Efficient Production of Fc Fusion Proteins in the Cytoplasm of Escherichia coli: Dissecting and Mitigating Redox Heterogeneity
Abstract
:1. Introduction
2. Results and Discussion
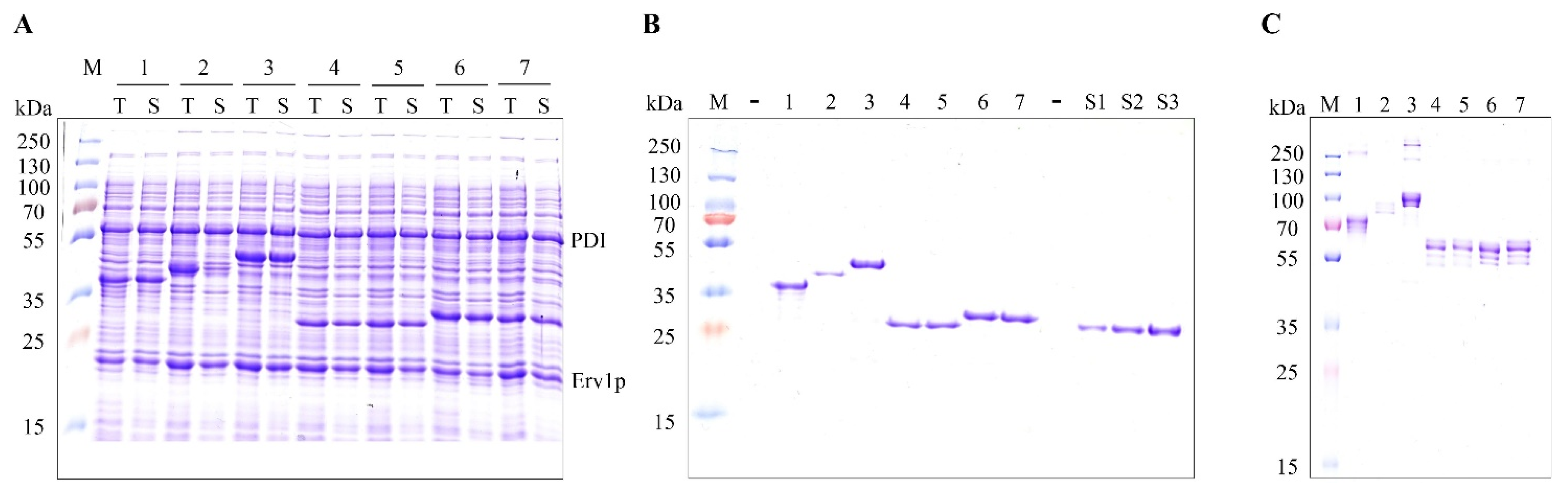
2.1. Soluble Production of Fc Fusion Proteins in the E. coli Cytoplasm
2.2. Identifying the Cause of Redox Heterogeneity in Fc Fusion Proteins
2.3. Mitigating the Cause of Redox Heterogeneity in Fc Fusion Proteins
2.4. Effect of Mutations on Protein Stability
3. Materials and Methods
3.1. Cloning
3.2. Protein Expression
3.3. Protein Purification
3.3.1. Protein G-Based Purification
3.3.2. Cobalt–IMAC-Based Purification
3.3.3. Purification of Wild–Type IgG1 Fc Region
3.4. Protein Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DWP | deep well plate |
| NEM | N-Ethyl maleimide |
| MalPEG | Methoxypolyethylene glycol maleimide |
| IgG | immunoglobulin G |
| Fc | crystallizable fragment |
| FcRn | Neonatal Fc receptor |
| IMAC | immobilized metal affinity chromatography |
| EDTA | Ethylenediaminetetraacetic acid |
References
- Rettenbacher, L.A.; Arauzo-Aguilera, K.; Buscajoni, L.; Castillo-Corujo, A.; Ferrero-Bordera, B.; Kostopoulou, A.; Moran-Torres, R.; Núñez-Nepomuceno, D.; Öktem, A.; Palma, A.; et al. Microbial protein cell factories fight back? Trends Biotechnol. 2021, 40, 576–590. [Google Scholar] [CrossRef]
- Choi, J.H.; Keum, K.C.; Lee, S.Y. Production of recombinant proteins by high cell density culture of Escherichia coli. Chem. Eng. Sci. 2006, 61, 876–885. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Hatahet, F.; Salo, K.E.; Enlund, E.; Zhang, C.; Ruddock, L.W. Pre-expression of a sulfhydryl oxidase significantly increases the yields of eukaryotic disulfide bond containing proteins expressed in the cytoplasm of E.coli. Microb. Cell Factories 2011, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Gaciarz, A.; Veijola, J.; Uchida, Y.; Saaranen, M.J.; Wang, C.; Hörkkö, S.; Ruddock, L.W. Systematic screening of soluble expression of antibody fragments in the cytoplasm of E. coli. Microb. Cell Factories 2016, 15, 22. [Google Scholar] [CrossRef] [Green Version]
- Gąciarz, A.; Khatri, N.K.; Velez-Suberbie, M.L.; Saaranen, M.J.; Uchida, Y.; Keshavarz-Moore, E.; Ruddock, L.W. Efficient soluble expression of disulfide-bonded proteins in the cytoplasm of Escherichia coli in fed-batch fermentation on chemically defined minimal media. Microb. Cell Factories 2017, 16, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamoto, G.; Gegg, C.; Boone, T.; Queva, C. Peptibodies: A flexible alternative format to antibodies. mAbs 2012, 4, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Cavaco, M.; Castanho, M.; Neves, V. Peptibodies: An elegant solution to a long-standing problem. Pept. Sci. 2018, 110, e2309. [Google Scholar] [CrossRef] [Green Version]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Sun, Y.N. Pharmacokinetics of Peptide–Fc Fusion Proteins. J. Pharm. Sci. 2013, 103, 53–64. [Google Scholar] [CrossRef]
- Chamow, S.M.; Ryll, T.; Lowman, H.B.; Farson, D. Therapeutic Fc-Fusion Proteins; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Saaranen, M.J.; Ruddock, L.W. Applications of catalyzed cytoplasmic disulfide bond formation. Biochem. Soc. Trans. 2019, 47, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.A.; Gaikwad, M.; Khadka, P.; Saaranen, M.J.; Ruddock, L.W. Production of Extracellular Matrix Proteins in the Cytoplasm of E. coli: Making Giants in Tiny Factories. Int. J. Mol. Sci. 2020, 21, 688. [Google Scholar] [CrossRef] [Green Version]
- Posfai, G.; Plunkett, I.I.I.; Fehér, T.; Frisch, D.; Keil, G.M.; Umenhoffer, K.; Kolisnychenko, V.; Stahl, B.; Sharma, S.S.; de Arruda, M.; et al. Emergent properties of reduced-genome Escherichia coli. Science 2006, 312, 1044–1046. [Google Scholar] [CrossRef] [Green Version]
- Maas, C.; Hermeling, S.; Bouma, B.; Jiskoot, W.; Gebbink, M.F. A role for protein misfolding in immunogenicity of biopharmaceuticals. J. Biol. Chem. 2007, 282, 2229–2236. [Google Scholar] [CrossRef] [Green Version]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thies, M.J.; Talamo, F.; Mayer, M.; Bell, S.; Ruoppolo, M.; Marino, G.; Buchner, J. Folding and oxidation of the antibody domain CH3. J. Mol. Biol. 2002, 319, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Makmura, L.; Hamann, M.; Areopagita, A.; Furuta, S.; Muñoz, A.; Momand, J. Development of a sensitive assay to detect reversibly oxidized protein cysteine sulfhydryl groups. Antioxid. Redox Signal. 2001, 3, 1105–1118. [Google Scholar] [CrossRef]
- Gevondyan, N.M.; Volynskaia, A.M.; Gevondyan, V.S. Four free cysteine residues found in human IgG1 of healthy donors. Biochemistry 2006, 71, 279–284. [Google Scholar] [CrossRef]
- Huh, J.H.; White, A.J.; Brych, S.R.; Franey, H.; Matsumura, M. The identification of free cysteine residues within antibodies and a potential role for free cysteine residues in covalent aggregation because of agitation stress. J. Pharm. Sci. 2013, 102, 1701–1711. [Google Scholar] [CrossRef]
- Schauenstein, E.; Sorger, S.; Reiter, M.; Dachs, F. Free thiol groups and labile disulfide bonds in the IgG fraction of human serum. J. Immunol. Methods 1982, 50, 51–56. [Google Scholar] [CrossRef]
- Xiang, T.; Chumsae, C.; Liu, H. Localization and quantitation of free sulfhydryl in recombinant monoclonal antibodies by differential labeling with 12C and 13C iodoacetic acid and LC-MS analysis. Anal. Chem. 2009, 81, 8101–8108. [Google Scholar] [CrossRef] [PubMed]
- Akazawa-Ogawa, Y.; Nagai, H.; Hagihara, Y. Heat denaturation of the antibody, a multi-domain protein. Biophys. Rev. 2018, 10, 255–258. [Google Scholar] [CrossRef] [PubMed]




| Fc Fusion Proteins (Wild Type) | Purified Yields (mg/L) |
|---|---|
| Trebananib | 178 ± 7 |
| Leptin–Fc | 61 ± 4 |
| hGH–Fc | 196 ± 2 |
| Angiotensin–Fc | 148 ± 5 |
| Substance P–Fc | 138 ± 8 |
| Gastrin–Fc | 142 ± 4 |
| Katacalcin–Fc | 131 ± 1 |
| Fc Fusion Proteins (Mutants) | Purified Yields (mg/L) |
|---|---|
| Trebananib | 117 ± 4 |
| Leptin–Fc | 37 ± 4 |
| hGH–Fc | 216 ± 2 |
| Angiotensin–Fc | 157 ± 4 |
| Substance P–Fc | 221 ± 5 |
| Gastrin–Fc | 105 ± 5 |
| Katacalcin–Fc | 230 ± 4 |
| Protein of Interest (POI) | Melting Temperatures (Tm) (°C) |
|---|---|
| IgG1 CH3 domain | |
| Wild type (+CyDisCo) | 72.1 ± 0.1 |
| Wild type (-CyDisCo) | 71.6 ± 0.1 |
| Mutant (C250A, C308A) | 71.5 ± 0.1 |
| IgG1 Fc region | |
| Wild type | 59.9 ± 0.1; 73.7 ± 0.1 |
| Mutant (C250A, C308A) | 59.6 ± 1.1; 72.9 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungekar, A.A.; Ruddock, L.W. Efficient Production of Fc Fusion Proteins in the Cytoplasm of Escherichia coli: Dissecting and Mitigating Redox Heterogeneity. Int. J. Mol. Sci. 2022, 23, 14740. https://doi.org/10.3390/ijms232314740
Tungekar AA, Ruddock LW. Efficient Production of Fc Fusion Proteins in the Cytoplasm of Escherichia coli: Dissecting and Mitigating Redox Heterogeneity. International Journal of Molecular Sciences. 2022; 23(23):14740. https://doi.org/10.3390/ijms232314740
Chicago/Turabian StyleTungekar, Aatir A., and Lloyd W. Ruddock. 2022. "Efficient Production of Fc Fusion Proteins in the Cytoplasm of Escherichia coli: Dissecting and Mitigating Redox Heterogeneity" International Journal of Molecular Sciences 23, no. 23: 14740. https://doi.org/10.3390/ijms232314740
APA StyleTungekar, A. A., & Ruddock, L. W. (2022). Efficient Production of Fc Fusion Proteins in the Cytoplasm of Escherichia coli: Dissecting and Mitigating Redox Heterogeneity. International Journal of Molecular Sciences, 23(23), 14740. https://doi.org/10.3390/ijms232314740






