HR [95% CI] | HR [95% CI] | ||||
| Galectin-3 | 2.48 [1.70–3.63] | <0.0001 | 3.44 [2.45–4.84] | <0.0001 | 0.20 | |
| Galectin-3 | 1.31 [0.74–2.32] | 0.35 | 1.87 [1.16–3.03] | 0.010 | 0.28 | |
| +sex & age | ||||||
| Galectin-3 | 1.05 [0.53–2.09] | 0.87 | 1.44 [0.83–2.48] | 0.20 | 0.82 | |
| +fully adjusted * | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozder, C.; Screever, E.M.; van der Velde, A.R.; Silljé, H.H.; Suwijn, J.; de Rond, S.; Kleber, M.E.; Delgado, G.; Schuringa, J.J.; van Gilst, W.H.; et al. Galectin-3 and Blood Group: Binding Properties, Effects on Plasma Levels, and Consequences for Prognostic Performance. Int. J. Mol. Sci. 2023, 24, 4415. https://doi.org/10.3390/ijms24054415
Pozder C, Screever EM, van der Velde AR, Silljé HH, Suwijn J, de Rond S, Kleber ME, Delgado G, Schuringa JJ, van Gilst WH, et al. Galectin-3 and Blood Group: Binding Properties, Effects on Plasma Levels, and Consequences for Prognostic Performance. International Journal of Molecular Sciences. 2023; 24(5):4415. https://doi.org/10.3390/ijms24054415
Chicago/Turabian StylePozder, Carolin, Elles M. Screever, A. Rogier van der Velde, Herman H. Silljé, Janne Suwijn, Saskia de Rond, Marcus E. Kleber, Graciela Delgado, Jan Jacob Schuringa, Wiek H. van Gilst, and et al. 2023. "Galectin-3 and Blood Group: Binding Properties, Effects on Plasma Levels, and Consequences for Prognostic Performance" International Journal of Molecular Sciences 24, no. 5: 4415. https://doi.org/10.3390/ijms24054415
APA StylePozder, C., Screever, E. M., van der Velde, A. R., Silljé, H. H., Suwijn, J., de Rond, S., Kleber, M. E., Delgado, G., Schuringa, J. J., van Gilst, W. H., Meijers, W. C., März, W., & de Boer, R. A. (2023). Galectin-3 and Blood Group: Binding Properties, Effects on Plasma Levels, and Consequences for Prognostic Performance. International Journal of Molecular Sciences, 24(5), 4415. https://doi.org/10.3390/ijms24054415








