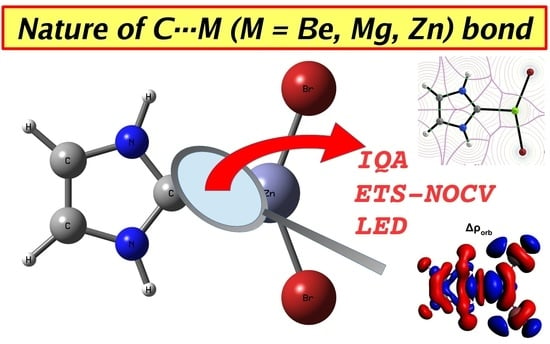
Nature of Beryllium, Magnesium, and Zinc Bonds in Carbene⋯MX2 (M = Be, Mg, Zn; X = H, Br) Dimers Revealed by the IQA, ETS-NOCV and LED Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characteristics of the Considered Dimers
2.2. IQA-Based Results
2.3. ETS-NOCV-Based Results
2.4. LED-Based Results
2.5. Flat vs. Perpendicular Structure of the Carben⋯MX Dimer
3. Methods and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QTAIM | Quantum Theory of Atoms in Molecules |
| IQA | the Interacting Quantum Atoms approach |
| ETS | the Extended Transition State method |
| NOCV | the Natural Orbitals for Chemical Valence method |
| DLPNO | Domain-based Localized Pair-Natural Orbital |
| CCSD(T) | the coupled cluster (CC) single-double-triple method |
| LED | the Local Energy Decomposition scheme |
References
- Kirmse, W. Carbene Chemistry; Academic Press: Cambridge, MA, USA, 1964. [Google Scholar]
- Hubert, A.J. Catalysis in C1 Chemistry; Springer: Dordrecht, The Netherlands, 1983. [Google Scholar]
- Schubert, U. Advances in Metal Carbene Chemistry; Springer: Dordrecht, The Netherlands, 1989. [Google Scholar]
- Herrmann, W.A.; Köcher, C. N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 2162–2187. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Bertrande, G. Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents; FontisMedia S.A.: Lausanne, Switzerland; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Moss, R.A.; Platz, M.S.; Jones, M., Jr. (Eds.) Reactive Intermediate Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Scott, N.M.; Nolan, S.P. Stabilization of Organometallic Species Achieved by the Use of N-Heterocyclic Carbene (NHC) Ligands. Eur. J. Inorg. Chem. 2005, 2005, 1815–1828. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes in Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Carey, F.A.; Sundberg, R.J. Carbenes, Part B: Reactions and Synthesis. Advanced Organic Chemistry; Springer: New York, NY, USA, 2007. [Google Scholar]
- Díez-González, S.; Nolan, S.P. Stereoelectronic parameters associated with N-heterocyclic carbene (NHC) ligands: A quest for understanding. Coord. Chem. Rev. 2007, 251, 874–883. [Google Scholar] [CrossRef]
- Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M–(NHC) (NHC = N-heterocyclic carbene) bond. Coord. Chem. Rev. 2009, 253, 687–703. [Google Scholar] [CrossRef]
- de Frémont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Moss, R.A.; Doyle, M.P. Contemporary Carbene Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Dagorne, S. Group 1 and 2 and Early Transition Metal Complexes Bearing N-Heterocyclic Carbene Ligands: Coordination Chemistry, Reactivity, and Applications. Chem. Rev. 2014, 114, 8747–8774. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Nesterov, V.; Reiter, D.; Bag, P.; Frisch, P.; Holzner, R.; Porzelt, A.; Inoue, S. NHCs in Main Group Chemistry. Chem. Rev. 2018, 118, 9678–9842. [Google Scholar] [CrossRef]
- Pople, J.A.; Raghavachari, K.; Frisch, M.J.; Binkley, J.S.; Schleyer, P.v.R. Comprehensive Theoretical Study of Isomers and Rearrangement Barriers of Even Electron Polyatomic Molecules HmABHn (A, B = C, N, O, and F). J. Am. Chem. Soc. 1983, 105, 6389–6398. [Google Scholar] [CrossRef]
- Pople, J.A. A theoretical search for the methylenefluoronium ylide. Chem. Phys. Lett. 1986, 132, 144–146. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Gamper, S.F.; Tamm, M.; Calabrese, J.C.; Davidson, F.; Craig, H.A. A Bis(carbene)–Proton Complex: Structure of a C–H–C Hydrogen Bond. J. Am. Chem. Soc. 1995, 117, 572–573. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. Carbenes and Silylenes as Hydrogen Bond Acceptors. J. Phys. Chem. 1996, 100, 19367–19370. [Google Scholar] [CrossRef]
- Jabłoński, M.; Palusiak, M. Divalent carbon atom as the proton acceptor in hydrogen bonding. Phys. Chem. Chem. Phys. 2009, 11, 5711–5719. [Google Scholar] [CrossRef]
- Giffin, N.A.; Makramalla, M.; Hendsbee, A.D.; Robertson, K.N.; Sherren, C.; Pye, C.C.; Masuda, J.D.; Clyburne, J.A.C. Anhydrous TEMPO-H: Reactions of a good hydrogen atom donor with low-valent carbon centres. Org. Biomol. Chem. 2011, 9, 3672–3680. [Google Scholar] [CrossRef] [Green Version]
- Gerbig, D.; Ley, D. Computational methods for contemporary carbene chemistry. WIREs Comput. Mol. Sci. 2013, 3, 242–272. [Google Scholar] [CrossRef]
- Samanta, R.C.; De Sarkar, S.; Fröhlich, R.; Grimme, S.; Studer, A. N-Heterocyclic carbene (NHC) catalyzed chemoselective acylation of alcohols in the presence of amines with various acylating reagents. Chem. Sci. 2013, 4, 2177–2184. [Google Scholar] [CrossRef]
- Jabłoński, M. Coexistence of the Carbene⋯H-D Hydrogen Bond and Other Accompanying Interactions in Forty Dimers of N-Heterocyclic-Carbenes (I, IMe2, IiPr2, ItBu2, IMes2, IDipp2, IAd2; I = imidazol-2-ylidene) and Some Fundamental Proton Donors (HF, HCN, H2O, MeOH, NH3). Molecules 2022, 27, 5712. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Liu, Z.; Li, W.; Cheng, J.; Gong, B.; Sun, J. Ab Initio Study of Lithium-Bonded Complexes with Carbene as an Electron Donor. J. Phys. Chem. A 2009, 113, 14156–14160. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Abraham, M.Y.; Wei, P.; Schaefer, H.F., III; Schleyer, P.v.R.; Robinson, G.H. A Viable Anionic N-Heterocyclic Dicarbene. J. Am. Chem. Soc. 2010, 132, 14370–14372. [Google Scholar] [CrossRef] [PubMed]
- Zhi-Feng, L.; Sheng, Y.; Hui-Xue, L. Theoretical prediction characters of unconventional weak bond with carbene as electron donors and Li–Y (Y = OH, H, F, NC and CN) as electron acceptors. J. Mol. Struct. THEOCHEM 2010, 952, 56–60. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Runte, O.; Artus, G. Synthesis and structure of an ionic beryllium—“Carbene” complex. J. Organomet. Chem. 1995, 501, C1–C4. [Google Scholar] [CrossRef]
- Gilliard, R.J., Jr.; Abraham, M.Y.; Wang, Y.; Wei, P.; Xie, Y.; Quillian, B.; Schaefer, H.F., III; Schleyer, P.v.R.; Robinson, G.H. Carbene-Stabilized Beryllium Borohydride. J. Am. Chem. Soc. 2012, 134, 9953–9955. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, M.; Hill, M.S.; Kociok-Köhn, G.; MacDougall, D.J.; Mahon, M.F. Beryllium-Induced C–N Bond Activation and Ring Opening of an N-Heterocyclic Carbene. Angew. Chem. Int. Ed. 2012, 51, 2098–2100. [Google Scholar] [CrossRef]
- Walley, J.E.; Wong, Y.-O.; Freeman, L.A.; Dickie, D.A.; Gilliard, R.J., Jr. N-Heterocyclic Carbene-Supported Aryl- and Alk- oxides of Beryllium and Magnesium. Catalysts 2019, 9, 934. [Google Scholar] [CrossRef] [Green Version]
- Jabłoński, M. Study of Beryllium, Magnesium, and Spodium Bonds to Carbenes and Carbodiphosphoranes. Molecules 2021, 26, 2275. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Dias, H.V.R.; Davidson, F.; Harlow, R.L. Carbene adducts of magnesium and zinc. J. Organomet. Chem. 1993, 462, 13–18. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Hill, M.S.; MacDougall, D.J.; Mahon, M.F. A Hydride-Rich Magnesium Cluster. Angew. Chem. Int. Ed. 2009, 48, 4013–4016. [Google Scholar] [CrossRef]
- Arnold, P.L.; Casely, I.J.; Turner, Z.R.; Bellabarba, R.; Tooze, R.B. Magnesium and zinc complexes of functionalised, saturated N-heterocyclic carbene ligands: Carbene lability and functionalisation, and lactide polymerisation catalysis. Dalton Trans. 2009, 7236–7247. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Dias, H.V.R.; Calabrese, J.C.; Davidson, F. A Stable Carbene-Alane Adduct. J. Am. Chem. Soc. 1992, 114, 9724–9725. [Google Scholar] [CrossRef]
- Li, X.-W.; Su, J.; Robinson, G.H. Syntheses and molecular structure of organo-group 13 metal carbene complexes. Chem. Commun. 1996, 23, 2683–2684. [Google Scholar] [CrossRef]
- Hibbs, D.E.; Hursthouse, M.B.; Jones, C.; Smithies, N.A. Synthesis, crystal and molecular structure of the first indium trihydride complex, [InH3{CN(Pri)C2Me2N(Pri)}]. Chem. Commun. 1998, 8, 869–870. [Google Scholar] [CrossRef]
- Merceron, N.; Miqueu, K.; Baceiredo, A.; Bertrand, G. Stable (Amino)(phosphino)carbenes: Difunctional Molecules. J. Am. Chem. Soc. 2002, 124, 6806–6807. [Google Scholar] [CrossRef]
- Wang, Y.; Robinson, G.H. Unique homonuclear multiple bonding in main group compounds. Chem. Commun. 2009, 5201–5213. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Alkorta, I.; Elguero, J. Carbon–Carbon Bonding between Nitrogen Heterocyclic Carbenes and CO2. J. Phys. Chem. A 2017, 121, 8136–8146. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Li, W.; Cheng, J. Carbene tetrel-bonded complexes. Struct. Chem. 2017, 28, 823–831. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Abraham, M.Y.; Gilliard, R.J., Jr.; Wei, P.; Schaefer, H.F., III; Schleyer, P.v.R.; Robinson, G.H. Carbene-Stabilized Parent Phosphinidene. Organometallics 2010, 29, 4778–4780. [Google Scholar] [CrossRef]
- Abraham, M.Y.; Wang, Y.; Xie, Y.; Wei, P.; Schaefer, H.F., III; Schleyer, P.v.R.; Robinson, G.H. Carbene Stabilization of Diarsenic: From Hypervalency to Allotropy. Chem. Eur. J. 2010, 16, 432–435. [Google Scholar] [CrossRef]
- Patel, D.S.; Bharatam, P.V. Divalent N(I) Compounds with Two Lone Pairs on Nitrogen. J. Phys. Chem. A 2011, 115, 7645–7655. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, D.; Sun, Y.; Hao, J.; Cai, Z. Theoretical Investigations on the Weak Nonbonded C=S⋯CH2 Interactions: Chalcogen-Bonded Complexes With Singlet Carbene as an Electron Donor. Int. J. Quant. Chem. 2011, 111, 3881–3887. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Kline, M.; Calabrese, J.C.; Davidson, F. Synthesis of a Reverse Ylide from a Nucleophilic Carbene. J. Am. Chem. Soc. 1991, 113, 9704–9705. [Google Scholar] [CrossRef]
- Kuhn, N.; Kratz, T.; Henkel, G. A Stable Carbene Iodine Adduct: Secondary Bonding in 1,3-Diethyl-2-iodo-4,5-dimethylimidazolium Iodide. J. Chem. Soc. Chem. Commun. 1993, 1778–1779. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Liu, Z.; Li, W.; Cheng, J.; Gong, B.; Sun, J. An unconventional halogen bond with carbene as an electron donor: An ab initio study. Chem. Phys. Lett. 2009, 469, 48–51. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadirad, N. Insights into the strength and nature of carbene⋯halogen bond interactions: A theoretical perspective. J. Mol. Model. 2013, 19, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Sabouri, A. Carbene–aerogen bonds: An Ab Initio Study. Mol. Phys. 2017, 115, 971–980. [Google Scholar] [CrossRef]
- Cases, M.; Frenking, G.; Duran, M.; Solà, M. Molecular Structure and Bond Characterization of the Fischer-Type Chromium–Carbene Complexes (CO)5Cr=C(X)R (X = H, OH, OCH3, NH2, NHCH3 and R = H, CH3, CH=CH2, Ph, C≡CH). Organometallics 2002, 21, 4182–4191. [Google Scholar] [CrossRef]
- Nemcsok, D.; Wichmann, K.; Frenking, G. The Significance of π Interactions in Group 11 Complexes with N-Heterocyclic Carbenes. Organometallics 2004, 23, 3640–3646. [Google Scholar] [CrossRef]
- Frenking, G.; Solà, M.; Vyboishchikov, S.F. Chemical bonding in transition metal carbene complexes. J. Organomet. Chem. 2005, 690, 6178–6204. [Google Scholar] [CrossRef]
- Tonner, R.; Heydenrych, G.; Frenking, G. Bonding Analysis of N-Heterocyclic Carbene Tautomers and Phosphine Ligands in Transition-Metal Complexes: A Theoretical Study. Chem. Asian J. 2007, 2, 1555–1567. [Google Scholar] [CrossRef]
- Radius, U.; Bickelhaupt, F.M. Bonding of Imidazol-2-ylidene Ligands in Nickel Complexes. Organometallics 2008, 27, 3410–3414. [Google Scholar] [CrossRef]
- Srebro, M.; Michalak, A. Theoretical Analysis of Bonding in N-Heterocyclic Carbene–Rhodium Complexes. Inorg. Chem. 2009, 48, 5361–5369. [Google Scholar] [CrossRef]
- Andrada, D.M.; Holzmann, N.; Hamadi, T.; Frenking, G.; Beilstein, J. Direct estimate of the internal π-donation to the carbene centre within N-heterocyclic carbenes and related molecules. Org. Chem. 2015, 11, 2727–2736. [Google Scholar] [CrossRef]
- Santoro, O.; Nahra, F.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P.; Cazin, C.S.J. Synthesis, characterization and catalytic activity of stable [(NHC)H][ZnXY2] (NHC = N-Heterocyclic carbene, X, Y = Cl, Br) species. J. Mol. Catal. 2016, 423, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Dagorne, S. Recent Developments on N-Heterocyclic Carbene Supported Zinc Complexes: Synthesis and Use in Catalysis. Synthesis 2018, 50, 3662–3670. [Google Scholar] [CrossRef]
- Procter, R.J.; Uzelac, M.; Cid, J.; Rushworth, P.J.; Ingleson, M.J. Low-Coordinate NHC–Zinc Hydride Complexes Catalyze Alkyne C–H Borylation and Hydroboration Using Pinacolborane. ACS Catal. 2019, 9, 5760–5771. [Google Scholar] [CrossRef]
- Specklin, D.; Fliedel, C.; Dagorne, S. Recent Representative Advances on the Synthesis and Reactivity of N-Heterocyclic-Carbene-Supported Zinc Complexes. Chem. Rec. 2021, 21, 1130–1143. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Davidson, F.; Krafczyk, R.; Marshall, W.J.; Tamm, M. Adducts of Carbenes with Group II and XII Metallocenes. Organometallics 1998, 17, 3375–3382. [Google Scholar] [CrossRef]
- Abernethy, C.D.; Baker, R.J.; Cole, M.L.; Davies, A.J.; Jones, C. Reactions of a carbene stabilised indium trihydride complex, [InH3{CN(Mes)-C2H2N(Mes)}] Mes = mesityl, with transition metal complexes. Trans. Met. Chem. 2003, 28, 296–299. [Google Scholar] [CrossRef]
- Wang, D.; Wurst, K.; Buchmeiser, M.R. N-heterocyclic carbene complexes of Zn(II): Synthesis, X-ray structures and reactivity. J. Organomet. Chem. 2004, 689, 2123–2130. [Google Scholar] [CrossRef]
- Jensen, T.R.; Breyfogle, L.E.; Hillmyer, M.A.; Tolman, W.B. Stereoelective polymerization of D,L-lactide using N-heterocyclic carbene based compounds. Chem. Commun. 2004, 2504–2505. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.R.; Schaller, C.P.; Hillmyer, M.A.; Tolman, W.B. Zinc N-heterocyclic carbene complexes and their polymerization of D,L-lactide. J. Organomet. Chem. 2005, 690, 5881–5891. [Google Scholar] [CrossRef]
- Anantharaman, G.; Elango, K. Synthesis and Characterization of NHC-Stabilized Zinc Aryloxide and Zinc Hydroxyaryloxide. Organometallics 2007, 26, 1089–1092. [Google Scholar] [CrossRef]
- Budagumpi, S.; Endud, S. Group XII Metal–N-Heterocyclic Carbene Complexes: Synthesis, Structural Diversity, Intramolecular Interactions, and Applications. Organometallics 2013, 32, 1537–1562. [Google Scholar] [CrossRef]
- Schnee, G.; Fliedel, C.; Avilés, T.; Dagorne, S. Neutral and Cationic N-Heterocyclic Carbene Zinc Adducts and the BnOH/Zn(C6F5)2 Binary Mixture–Characterization and Use in the Ring-Opening Polymerization of β-Butyrolactone, Lactide, and Trimethylene Carbonate. Eur. J. Inorg. Chem. 2013, 2013, 3699–3709. [Google Scholar] [CrossRef]
- Fliedel, C.; Vila-Viçosa, D.; Calhorda, M.J.; Dagorne, S.; Avilés, T. Dinuclear Zinc–N-Heterocyclic Carbene Complexes for Either the Controlled Ring-Opening Polymerization of Lactide or the Controlled Degradation of Polylactide Under Mild Conditions. ChemCatChem 2014, 6, 1357–1367. [Google Scholar] [CrossRef]
- Collins, L.R.; Moffat, L.A.; Mahon, M.F.; Jones, M.D.; Whittlesey, M.K. Lactide polymerisation by ring-expanded NHC complexes of zinc. Polyhedron 2016, 103, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Chen, Y.; Vayer, M.; Djurovic, A.; Guillot, R.; Guermazi, R.; Dagorne, S.; Bour, C.; Gandon, V. Exploring the Limits of π-Acid Catalysis Using Strongly Electrophilic Main Group Metal Complexes: The Case of Zinc and Aluminium. Chem. Eur. J. 2020, 26, 12831–12838. [Google Scholar] [CrossRef]
- Roy, M.M.D.; Baird, S.R.; Ferguson, M.J.; Rivard, E. Toward N-heterocyclic carbene stabilized zinc sulfides. Mendeleev Commun. 2021, 31, 173–175. [Google Scholar] [CrossRef]
- Jabłoński, M. Theoretical Study of N-Heterocyclic-Carbene–ZnX2 (X = H, Me, Et) Complexes. Materials 2021, 14, 6147. [Google Scholar] [CrossRef]
- Schoeller, W.W. Electrophilicity and nucleophilicity in singlet carbenes. II. Electrophilic selectivity. Tetrahedron Lett. 1980, 21, 1509–1510. [Google Scholar] [CrossRef]
- Goumri-Magnet, S.; Polishchuk, O.; Gornitzka, H.; Marsden, C.J.; Baceiredo, A.; Bertrand, G. The Electrophilic Behavior of Stable Phosphanylcarbenes Towards Phosphorus Lone Pairs. Angew. Chem. Int. Ed. 1999, 38, 3727–3729. [Google Scholar] [CrossRef]
- Moss, R.A.; Wang, L.; Cang, H.; Krogh-Jespersen, K. Extremely reactive carbenes: Electrophiles and nucleophiles. J. Phys. Org. Chem. 2017, 30, e3555. [Google Scholar] [CrossRef]
- Jabłoński, M. The first theoretical proof of the existence of a hydride-carbene bond. Chem. Phys. Lett. 2018, 710, 78–83. [Google Scholar] [CrossRef]
- Jabłoński, M. In search for a hydride-carbene bond. J. Phys. Org. Chem. 2019, 32, e3949. [Google Scholar] [CrossRef]
- Yourdkhani, S.; Jabłoński, M. Physical nature of silane⋯carbene dimers revealed by state-of-the-art ab initio calculations. J. Comput. Chem. 2019, 40, 2643–2652. [Google Scholar] [CrossRef]
- Yáñez, M.; Sanz, P.; Mó, O.; Alkorta, I.; Elguero, J. Beryllium Bonds, Do They Exists? J. Chem. Theory Comput. 2009, 5, 2763–2771. [Google Scholar] [CrossRef]
- Martín-Sómer, A.; Lamsabhi, A.M.; Mó, O.; Yáñez, M. The importance of deformation on the strength of beryllium bonds. Comput. Theor. Chem. 2012, 998, 74–79. [Google Scholar] [CrossRef]
- Eskandari, K. Characteristics of beryllium bonds: A QTAIM study. J. Mol. Model. 2012, 18, 3481–3487. [Google Scholar] [CrossRef]
- Villanueva, E.F.; Mó, O.; Yáñez, M. On the existence and characteristics of π-beryllium bonds. Phys. Chem. Chem. Phys. 2014, 16, 17531–17536. [Google Scholar] [CrossRef]
- Zhong, A.; Chen, D.; Li, R. Revisiting the beryllium bonding interactions from energetic and wavefunction perspectives. Chem. Phys. Lett. 2015, 633, 265–272. [Google Scholar] [CrossRef]
- Eskandari, K. Nature of beryllium bonds in view of interacting quantum atoms and natural energy decomposition analysis. Comput. Theor. Chem. 2016, 1090, 74–79. [Google Scholar] [CrossRef]
- Montero-Campillo, M.M.; Mó, O.; Yáñez, M.; Alkorta, I.; Elguero, J. The beryllium bond. Adv. Inorg. Chem. 2019, 73, 73–121. [Google Scholar]
- Alkorta, I.; Legon, A.C. Non-Covalent Interactions Involving Alkaline-Earth Atoms and Lewis Bases B: An ab Initio Investigation of Beryllium and Magnesium Bonds, B⋯MR2 (M = Be or Mg, and R = H, F or CH3). Inorganics 2019, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Jabłoński, M. On the Uselessness of Bond Paths Linking Distant Atoms and on the Violation of the Concept of Privileged Exchange Channels. Chem. Open 2019, 8, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, Q.; Cheng, J.; Li, W. A new interaction mechanism of LiNH2 with MgH2: Magnesium bond. J. Mol. Model. 2013, 19, 247–253. [Google Scholar] [CrossRef]
- Xu, H.-L.; Li, Q.-Z.; Scheiner, S. Effect of magnesium bond on the competition between hydrogen and halogen bonds and the induction of proton and halogen transfer. ChemPhysChem 2018, 19, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Sanz, P.; Montero-Campillo, M.M.; Mó, O.; Yáñez, M.; Alkorta, I.; Elguero, J. Intramolecular magnesium bonds in malonaldehyde-like systems: A critical view of the resonance-assisted phenomena. Theor. Chem. Acc. 2018, 137, 97. [Google Scholar] [CrossRef] [Green Version]
- Lupinetti, A.J.; Jonas, V.; Thiel, W.; Strauss, S.H.; Frenking, G. Trends in Molecular Geometries and Bond Strengths of the Homoleptic d10 Metal Carbonyl Cations [M(CO)n]x+ (Mx+ = Cu+, Ag+, Au+, Zn2+, Cd2+, Hg2+; n = 1–6): A Theoretical Study. Chem. Eur. J. 1999, 5, 2573–2583. [Google Scholar] [CrossRef]
- Joy, J.; Jemmis, E.D. Contrasting Behavior of the Z Bonds in X–Z⋯Y Weak Interactions: Z = Main Group Elements Versus the Transition Metals. Inorg. Chem. 2017, 56, 1132–1143. [Google Scholar] [CrossRef]
- Wang, S.R.; Arrowsmith, M.; Braunschweig, H.; Dewhurst, R.D.; Dömling, M.; Mattock, J.D.; Pranckevicius, C.; Vargas, A. Monomeric 16-Electron π-Diborene Complexes of Zn(II) and Cd(II). J. Am. Chem. Soc. 2017, 139, 10661–10664. [Google Scholar] [CrossRef]
- Kalhor, P.; Wang, Y.; Yu, Z. The Structures of ZnCl2-Ethanol Mixtures, a Spectroscopic and Quantum Chemical Calculation Study. Molecules 2021, 26, 2498. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density–Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Stephens, M.E. Spatial Localization of the Electronic Pair and Number Distributions in Molecules. J. Am. Chem. Soc. 1975, 97, 7391–7399. [Google Scholar] [CrossRef]
- Fradera, X.; Austen, M.A.; Bader, R.F.W. The Lewis Model and Beyond. J. Phys. Chem. A 1999, 103, 304–314. [Google Scholar] [CrossRef]
- Fradera, X.; Poater, J.; Simon, S.; Duran, M.; Solà, M. Electron-pairing analysis from localization and delocalization indices in the framework of the atoms-in-molecules theory. Theor. Chem. Acc. 2002, 108, 214–224. [Google Scholar] [CrossRef]
- Firme, C.L.; Antunes, O.A.C.; Esteves, P.M. Relation between bond order and delocalization index of QTAIM. Chem. Phys. Lett. 2009, 468, 129–133. [Google Scholar] [CrossRef]
- Rafat, M.; Popelier, P.L.A. The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design; Matta, C.F., Boyed, R.J., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 121–140. [Google Scholar]
- García-Revilla, M.; Francisco, E.; Popelier, P.L.A.; Pendás, A.M. Domain-Averaged Exchange-Correlation Energies as a Physical Underpinning for Chemical Graphs. ChemPhysChem 2013, 14, 1211–1218. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Blanco, M.A.; Pendás, A.M.; Francisco, E. Interacting Quantum Atoms: A Correlated Energy Decomposition Scheme Based on the Quantum Theory of Atoms in Molecules. J. Chem. Theory Comput. 2005, 1, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Vela, J.M.; Francisco, E.; Rocha-Rinza, T.; Pendás, A.M. Interacting Quantum Atoms–A Review. Molecules 2020, 25, 4028. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the calculation of bonding energies by the Hartree Fock Slater method. Theoret. Chim. Acta (Berl.) 1977, 46, 1–10. [Google Scholar]
- Mitoraj, M.; Michalak, A. Donor–Acceptor Properties of Ligands from the Natural Orbitals for Chemical Valence. Organometallics 2007, 26, 6576–6580. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A. Natural orbitals for chemical valence as descriptors of chemical bonding in transition metal complexes. J. Mol. Model. 2007, 13, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Mitoraj, M.; Ziegler, T. Bond Orbitals from Chemical Valence Theory. J. Phys. Chem. A 2008, 112, 1933–1939. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. On the Nature of the Agostic Bond between Metal Centers and β-Hydrogen Atoms in Alkyl Complexes. An Analysis Based on the Extended Transition State Method and the Natural Orbitals for Chemical Valence Scheme (ETS-NOCV). Organometallics 2009, 28, 3727–3733. [Google Scholar] [CrossRef]
- Saebø, S.; Pulay, P. Local Treatment of Electron Correlation. Annu. Rev. Phys. Chem 1993, 44, 213–236. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A. Efficient and accurate local approximations to coupled-electron pair approaches: An attempt to revive the pair natural orbital method. J. Chem. Phys. 2009, 130, 114108. [Google Scholar] [CrossRef]
- Neese, F.; Hansen, A.; Liakos, D.G. Efficient and accurate approximations to the local coupled cluster singles doubles method using a truncated pair natural orbital basis. J. Chem. Phys. 2009, 131, 064103. [Google Scholar] [CrossRef]
- Riplinger, C.; Sandhoefer, B.; Hansen, A.; Neese, F. Natural triple excitations in local coupled cluster calculations. J. Chem. Phys. 2013, 139, 134101. [Google Scholar] [CrossRef]
- Guo, Y.; Riplinger, C.; Becker, U.; Liakos, D.G.; Minenkov, Y.; Cavallo, L.; Neese, F. Communication: An improved linear scaling perturbative triples correction for the domain based local pair-natural orbital based singles and doubles coupled cluster method [DLPNO-CCSD(T)]. J. Chem. Phys. 2018, 148, 011101. [Google Scholar] [CrossRef]
- Altun, A.; Saitow, M.; Neese, F.; Bistoni, G. Local Energy Decomposition of Open-Shell Molecular Systems in the Domain-Based Local Pair Natural Orbital Coupled Cluster Framework. J. Chem. Theory Comput. 2019, 15, 1616–1632. [Google Scholar] [CrossRef] [Green Version]
- Altun, A.; Izsák, R.; Bistoni, G. Local energy decomposition of coupled-cluster interaction energies: Interpretation, benchmarks, and comparison with symmetry-adapted perturbation theory. Int. J. Quantum Chem. 2021, 121, e26339. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Ritchie, J.P.; Bachrach, S.M. Some Methods and Applications of Electron Density Distribution Analysis. J. Comput. Chem. 1987, 8, 499–509. [Google Scholar] [CrossRef]
- Ritchie, J.P. Electron Density Distribution Analysis for Nitromethane, Nitromethide, and Nitramide. J. Am. Chem. Soc. 1985, 107, 1829–1837. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Rablen, P.R. Atomic Charges. J. Org. Chem. 2018, 83, 15463–15469. [Google Scholar] [CrossRef]
- Jabłoński, M.; Krygowski, T.M. Study of the influence of intermolecular interaction on classical and reverse substituent effects in para-substituted phenylboranes. New J. Chem. 2020, 44, 9656–9670. [Google Scholar] [CrossRef]
- Walsh, A.D. The Electronic Orbitals, Shapes, and Spectra of Polyatomic Molecules. Part I. AH2 Molecules. J. Chem. Soc. 1953, 2260–2266. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.v.R. Efficient Diffuse Function-Augmented Basis Sets for Anion Calculations. III. The 3-21+G Basis Set for First-Row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Curtiss, L.A.; McGrath, M.P.; Blandeau, J.-P.; Davis, N.E.; Binning, R.C., Jr.; Radom, L. Extension of Gaussian-2 theory to molecules containing third-row atoms Ga–Kr. J. Chem. Phys. 1995, 103, 6104–6113. [Google Scholar] [CrossRef]
- Jensen, F. Introduction to Computational Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Keith, T.A. AIMAll (Version 15.05.18); TK Gristmill Software: Overland Park, KS, USA, 2015; Available online: Aim.tkgristmill.com (accessed on 17 September 2022).
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 Energy Evaluation by Direct Methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Becke, A.D. Density-fnnctional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijder, J.G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- Snijder, J.G.; Sadlej, A.J. Perturbation versus variation treatment of regular relativistic Hamiltonians. Chem. Phys. Lett. 1996, 252, 51–61. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ziegler, T.; Atkins, A.J.; Autschbach, J.; Baseggio, O.; Bashford, D.; Bééces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; et al. ADF 2022.1; SCM-Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Sagan, F.; Mitoraj, M.P. Non-covalent Interactions in Selected Transition Metal Complexes. In Transition Metals in Coordination Environments: Computational Chemistry and Catalysis Viewpoints; Broclawik, E., Borowski, T., Radoń, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 65–89. [Google Scholar]
- Stasyuk, O.A.; Sedlak, R.; Fonseca-Guerra, C.; Hobza, P. Comparison of the DFT-SAPT and Canonical EDA Schemes for the Energy Decomposition of Various Types of Noncovalent Interactions. J. Chem. Theory Comput. 2018, 14, 3440–3450. [Google Scholar] [CrossRef]





| MX | CT a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| cyclopropenylidene | |||||||||
| BeH | 1.743 | 1.374 | 0.039 | 135.1 | 112.4 | 56.6 | 0.8 | 29.1 | −0.317 |
| MgH | 2.268 | 1.740 | 0.035 | 148.3 | 105.9 | 56.7 | 0.9 | 20.9 | −0.273 |
| ZnH | 2.121 | 1.575 | 0.036 | 144.6 | 107.7 | 57.3 | 0.9 | 15.2 | −0.279 |
| BeBr | 1.764 | 2.043 | 0.093 | 134.5 | 112.8 | 57.3 | 1.5 | 35.4 | −0.414 |
| MgBr | 2.206 | 2.376 | 0.052 | 145.4 | 107.3 | 57.0 | 1.3 | 32.1 | −0.331 |
| ZnBr | 2.061 | 2.298 | 0.083 | 137.1 | 111.5 | 57.3 | 1.5 | 28.2 | −0.380 |
| imidazol-2-ylidene | |||||||||
| BeBr | 1.765 | 2.059 | 0.109 | 132.9 | 113.6 | 104.2 | 3.5 | 48.6 | −0.464 |
| MgBr | 2.173 | 2.393 b | 0.069 c | 147.9 | 106.1 d | 103.5 | 2.8 | 43.7 | −0.363 |
| ZnBr | 2.037 | 2.311 | 0.097 | 139.2 | 110.4 | 104.2 | 3.4 | 41.2 | −0.432 |
| MX | ||||||||
|---|---|---|---|---|---|---|---|---|
| cyclopropenylidene | ||||||||
| BeH | −12.2839 | 7.2846 | 4.6505 | 4.6963 | −0.0458 | 13.1 | −0.3030 | −0.3488 |
| MgH | −32.4999 | 16.8017 | 15.5098 | 15.5405 | −0.0307 | 16.3 | −0.1577 | −0.1884 |
| ZnH | −90.1277 | 44.8987 | 45.0634 | 45.1726 | −0.1091 | 65.9 | −0.0564 | −0.1655 |
| BeBr | −12.1017 | 7.1990 | 4.5383 | 4.5810 | −0.0428 | 11.7 | −0.3217 | −0.3644 |
| MgBr | −33.3934 | 17.2713 | 15.9040 | 15.9383 | −0.0343 | 15.7 | −0.1838 | −0.2181 |
| ZnBr | −92.5816 | 46.2217 | 46.1682 | 46.2894 | −0.1212 | 63.2 | −0.0705 | −0.1917 |
| imidazol-2-ylidene | ||||||||
| BeBr | −11.3609 | 7.1976 | 4.1093 | 4.1554 | −0.0461 | 85.5 | −0.0079 | −0.0539 |
| MgBr | −32.0341 | 17.5351 | 14.5370 | 14.5767 | −0.0397 | −104.7 | 0.0777 | 0.0379 |
| ZnBr | −88.3086 | 46.7541 | 41.5418 | 41.6735 | −0.1317 | 1037.0 | 0.1190 | −0.0127 |
| MX | |||||
|---|---|---|---|---|---|
| cyclopropenylidene | |||||
| BeH | −143.28 | −73.68 | 51.4 | −69.60 | 48.6 |
| MgH | −71.81 | −38.55 | 53.7 | −33.25 | 46.3 |
| ZnH | −91.45 | −14.86 | 16.2 | −76.59 | 83.8 |
| BeBr | −162.74 | −90.98 | 55.9 | −71.76 | 44.1 |
| MgBr | −88.25 | −49.41 | 56.0 | −38.85 | 44.0 |
| ZnBr | −105.00 | −18.61 | 17.7 | −86.38 | 82.3 |
| imidazol-2-ylidene | |||||
| BeBr | −190.24 | −110.00 | 57.8 | −80.27 | 42.2 |
| MgBr | −113.04 | −63.87 | 56.5 | −49.17 | 43.5 |
| ZnBr | −127.25 | −26.19 | 20.6 | −101.05 | 79.4 |
| MX | ||||||||
|---|---|---|---|---|---|---|---|---|
| cyclopropenylidene | ||||||||
| BeH | −45.69 | −69.29 (56%) | 77.03 | −2.56 (2%) | −50.87 (41%) | −40.33 | −10.11 | n/a |
| MgH | −26.20 | −45.85 (70%) | 39.55 | −2.58 (4%) | −17.32 (26%) | −14.16 | −2.88 | n/a |
| ZnH | −23.63 | −75.38 (68%) | 87.86 | −2.87 (3%) | −33.24 (30%) | −27.31 | −5.19 | n/a |
| BeBr | −52.23 | −82.67 (58%) | 90.98 | −4.90 (3%) | −55.64 (39%) | −47.43 | −6.34 | n/a |
| MgBr | −38.57 | −57.23 (69%) | 44.25 | −4.14 (5%) | −21.44 (26%) | −17.67 | −2.56 | n/a |
| ZnBr | −37.74 | −95.45 (67%) | 104.58 | −4.52 (3%) | −42.34 (30%) | −36.96 | −3.53 | n/a |
| imidazol-2-ylidene | ||||||||
| BeBr | −66.86 | −103.25 (61%) | 102.10 | −6.61 (4%) | −59.10 (35%) | −47.92 | −5.24 | −1.71 |
| MgBr | −50.49 | −77.00 (71%) | 58.24 | −6.01 (5%) | −25.72 (24%) | −18.57 | −3.13 | −1.10 |
| ZnBr | −50.92 | −122.82 (68%) | 128.22 | −6.45 (4%) | −49.87 (28%) | −42.15 | −3.70 | −1.11 |
| cpy–BeBr | cpy–MgBr | cpy–ZnBr | imi–BeBr | imi–MgBr | imi–ZnBr | |
|---|---|---|---|---|---|---|
| E | −39.60 | −34.28 | −29.08 | −54.28 | −46.37 | −42.63 |
| E | −218.44 | −152.51 | −327.12 | −251.84 | −190.31 | −393.52 |
| E | −20.12 | −11.84 | −33.73 | −20.84 | 14.59 | −39.32 |
| E | 14.88 | 6.23 | 10.76 | 17.58 | 6.73 | 11.24 |
| E | 190.33 | 126.75 | 325.62 | 210.63 | 158.28 | 387.67 |
| E | -0.69 | 0.12 | 1.91 | −2.74 | −1.83 | −0.17 |
| E | −5.35 | −2.93 | −6.14 | −6.43 | −4.10 | −7.67 |
| E | −0.21 | −0.10 | −0.38 | −0.63 | −0.55 | −0.85 |
| CT 1→2 | −16.96 | −9.52 | −13.44 | −19.17 | −11.11 | −16.32 |
| CT 2→1 | −3.33 | −2.43 | −7.58 | −3.56 | −2.53 | −8.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagan, F.; Mitoraj, M.; Jabłoński, M. Nature of Beryllium, Magnesium, and Zinc Bonds in Carbene⋯MX2 (M = Be, Mg, Zn; X = H, Br) Dimers Revealed by the IQA, ETS-NOCV and LED Methods. Int. J. Mol. Sci. 2022, 23, 14668. https://doi.org/10.3390/ijms232314668
Sagan F, Mitoraj M, Jabłoński M. Nature of Beryllium, Magnesium, and Zinc Bonds in Carbene⋯MX2 (M = Be, Mg, Zn; X = H, Br) Dimers Revealed by the IQA, ETS-NOCV and LED Methods. International Journal of Molecular Sciences. 2022; 23(23):14668. https://doi.org/10.3390/ijms232314668
Chicago/Turabian StyleSagan, Filip, Mariusz Mitoraj, and Mirosław Jabłoński. 2022. "Nature of Beryllium, Magnesium, and Zinc Bonds in Carbene⋯MX2 (M = Be, Mg, Zn; X = H, Br) Dimers Revealed by the IQA, ETS-NOCV and LED Methods" International Journal of Molecular Sciences 23, no. 23: 14668. https://doi.org/10.3390/ijms232314668
APA StyleSagan, F., Mitoraj, M., & Jabłoński, M. (2022). Nature of Beryllium, Magnesium, and Zinc Bonds in Carbene⋯MX2 (M = Be, Mg, Zn; X = H, Br) Dimers Revealed by the IQA, ETS-NOCV and LED Methods. International Journal of Molecular Sciences, 23(23), 14668. https://doi.org/10.3390/ijms232314668