Cyclic Dimers of 4-n-Propyloxybenzoic Acid with Hydrogen Bonds in the Gaseous State
Abstract
:1. Introduction
2. Results and Discussions
2.1. Peculiarity of GED Data Structural Analysis of POBA Vapor
2.2. Conformers of the Monomeric and Dimeric Forms of POBA. Possibilities of Gas Electron Diffraction Method
2.3. Mass Spectrometric Confirmation of the Existence of Dimeric Forms in a Vapor over POBA
2.4. Comparison of the Results of GED Studies of Three Carboxylic Acids: AA—Acetic Acid, BA—Benzoic Acid, and POBA—4-n-Propyloxybenzoic Acid
2.5. NBO-Analysis and HB Energy
3. Materials and Methods
3.1. Conditions of a Synchronous GED/MS Experiment
3.2. The Separate MS Experiment
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdy, M.; Murdoch, A.; Martínez-Felipe, A. New insights into the role of hydrogen bonding on the liquid crystal behavior of 4-alkoxybenzoic acids: A detailed IR spectroscopy study. Liq. Cryst. 2016, 43, 2191–2207. [Google Scholar] [CrossRef]
- Giricheva, N.; Fedorov, M.; Shpilevaya, K.; Syrbu, S.; Ditsina, O. Characteristics of the hydrogen bond and the structure of H-complexes of p-n-propyloxybenzoic acid and p-n-propyloxy-p′-cyanobiphenyl. J. Struct. Chem. 2017, 58, 9–16. [Google Scholar] [CrossRef]
- Painter, P.; Cleveland, C.; Coleman, M. An Infrared Spectroscopic Study of p-n-Alkoxybenzoic Acids. Molecular Crystals and Liquid Crystals Science and Technology. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 2000, 348, 269–293. [Google Scholar] [CrossRef]
- Kato, T.; Jin, C.; Kaneuchi, F.; Uryu, T. Effect of the Molecular Orientation on the Stability of Hydrogen-Bonded Benzoic Acid Dimers. Infrared Study of Liquid-Crystalline 4-Alkylbenzoic Acids. Bull. Chem. Soc. Jpn. 1993, 66, 3581–3584. [Google Scholar] [CrossRef]
- Demus, D.; Zaschke, H. Flussiqe Kristalle in Tabellen II; VEB Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1984; 468p. [Google Scholar] [CrossRef]
- Giricheva, N.; Syrbu, S.; Bubnova, K.; Fedorov, M.; Kiselev, M.; Girichev, G. H-complexes in the “4-n-alkoxybenzoic acid: 4-pyridyl 4′-n-alkoxybenzoate” system. IR spectroscopy and quantum chemical calculations. J. Mol. Liq. 2019, 277, 833–842. [Google Scholar] [CrossRef]
- Yaremko, A.; Ratajczak, H.; Barnes, A.; Baran, J.; Durlak, P.; Latajka, Z. Fermi resonance and strong anharmonic effects in the absorption spectra of the m-OH (m-OD) vibration of solid H- and D-benzoic acid. Chem. Phys. 2009, 364, 51–63. [Google Scholar] [CrossRef]
- Paterson, D.; Martinez-Felipe, A.; Jansze, S.; Marcelis, A.; Storey, J.; Imrie, C. New insights into the liquid crystal behavior of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 5–6, 928–939. [Google Scholar] [CrossRef]
- Meganathan, C.; Sebastian, S.; Kurt, M.; Lee, K.; Sundaraganesan, N. Molecular structure, spectroscopic (FTIR, FTIR gas phase, FT-Raman) first-order hyperpolarizability and HOMO–LUMO analysis of 4-methoxy-2-methyl benzoic acid. J. Raman Spectrosc. 2010, 41, 1369–1378. [Google Scholar] [CrossRef]
- Bakker, J.; Aleese, L.; von Helden, G.; Meijer, G. The infrared absorption spectrum of the gas phase neutral benzoic acid monomer and dimer. J.Chem. Phys. 2003, 119, 11180. [Google Scholar] [CrossRef]
- Reva, I.; Stepanian, S. An infrared study on matrix-isolated benzoic acid. J. Mol. Struct. 1995, 349, 337–340. [Google Scholar] [CrossRef]
- Stepanian, S.; Reva, I.; Radchenko, E.; Sheina, G. Infrared spectra of benzoic acid monomers and dimers in argon matrix. Vib. Spectrosc. 1996, 11, 123–133. [Google Scholar] [CrossRef]
- Florio, G.; Silbert III, E.; Zwier, T. Fluorescence-dip IR spectra of jet-cooled benzoic acid dimer in its ground and First excited singlet states. Faraday Discuss. 2001, 118, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Vishnevskiy, Y.; Blomeyer, S.; Reuter, C. Low Pressure Gas Electron Diffraction: An Experimental Setup and Case Studies. Rev. Sci. Instrum. 2020, 91, 074104. [Google Scholar] [CrossRef] [PubMed]
- Aarset, K.; Page, E.; Rice, D. Molecular Structures of Benzoic Acid and 2-Hydroxybenzoic Acid, Obtained by Gas-Phase Electron Diffraction and Theoretical Calculations. J. Phys. Chem. A. 2006, 110, 9014–9019. [Google Scholar] [CrossRef] [PubMed]
- Vishnevskiy, Y.; Blomeyer, S.; Reuter, C.; Pimenov, O.; Shlykov, S. Combined gas electron diffraction and mass spectrometric experimental setup at Bielefeld University. Rev Sci Instrum. 2020, 91, 73103. [Google Scholar] [CrossRef]
- Andersen, B.; Seip, H.; Strand, T.; Stolevik, R. Procedure and Computer Programs for the Structure Determination of Gaseous Molecules from Electron Diffraction Data. Acta Chem. Scand. 1969, 23, 3224–3234. [Google Scholar] [CrossRef] [Green Version]
- Vishnevskiy, Y.; Zhabanov, Y. New implementation of the first-order perturbation theory for calculation of interatomic vibrational amplitudes and corrections in gas electron diffraction. J. Phys. Conf. Ser. 2015, 633, 012076. [Google Scholar] [CrossRef]
- Giricheva, N.; Fedorov, M.; Shpilevaya, K.; Syrbu, S.; Girichev, G. Intramolecular vibrations and structural nonrigidity of hydrogen-bonded complexes. Liq. Cryst. Their Appl. 2018, 18, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Zhabanov, Y.; Zakharov, A.; Giricheva, N.; Shlykov, S.; Koifman, O.; Girichev, G. To the limit of gas-phase electron diffraction: Molecular structure of magnesium octa(m-trifluoromethylphenyl)porphyrazine. J. Mol. Struct. 2015, 1092, 104–112. [Google Scholar] [CrossRef]
- Hamilton, W. Significance tests on the crystallographic R factor. Acta Crystallogr. 1965, 18, 502–510. [Google Scholar] [CrossRef]
- Pelipets, O.V. Investigation of the Thermodynamics of ErCl3, EuBr2 and EuCl2 Evaporation and the Structure of Molecular Forms According to High-Temperature Mass Spectrometry and Gas Electron Diffraction Data; ISU: Ivanovo, Russia, 2000; 146p. (In Russian) [Google Scholar]
- Lebedev, A. Mass Spectrometry in Organic Chemistry; BINOM: Moscow, Russia, 2003; 493p. (In Russian) [Google Scholar]
- Mori, Y.; Kitagawa, T.; Yamamoto, T.; Yanada, K.; Nagahara, S. Mass Spectrometric Studies of Some Carboxylic Acid Dimers Produced by a Nozzle Beam: HCO2H, CH3CO2H, C2H5CO2H, and n-C3H7CO2H. Bull. Chem. Soc. Jpn. 1980, 53, 3492–3495. [Google Scholar] [CrossRef] [Green Version]
- Karle, J.; Brockway, L. An Electron Diffraction Investigation of the Monomers and Dimers of Formic, Acetic and Trifluoroacetic Acids and the Dimer of Deuterium Acetate. J.Am. Chem. Soc. 1944, 66, 574–584. [Google Scholar] [CrossRef]
- Derissen, J. A reinvestigation of the molecular structure of acetic acid monomer and dimer by gas electron diffraction. J. Mol. Struct. 1971, 7, 67–80. [Google Scholar] [CrossRef]
- Giricheva, N.; Fedorov, M.; Syrbu, S.; Shpilevaya, K.; Fedoseev, O. Geometric and electronic characteristics of H-complexes based on aromatic carboxylic acids with N···H–O type hydrogen bonds. In Organic and Hybrid Nanomaterials: Preparation and Prospects of Application: Monograph; Razumova, V., Klyuev, M., Eds.; Ivanovo State University: Ivanovo, Russia, 2017; pp. 149–174. (In Russian) [Google Scholar]
- Bryan, R.F.; Hartley, P. An X-Ray Study of the p-n-Alkoxybenzoic Acids. Part V. Crystal Structures of the Nematogenic Acids Having Three and Five Alkyl-Chain Carbon Atoms. Mol. Cryst. Liq. Cryst. 1980, 62, 259–279. [Google Scholar] [CrossRef]
- Giricheva, N.; Bubnova, K.; Zhabanov, Y.; Fedorov, M.; Girichev, G. Structural and dynamic non-rigidity of hydrogen-bonded complexes of A∙∙∙A and A∙∙∙B∙∙∙A types and odd-even effect. J. Mol. Liq. 2022, 350, 118521. [Google Scholar] [CrossRef]
- Girichev, G.; Utkin, A.; Revichev, Y. Modernization of electron diffraction recorder EMR-100 for gas research. Prib. Tekh. Eksp. 1984, 27, 187–190. (In Russian) [Google Scholar]
- Girichev, G.; Shlykov, S.; Revichev, Y. Equipment for studying the structure of molecules of valence-unsaturated compounds. Prib. Tekh. Eksp. 1986, 4, 167. (In Russian) [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.02.; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Compt. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- McLean, A.; Chandler, G. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Zhurko, G.; Zhurko, D. Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 21 March 2022).
- Glendening, E.; Reed, A.; Carpenter, J.; Weinhold, F. NBO; Version 3.1; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
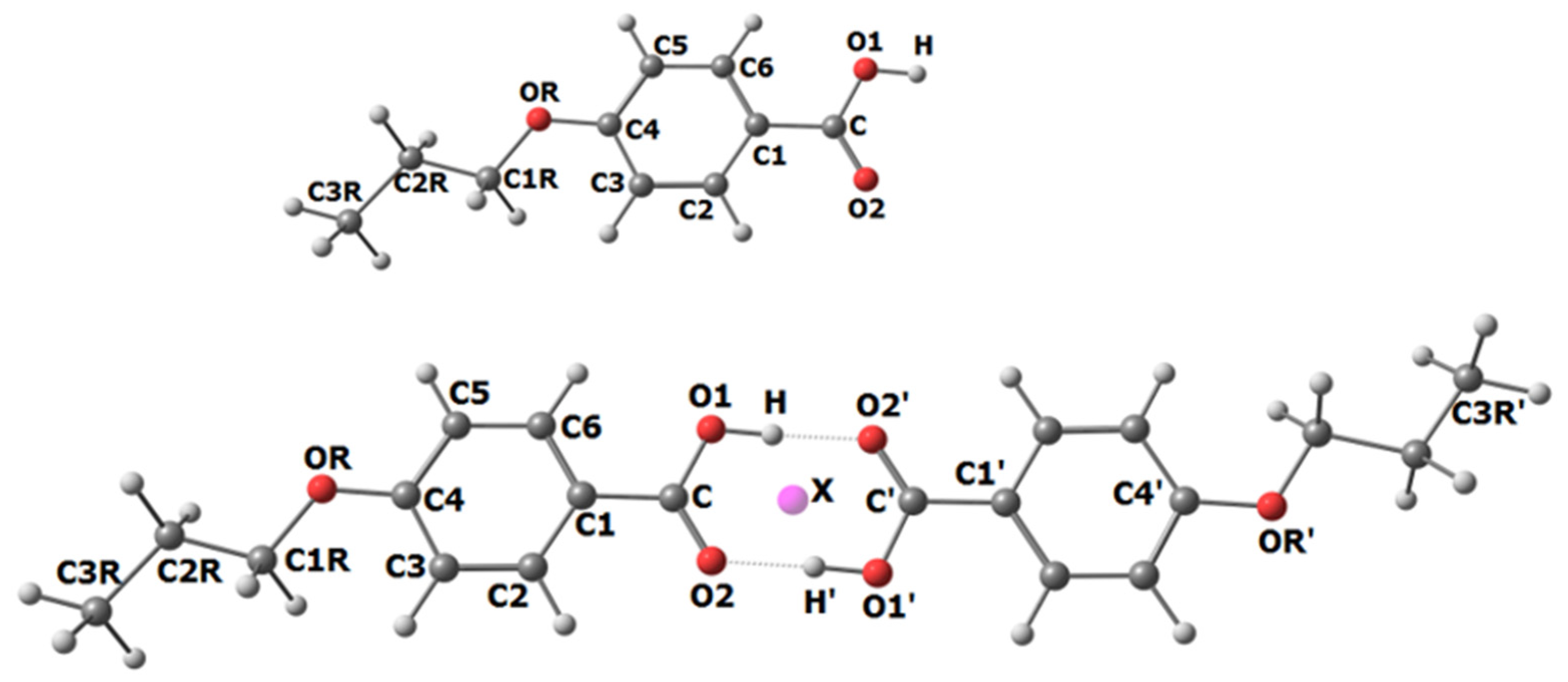




| Monomer | Dimer | |||
|---|---|---|---|---|
| Parameters a | QC re Structure | GED b rh1 Structure | QC re Structure | GED rh1 Structure |
| (C-C)av (Ph) | 1.404 | 1.405(4) p1c | 1.405 | 1.406(4) |
| C-O1 | 1.375 | 1.368(7) p2 | 1.332 | 1.325(7) |
| C=O2 | 1.219 | 1.212(7) (p2) | 1.242 | 1.234(7) |
| O1-H | 0.971 | 0.971 | 1.010 | 1.010 |
| C1-C | 1.483 | 1.484(4) (p1) | 1.484 | 1.484(4) |
| C1-C6 | 1.413 | 1.414(4) (p1) | 1.413 | 1.414(4) |
| C-Hav(Ph) | 1.087 | 1.082(5) p3 | 1.087 | 1.082(5) |
| C4-OR | 1.362 | 1.354(7) (p2) | 1.362 | 1.355(7) |
| OR-C1R | 1.443 | 1.436(7) (p2) | 1.442 | 1.435(7) |
| C1R-C2R | 1.524 | 1.525(4) (p1) | 1.524 | 1.525(4) |
| C-Hav (R) | 1.101 | 1.094(5) (p3) | 1.099 | 1.095(5) |
| O2=C-O1 | 121.6 | 121.8(30) p4 | 123.2 | 123.2(30) |
| C1-C-O1 | 112.9 | 114.4(13) p5 | 114.6 | 113.1(13) |
| H-O1-C | 105.9 | 105.9 | 110.1 | 110.1 |
| C6-C1-C | 123.0 | 122.9(4) p6 | 122.1 | 122.0(4) |
| H-C6-C1 | 119.6 | 124.6(18) p7 | 119.4 | 124.3(18) |
| C4-OR-C1R | 118.5 | 117.4(23) p8 | 118.5 | 117.4(23) |
| H-C1R-OR | 109.3 | 111.6(80) p9 | 109.4 | 111.6(80) |
| C2-C1-C | 118.3 | 116.5(25) p10 | 119.1 | 119.0(25) |
| O1-C-C1-C2 | 180.0 | 180.0 | 179.2 | 179.2 |
| C5-C4-OR-C1R | 180.0 | 180.0 | 178.6 | 178.7 |
| C3R-C2R-C1R-OR | 180.0 | 180.0 | 179.0 | 179.0 |
| H-C1R-OR-C4 | −59.2 | −59.2 | −60.0 | −62.9 |
| H-C3R-C2R-C1R | 180.0 | 180.0 | 180.0 | 180.0 |
| χ, mol.fr. | 0.80(10) | 0.20(10) | ||
| Ion Mass a.m.u. | Elemental Composition of Ion a | I, % this Work | I, % NIST |
|---|---|---|---|
| 39 | C3H3 | 10 | 22 |
| 41–43 b | C3Hn | 15 | 21 |
| 50–53 | C4Hn | 2 | 8 |
| 63–65 | C5Hn | 18 | 23 |
| 81 | C5H4OH | 2 | 7 |
| 93 | C6H4-OH | 12 | 12 |
| 121 | HOOC-C6H4 | 68 | 92 |
| 138 | HOOC-C6H4-OH | 100 | 100 |
| 180 | HOOC-C6H4-OC3H7 | 50 | 25 |
| 198 c | –COOH….HOOC–C6H4-OCH3 | 0.05 | |
| 207 | H7C3O–COOH….HOOC–OC3H7 | 0.14 | |
| 214–212 | H3CO–COOH….HOOC–C6H4-CH3 | 0.01 | |
| 221–222 | H6C3–COOH….HOOC–C6H4-CH2 -C6H4-B-C6H4-CH2- | 0.004 <0.01 | |
| 233–232 | H5C6-A-C6H4-OH | <0.01 | |
| 248 | HO-H4C6-A-C6H4-OH | 0.01 | |
| 256–258 | H7C3O–COOH….HOOC–C6H4-OCH3 | <0.01 | |
| 264 | H4C2-C6H4-B-C6H4-C2H4 | <0.01 | |
| 285 | H7C3O–COOH….HOOC–C6H4-OC3H7 | <0.01 | |
| 296 | H4C2O-C6H4-B-C6H4-OC2H4 | <0.01 | |
| 302–300 | H2CO-C6H4–COOH….HOOC–C6H4-OCH2 | <0.01 |
| POBA this Work | AA [16] a | BA [14,15] a | ||||
|---|---|---|---|---|---|---|
| Parameters | Monomer rh1 Structure T = 360 K | Dimer rh1 Structure T = 360 K | Monomer re Structure T = 296 K | Dimer re Structure T = 296 K | Monomer ra Structure T = 288 K | Monomer ra Structure T = 405 K |
| (CPh-CPh)av | 1.405(4) | 1.406(4) | - | - | 1.393(12) | 1.401(2) |
| C-O | 1.368(7) | 1.325(7) | 1.350(8) | 1.315(10) | 1.350(10) | 1.367(8) |
| C=O | 1.212(7) | 1.234(7) | 1.207(10) | 1.226(12) | 1.205(10) | 1.225(6) |
| C1Ph-C | 1.484(4) | 1.484(4) | 1.490(12) | 1.493(12) | 1.493(10) | 1.484(7) |
| C-Hav(Ph) | 1.082(5) | 1.095(5) | 1.090(17) | 1.090(17) | 1.103(10) | 1.102(8) |
| O…H | 1.565(8) | 1.657(23) | ||||
| O…O | 2.574(12) | |||||
| O–C=O | 121.8(30) | 123.2(30) | 120.5(9) | 123.0(12) | - | 120.3(31) |
| χmon | 0.80(10) | 0.69(5) | 1.0 | 1.0 | ||
| Nozzle-to-Plate Distance, mm | 338 | 598 |
|---|---|---|
| Number of recorded plates | 6 | 6 |
| Electron beam current, μA | 1.35 | 0.65 |
| Wavelength of electrons, Å | 0.03969(3) | 0.03981(4) |
| Temperature of the effusion cell, K | 360(15) | 360(15) |
| Average exposure time, s | 105 | 85 |
| Residual gas pressure in diffraction chamber, Torr | 1.3 · 10−6 | 1.8 · 10−6 |
| s-range/∆s, Å−1 | 2.6–27.0/0.1 | 1.3–15.0/0.1 |
| Ionization voltage, V | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giricheva, N.I.; Bubnova, K.E.; Krasnov, A.V.; Girichev, G.V. Cyclic Dimers of 4-n-Propyloxybenzoic Acid with Hydrogen Bonds in the Gaseous State. Int. J. Mol. Sci. 2022, 23, 15079. https://doi.org/10.3390/ijms232315079
Giricheva NI, Bubnova KE, Krasnov AV, Girichev GV. Cyclic Dimers of 4-n-Propyloxybenzoic Acid with Hydrogen Bonds in the Gaseous State. International Journal of Molecular Sciences. 2022; 23(23):15079. https://doi.org/10.3390/ijms232315079
Chicago/Turabian StyleGiricheva, Nina I., Ksenia E. Bubnova, Alexander V. Krasnov, and Georgiy V. Girichev. 2022. "Cyclic Dimers of 4-n-Propyloxybenzoic Acid with Hydrogen Bonds in the Gaseous State" International Journal of Molecular Sciences 23, no. 23: 15079. https://doi.org/10.3390/ijms232315079
APA StyleGiricheva, N. I., Bubnova, K. E., Krasnov, A. V., & Girichev, G. V. (2022). Cyclic Dimers of 4-n-Propyloxybenzoic Acid with Hydrogen Bonds in the Gaseous State. International Journal of Molecular Sciences, 23(23), 15079. https://doi.org/10.3390/ijms232315079







