Hepatic Oleate Regulates Insulin-like Growth Factor-Binding Protein 1 Partially through the mTORC1-FGF21 Axis during High-Carbohydrate Feeding
Abstract
:1. Introduction
2. Results
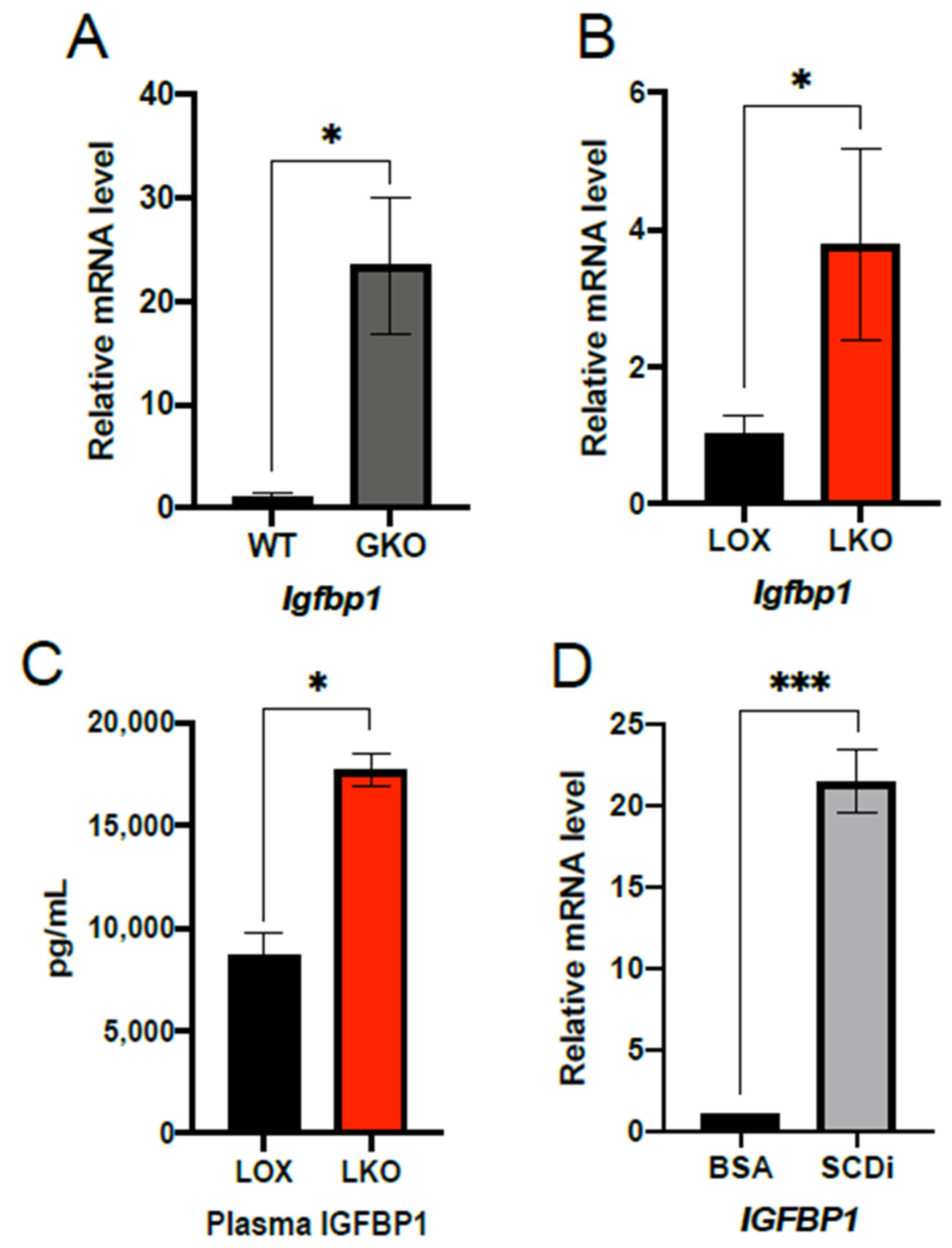
2.1. IGFBP1 Expression Is Enhanced by SCD1 Deletion and Inhibition of Enzyme Activity
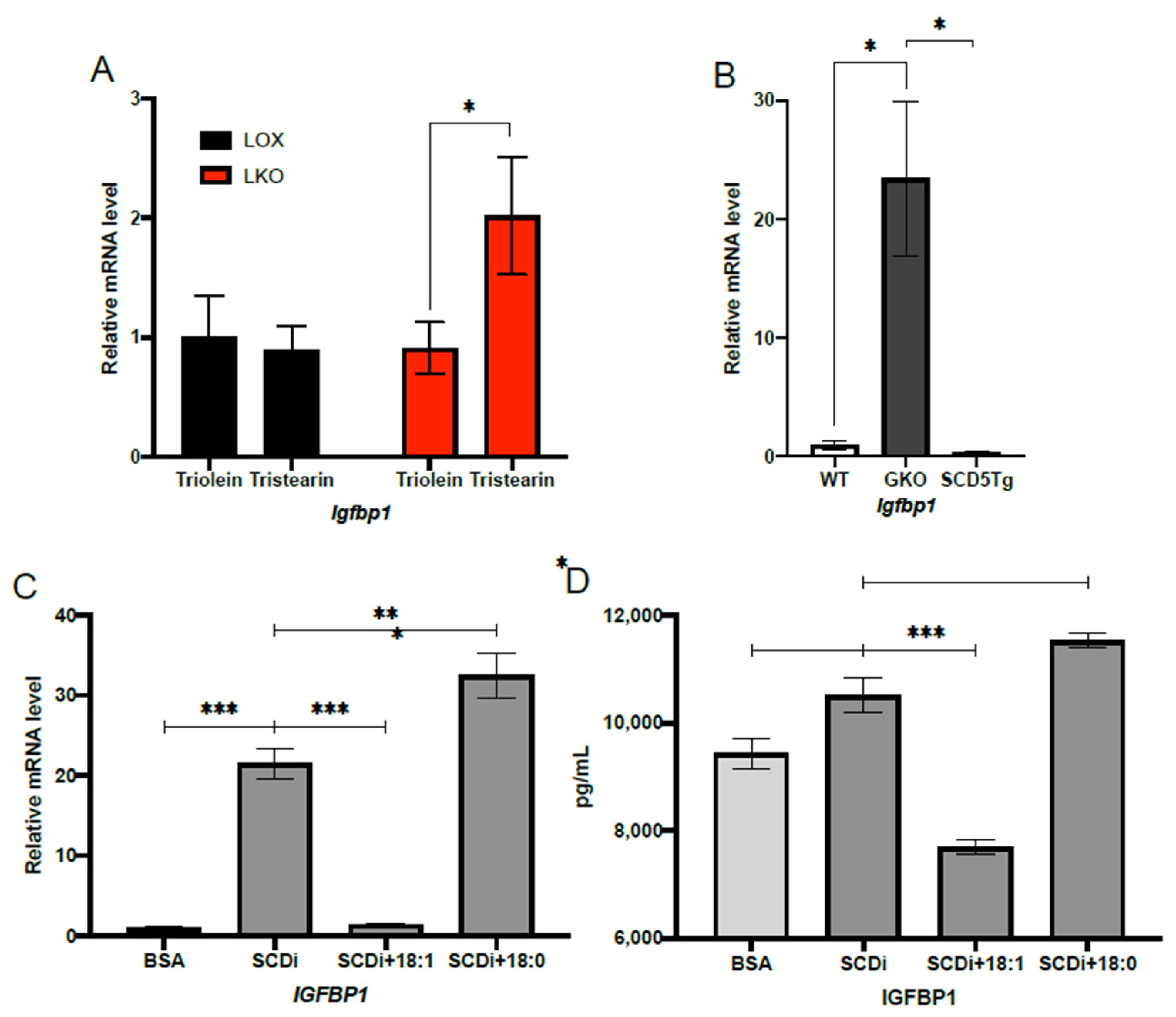
2.2. Oleic Acid Suppresses IGFBP1 Expression under HCD Feeding Conditions
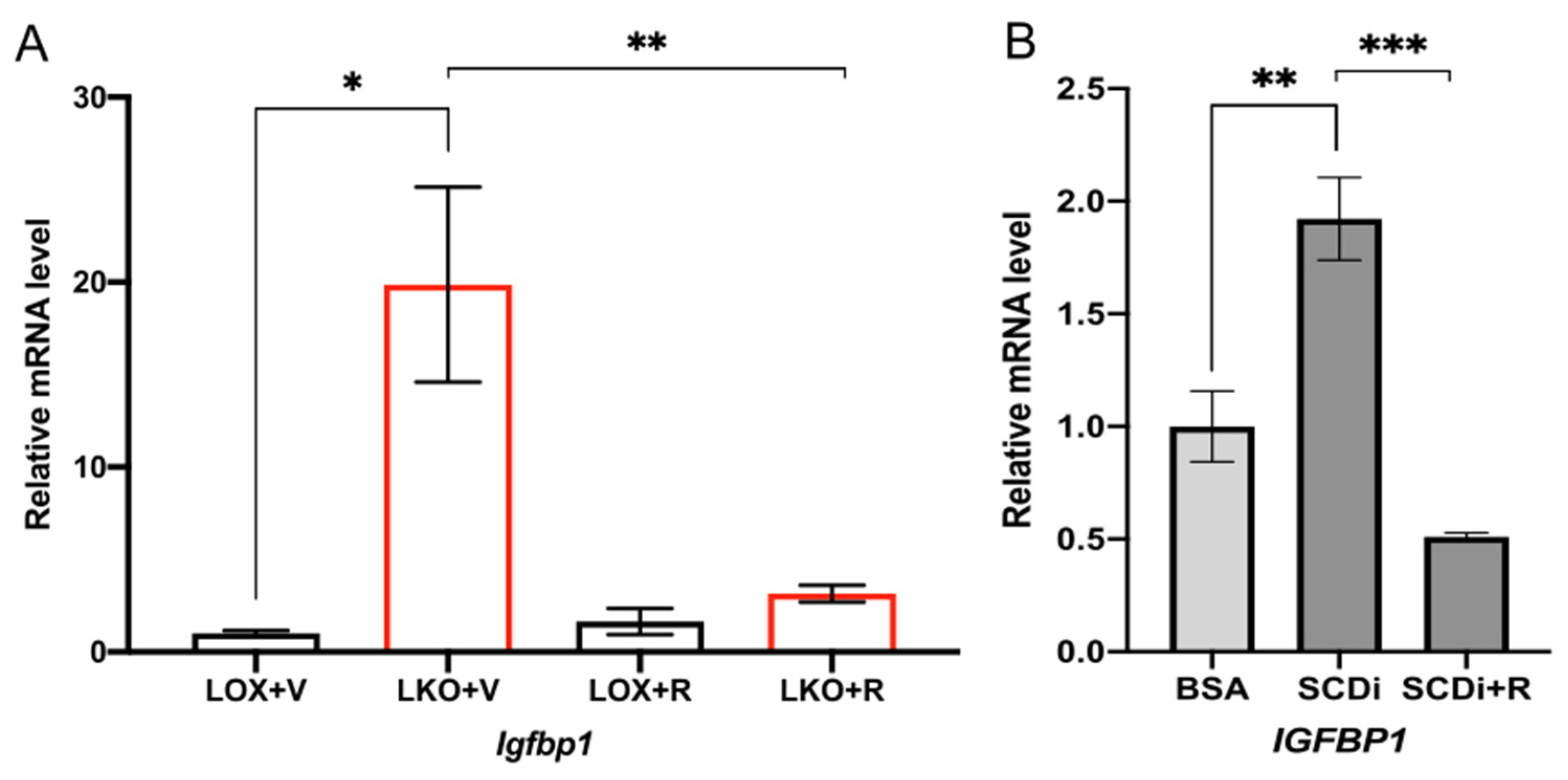
2.3. SCD1 Deficiency Enhances IGFBP1 through mTORC1
2.4. Oleic Acid Regulates IGFBP1 through the mTORC1-FGF21 Axis
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Quantitative Real-Time PCR
4.3. Cell Culture and Treatments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ravussin, E.; Ryan, D.H. Three New Perspectives on the Perfect Storm: What’s Behind the Obesity Epidemic? Obesity 2018, 26, 9–10. [Google Scholar] [CrossRef]
- Slyper, A.H. The pediatric obesity epidemic: Causes and controversies. J. Clin. Endocrinol. Metab. 2004, 89, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kuczmarski, R.J.; Johnson, C.L. Overweight and obesity in the United States: Prevalence and trends, 1960-1994. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.T.; Ntambi, J.M. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim. Biophys. Acta 2009, 1791, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, M.; Flowers, M.T.; Sampath, H.; Chu, K.; Otzelberger, C.; Liu, X.; Ntambi, J.M. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007, 6, 484–496. [Google Scholar] [CrossRef] [Green Version]
- Oballa, R.M.; Belair, L.; Black, W.C.; Bleasby, K.; Chan, C.C.; Desroches, C.; Du, X.; Gordon, R.; Guay, J.; Guiral, S.; et al. Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia. J. Med. Chem. 2011, 54, 5082–5096. [Google Scholar] [CrossRef] [PubMed]
- Man, W.C.; Miyazaki, M.; Chu, K.; Ntambi, J.M. Membrane topology of mouse stearoyl-CoA desaturase 1. J. Biol. Chem. 2006, 281, 1251–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, M.; Bruggink, S.M.; Ntambi, J.M. Identification of mouse palmitoyl-coenzyme A Delta9-desaturase. J. Lipid Res. 2006, 47, 700–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, M.; Kim, Y.C.; Ntambi, J.M. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J. Lipid Res. 2001, 42, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R.; Leaver, M.J.; Hodgson, P.A. Recent advances in the biochemistry and molecular biology of fatty acyl desaturases. Prog. Lipid. Res. 1998, 37, 73–117. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Lounis, M.A.; Bergeron, K.F.; Burhans, M.S.; Ntambi, J.M.; Mounier, C. Oleate activates SREBP-1 signaling activity in. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E710–E720. [Google Scholar] [CrossRef] [PubMed]
- Takada, R.; Satomi, Y.; Kurata, T.; Ueno, N.; Norioka, S.; Kondoh, H.; Takao, T.; Takada, S. Monounsaturated Fatty Acid Modification of Wnt Protein: Its Role in Wnt Secretion. Dev. Cell 2006, 11, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, M.; Sampath, H.; Liu, X.; Flowers, M.T.; Chu, K.; Dobrzyn, A.; Ntambi, J.M. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem. Biophys. Res. Commun. 2009, 380, 818–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, H.; Flowers, M.T.; Liu, X.; Paton, C.M.; Sullivan, R.; Chu, K.; Zhao, M.; Ntambi, J.M. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J. Biol. Chem. 2009, 284, 19961–19973. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.T.; Keller, M.P.; Choi, Y.; Lan, H.; Kendziorski, C.; Ntambi, J.M.; Attie, A.D. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol. Genom. 2008, 33, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Liu, K.-C.; Schulz, N.; Karampelias, C.; Charbord, J.; Hilding, A.; Rautio, L.; Bertolino, P.; Östenson, C.-G.; Brismar, K.; et al. IGFBP1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. EMBO J. 2016, 35, 2026–2044. [Google Scholar] [CrossRef]
- Haywood, N.J.; Cordell, P.A.; Tang, K.Y.; Makova, N.; Yuldasheva, N.Y.; Imrie, H.; Viswambharan, H.; Bruns, A.F.; Cubbon, R.M.; Kearney, M.T.; et al. Insulin-Like Growth Factor Binding Protein 1 Could Improve Glucose Regulation and Insulin Sensitivity Through Its RGD Domain. Diabetes 2017, 66, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Rajwani, A.; Ezzat, V.; Smith, J.; Yuldasheva, N.Y.; Duncan, E.R.; Gage, M.; Cubbon, R.M.; Kahn, M.B.; Imrie, H.; Abbas, A.; et al. Increasing Circulating IGFBP1 Levels Improves Insulin Sensitivity, Promotes Nitric Oxide Production, Lowers Blood Pressure, and Protects Against Atherosclerosis. Diabetes 2012, 61, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Mashili, F.L.; Austin, R.L.; Deshmukh, A.S.; Fritz, T.; Caidahl, K.; Bergdahl, K.; Zierath, J.R.; Chibalin, A.V.; Moller, D.E.; Kharitonenkov, A.; et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: Implications for type 2 diabetes and obesity. Diabetes Metab. Res. Rev. 2011, 27, 286–297. [Google Scholar] [CrossRef]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Stanislaus, S.; Chinookoswong, N.; Lau, Y.Y.; Hager, T.; Patel, J.; Ge, H.; Weiszmann, J.; Lu, S.-C.; Graham, M.; et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—Association with liver and adipose tissue effects. Am. J. Physiol. Metab. 2009, 297, E1105–E1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Cao, H.; Hou, Y.; Sun, G.; Li, D.; Wang, W. Liver Plays a Major Role in FGF-21 Mediated Glucose Homeostasis. Cell. Physiol. Biochem. 2018, 45, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, W.; Krzeszinski, J.Y.; Wang, Y.; Wan, Y. A Liver-Bone Endocrine Relay by IGFBP1 Promotes Osteoclastogenesis and Mediates FGF21-Induced Bone Resorption. Cell Metab. 2015, 22, 811–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.; Kliewer, S.A. Inhibition of Growth Hormone Signaling by the Fasting-Induced Hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljohani, A.; Khan, M.I.; Syed, D.N.; Abram, B.; Lewis, S.; Neill, L.O.; Mukhtar, H.; Ntambi, J.M. Hepatic Stearoyl-CoA desaturase-1 deficiency-mediated activation of mTORC1- PGC-1α axis regulates ER stress during high-carbohydrate feeding. Sci. Rep. 2019, 9, 15761. [Google Scholar] [CrossRef] [Green Version]
- Aljohani, A.; Khan, M.I.; Bonneville, A.; Guo, C.; Jeffery, J.; O’Neill, L.; Syed, D.N.; Lewis, S.; Burhans, M.; Mukhtar, H.; et al. Hepatic stearoyl CoA desaturase 1 deficiency increases glucose uptake in adipose tissue partially through the PGC-1α–FGF21 axis in mice. J. Biol. Chem. 2019, 294, 19475–19485. [Google Scholar] [CrossRef]
- Burhans, M.S.; Flowers, M.T.; Harrington, K.; Bond, L.M.; Guo, C.-A.; Anderson, R.M.; Ntambi, J.M. Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation. J. Lipid Res. 2015, 56, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.H.; Cruickshank, J.K.; Riste, L.K.; Cade, J.E.; Anderson, S.; Greenhalgh, A.; Sampayo, J.; Taylor, W.; Fraser, W.; White, A.; et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia 2001, 44, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Gokulakrishnan, K.; Velmurugan, K.; Ganesan, S.; Mohan, V. Circulating levels of insulin-like growth factor binding protein-1 in relation to insulin resistance, type 2 diabetes mellitus, and metabolic syndrome (Chennai Urban Rural Epidemiology Study 118). Metabolism 2012, 61, 43–46. [Google Scholar] [CrossRef]
- Liew, C.F.; Wise, S.D.; Yeo, K.P.; Lee, K.-O. Insulin-like growth factor binding protein-1 is independently affected by ethnicity, insulin sensitivity, and leptin in healthy, glucose-tolerant young men. J. Clin. Endocrinol. Metab. 2005, 90, 1483–1488. [Google Scholar] [CrossRef] [Green Version]
- Rajpathak, S.N.; McGinn, A.P.; Strickler, H.D.; Rohan, T.E.; Pollak, M.; Cappola, A.R.; Kuller, L.; Xue, X.; Newman, A.B.; Strotmeyer, E.S.; et al. Insulin-like growth factor-(IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm. IGF Res. 2008, 18, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogul, H.R.; Marshall, M.; Frey, M.; Burke, H.B.; Wynn, P.S.; Wilker, S.; Southern, A.L.; Gambert, S.R. Insulin like growth factor-binding protein-1 as a marker for hyperinsulinemia in obese menopausal women. J. Clin. Endocrinol. Metab. 1996, 81, 4492–4495. [Google Scholar]
- Mohamed-Ali, V.; Pinkney, J.H.; Panahloo, A.; Cwyfan-Hughes, S.; Holly, J.M.; Yudkin, J.S. Insulin-like growth factor binding protein-1 in NIDDM: Relationship with the insulin resistance syndrome. Clin. Endocrinol. 1999, 50, 221–228. [Google Scholar] [CrossRef]
- Travers, S.H.; Labarta, J.I.; Gargosky, S.E.; Rosenfeld, R.G.; Jeffers, B.W.; Eckel, R.H. Insulin-Like Growth Factor Binding Protein-I Levels Are Strongly Associated with Insulin Sensitivity and Obesity in Early Pubertal Children1. J. Clin. Endocrinol. Metab. 1998, 83, 1935–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh, H.; Kamoda, T.; Nakahara, S.; Hirano, T.; Nakamura, N. Serum concentrations of insulin, insulin-like growth factor(IGF)-I, IGF binding protein (IGFBP)-1 and -3 and growth hormone binding protein in obese children: Fasting IGFBP-1 is suppressed in normoinsulinaemic obese children. Clin. Endocrinol. 1998, 48, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Borai, A.; Livingstone, C.; Zarif, H.; Ferns, G. Serum insulin-like growth factor binding protein-1: An improvement over other simple indices of insulin sensitivity in the assessment of subjects with normal glucose tolerance. Ann. Clin. Biochem. Int. J. Lab. Med. 2009, 46, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Petersson, U.; Östgren, C.; Brudin, L.; Brismar, K.; Nilsson, P. Low levels of insulin-like growth-factor-binding protein-1 (IGFBP-1) are prospectively associated with the incidence of type 2 diabetes and impaired glucose tolerance (IGT): The Söderåkra Cardiovascular Risk Factor Study. Diabetes Metab. 2009, 35, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Burhans, M.S.; Flowers, M.T.; Ntambi, J.M. Hepatic oleate regulates liver stress response partially through PGC-1α during high-carbohydrate feeding. J. Hepatol. 2016, 65, 103–112. [Google Scholar] [CrossRef]
- Schaap, F.G.; Kremer, A.E.; Lamers, W.H.; Jansen, P.L.; Gaemers, I.C. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie 2013, 95, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.; Tomkiewicz, C.; Magne, L.; Barouki, R.; Garlatti, M. Endoplasmic Reticulum Stress Induction of Insulin-like Growth Factor-binding Protein-1 Involves ATF4. J. Biol. Chem. 2006, 281, 19124–19133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [PubMed]
- Zou, Y.; Wang, Y.-N.; Ma, H.; He, Z.-H.; Tang, Y.; Guo, L.; Liu, Y.; Ding, M.; Qian, S.-W.; Tang, Q.-Q. SCD1 promotes lipid mobilization in subcutaneous white adipose tissue. J. Lipid Res. 2020, 61, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Sera, R.K.; McBride, J.H.; Higgins, S.A.; Rodgerson, D.O. Evaluation of reference ranges for fatty acids in serum. J. Clin. Lab. Anal. 1994, 8, 81–85. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, L.M.; Phang, Y.X.; Liu, Z.; Lewis, S.A.; Aljohani, A.; McGahee, A.; Wade, G.; Kalyesubula, M.; Simcox, J.; Ntambi, J.M. Hepatic Oleate Regulates Insulin-like Growth Factor-Binding Protein 1 Partially through the mTORC1-FGF21 Axis during High-Carbohydrate Feeding. Int. J. Mol. Sci. 2022, 23, 14671. https://doi.org/10.3390/ijms232314671
O’Neill LM, Phang YX, Liu Z, Lewis SA, Aljohani A, McGahee A, Wade G, Kalyesubula M, Simcox J, Ntambi JM. Hepatic Oleate Regulates Insulin-like Growth Factor-Binding Protein 1 Partially through the mTORC1-FGF21 Axis during High-Carbohydrate Feeding. International Journal of Molecular Sciences. 2022; 23(23):14671. https://doi.org/10.3390/ijms232314671
Chicago/Turabian StyleO’Neill, Lucas M., Yar Xin Phang, Zhaojin Liu, Sarah A. Lewis, Ahmed Aljohani, Ayren McGahee, Gina Wade, Mugagga Kalyesubula, Judith Simcox, and James M. Ntambi. 2022. "Hepatic Oleate Regulates Insulin-like Growth Factor-Binding Protein 1 Partially through the mTORC1-FGF21 Axis during High-Carbohydrate Feeding" International Journal of Molecular Sciences 23, no. 23: 14671. https://doi.org/10.3390/ijms232314671
APA StyleO’Neill, L. M., Phang, Y. X., Liu, Z., Lewis, S. A., Aljohani, A., McGahee, A., Wade, G., Kalyesubula, M., Simcox, J., & Ntambi, J. M. (2022). Hepatic Oleate Regulates Insulin-like Growth Factor-Binding Protein 1 Partially through the mTORC1-FGF21 Axis during High-Carbohydrate Feeding. International Journal of Molecular Sciences, 23(23), 14671. https://doi.org/10.3390/ijms232314671






