Abstract
To study the distance-dependent electromagnetic field effects related to the enhancement and quenching mechanism of surface-enhanced Raman scattering (SERS) or fluorescence, it is essential to precisely control the distance from the surface of the metal nanoparticle (NP) to the target molecule by using a dielectric layer (e.g., SiO2, TiO2, and Al2O3). However, precisely controlling the thickness of this dielectric layer is challenging. Herein, we present a facile approach to control the thickness of the silica shell on silver nanoparticle-assembled silica nanocomposites, SiO2@Ag NPs, by controlling the number of reacting SiO2@Ag NPs and the silica precursor. Uniform silica shells with thicknesses in the range 5–40 nm were successfully fabricated. The proposed method for creating a homogeneous, precise, and fine silica coating on nanocomposites can potentially contribute to a comprehensive understanding of the distance-dependent electromagnetic field effects and optical properties of metal NPs.
1. Introduction
Core-shell nanomaterials comprising an inner core and an outer shell have attracted considerable attention due to their properties derived from both the core and the shell materials [1,2,3,4,5]. It has been reported that the surface reactivity [6,7,8,9], thermal stability [10,11], colloidal stability [12,13], dispersibility [14,15], absorption [16,17,18], chemical configuration transition [19,20], and releasing properties [21,22] of core-shell nanomaterials mostly depend on the chemical and physical properties of the shell material. Various encapsulation methods have been extensively studied to prepare core-shell hybrid nanomaterials with excellent functionalities [2].
Shell materials, such as metals [23,24,25], silica [26,27,28], polymers [29,30], and glucose [31,32], are often chosen to extend the applications of nanoparticles (NPs). Among these shell materials, silica has been used as an ideal material for preparing core-shell nanomaterials, for example, silica based-metals, -ceramics, -semiconductors, and -magnetics [33], due to its low cost and its excellent properties, such as easy surface modification, optical transparency, chemical inertness, biocompatibility, and easy bio-binding with functional groups [33,34].
The thickness of the silica coating on NPs considerably impacts the physico-chemical properties of core-shell NPs. Phenomena such as metal-enhanced fluorescence (MEF) and surface-enhanced Raman scattering (SERS), which occur on the surfaces of metal NPs due to the intense plasmon-induced electric field, depend strongly on the distance between the metallic surfaces and the coating molecules. Therefore, accurate and fine control of the silica coating of the nanocore particles is necessary. The shell thickness of magnetic NPs affects the distance dependent magnetic field properties of the NPs. However, previous studies on the control of silica shell thickness were mainly conducted on single metal NPs, and there are few studies on the extensive fine control and uniformity of the shell [35,36,37,38]. In addition, complicated composite NPs as a core-multi-shell NPs with multiplex functions have been studied recently. The disorder of chemical configuration during synthesis leads to a variety of core-multi-shell nanomaterials [20]. Therefore, it is also necessary to study a method for the fine-thickness-control of the coating of composite NPs rather than single metal NPs.
Our research group has developed several silver NP-assembled silica (SiO2@Ag) NPs that exhibit multiple properties, such as fluorescence, magnetism, and SERS [39,40]. The as-prepared NPs showed strong and reproducible Raman enhancement and/or fluorescence at a single NP level [41,42,43,44]. In this study, we present a new approach to obtain a fine silica shell coating on composite SiO2@Ag NPs (SiO2@Ag@SiO2). The silica shell thickness was finely controlled in the range 5–40 nm by adjusting two parameters: the number of NPs and the amount of silica precursor (sodium silicate (Na2SiO3) and tetraethylorthosilicate (TEOS)) during the silica coating process. This simple method resulted in the formation of a uniform silica shell on the surface of the SiO2@Ag NPs. This technique is expected to be useful in the optimization of the fluorescence and the SERS signal of SiO2@Ag NPs and opens up an opportunity for the fine control of the silica shell thickness of other nanocomposites.
2. Results and Discussion
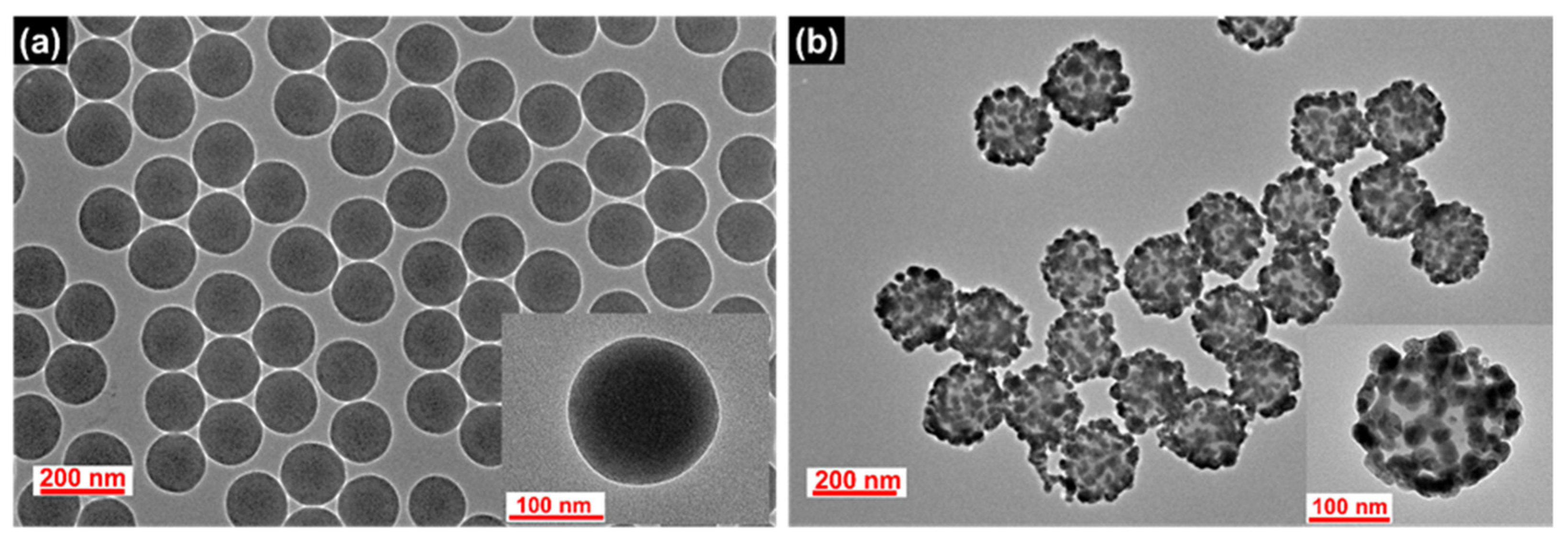
SiO2@Ag NPs were prepared as a nanocomposite prior to the silica shell coating. SiO2 NPs, a template for the deposition of Ag NPs, were synthesized by the Stöber method [45]. The surface of the SiO2 NPs was modified with thiol groups by incubating the NPs with 3-mercaptopropyl trimethoxysilane (MPTS), and then Ag NPs were introduced on the SiO2 surface by the in situ reduction of Ag+ ions with a reducing agent (octylamine). The TEM images of the SiO2 NPs and the resultant SiO2@Ag NPs are shown in Figure 1. The SiO2 and SiO2@Ag NPs were found to be homogeneous and well dispersed without aggregation. The average diameter of the SiO2 NPs is 153 ± 2.4 nm as shown in Figure 1a. After the assembling of the Ag NPs, the average diameter of SiO2@Ag NPs increased up to 192 ± 7.5 nm, and the Ag NPs with an average size of 21.5 ± 6.1 nm were densely assembled on the SiO2 surface (Figure 1b). As shown in Figure S1, the absorption intensity and the extinction maxima of the plasmonic resonance bands significantly changed after the assembly of the Ag NPs on the SiO2 NPs surface. Broad absorption in the range from 322 to 800 nm with a maximum peak at 430 nm was exhibited by the SiO2@Ag NPs suspension, indicating that Ag NPs aggregated on the surface of SiO2 NPs [24], which is consistent with the results shown in Figure 1.
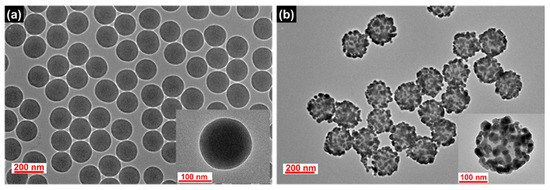
Figure 1.
TEM images of the synthesized (a) SiO2 NPs and (b) SiO2@Ag NPs.
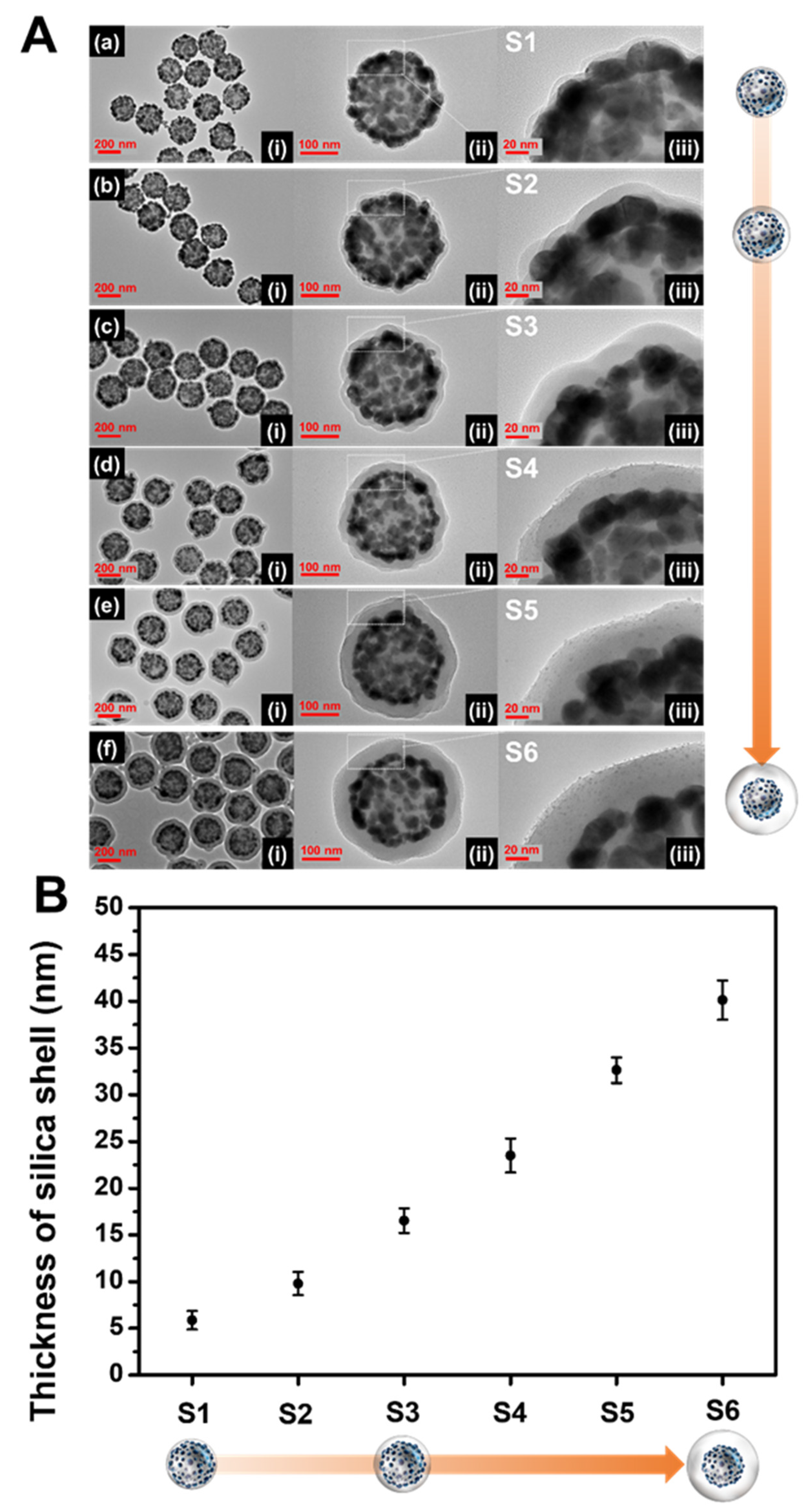
The thickness of the SiO2 shell is highly sensitive to the experimental conditions, which may generate inaccurate and unstable results. It is difficult to evenly form a silica shell with sub-nanometer thickness on the NPs because excessive shell-forming reactions should be excluded to prevent the formation of non-core silica structures such as silica NPs. In our procedure, Na2SiO3 as a silica precursor was added to the aqueous SiO2@Ag NPs solution, and then the aqueous dispersion was transferred into ethanol. The solvent exchange procedure serves to precipitate the silicate portion (monomer or oligomer) remaining in the solution due to a sharp decrease in solubility, forming a silica shell [46,47]. One would expect that a thicker silica shell would form as the amount of Na2SiO3 is increased. However, experimental results showed that the thick silica shell formation was difficult, and new non-core SiO2 NPs were generated (Figure S2). On the other hand, it was found that the number of SiO2@Ag NPs is a key parameter in controlling the thickness of the silica shell (Figure 2). The amount of SiO2@Ag NPs added was adjusted by changing the quantity of SiO2 (5, 10, and 20 mg), while the amount of Na2SiO3 was fixed at 90 µmol. The average number of SiO2 NPs per mg was calculated to be 2.01 × 1011 ± 9.35 × 109. Therefore, the average number of 5, 10, and 20 mg SiO2 are 1.01 × 1012; 2.01 × 1012, and 4.02 × 1012, respectively (Table S1). The resultant silica shell thickness of each sample was 5.8 ± 0.9 nm (S1), 9.7 ±1.2 nm (S2), and 16.5 nm ±1.3 (S3), which correspond to 4.02 × 1012 SiO2 NPs, 2.01 × 1012 SiO2 NPs, and 1.01 × 1012 SiO2 NPs, respectively (Figure 2a–c). When the number of SiO2@Ag NPs is doubled (from 1.01 × 1012 NPs (S3) to 2.01 × 1012 NPs (S2), and from 2.01 × 1012 NPs (S2) to 4.02 × 1012 NPs (S1)), the thickness of the silica shell decreased by 6.8 and 3.9 nm, respectively. Therefore, the thickness of the silica layer in S1 to S3 was observed to increase as the number of nanoparticles decreased. The shell thickness is inversely proportional to the cubic root of the number of nanoparticles as shown in the following equation:
where n is the number of mols of Na2SiO3 or TEOS, M is the molecular weight of SiO2, ρ is the density of SiO2, N is number of NPs, V is the volume of SiO2@Ag, and d is the diameter of SiO2@Ag. Indeed, the thicknesses of the silica shells of the nanoparticles (S1 to S3) were found to be consistent with the calculated silica shell values in Table S2, even though the observed values were thicker than the calculated values (~1.5–1.6 nm).
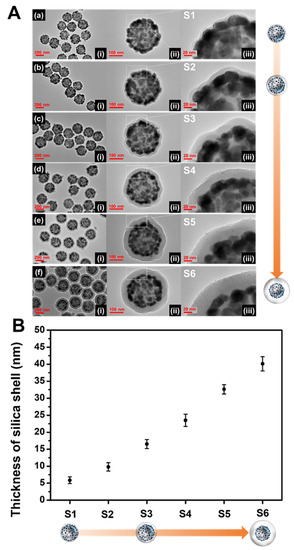
Figure 2.
(A) TEM images at (i) low and (ii, iii) high magnifications. (B) The thickness of the silica shell layer on the surface of the SiO2@Ag NPs synthesized under various conditions (a–f).
Although the thickness control of the silica shell was successful as described above, reducing the number of SiO2@Ag NPs leads to a low yield of the SiO2@Ag@SiO2 product. To generate a thick silica shell coating on the surface of SiO2@Ag NPs with a desired and reasonable product yield, a secondary silica shell precursor was added into the suspension of SiO2@Ag NPs coated with silica and Na2SiO3. Tetraethyl orthosilicate (TEOS) was used as a secondary silica shell precursor in the presence of NH4OH (as base catalyst in hydrolysis of TEOS). Homogeneous silica shells with variable thickness were grown on SiO2@Ag NPs by the addition of different volumes of TEOS. TEOS was added into the S2 suspension (SiO2@Ag NPs coated by Na2SiO3). TEOS volumes of 88.7, 177, and 266 μmol were added into the suspension, which corresponded to the final TEOS concentration of 1.16, 2.33, and 3.49 mM, respectively. Figure 2d–f shows the TEM images of the SiO2@Ag@SiO2 NPs generated by controlling the molar concentration of TEOS in the suspension. The silica shell thicknesses of the SiO2@Ag@SiO2 NPs were obtained to be 23.5 ±1.8 nm (S4), 32.6 ±1.3 nm (S5), and 40.1 ± 2.1 nm (S6). The thickness of the silica shell increased by approximately 7–9 nm as the final concentration of TEOS increased from 1.16 to 3.49 mM. The observed silica thicknesses of S4–S6 were thicker than those of the calculated values (Table S2). In particular, the ratio of the observed and calculated values of the silica shell thickness was consistent and was higher when TEOS was added to the suspension. Based on these results, we concluded that the number of SiO2@Ag NPs is effective for controlling thin silica coatings and the addition of TEOS is effective for controlling thick silica coatings of SiO2@Ag NPs. The absorbance spectra of SiO2@Ag@SiO2 NPs are shown in Figure S3. The spectra of thin silica coated SiO2@Ag NPs were insignificantly different from that of the SiO2@Ag NPs, indicating that no leakage of Ag NPs occurred from the surface of the SiO2@Ag NPs during the silica shell coating. As expected, a thin silica shell coating does not seriously affect the plasmonic resonance properties of thin shell silica coated SiO2@Ag NPs [38]. To demonstrate the stability during storage, SiO2@Ag@SiO2 NPs were stored in EtOH and water for 10 days. The shapes of both SiO2@Ag@SiO2 NPs synthesized using Na2SiO3 with or without TEOS did not show any significant differences after 10 days in water and EtOH (Figure S4). This study indicates that the SiO2@Ag@SiO2 NPs using Na2SiO3 with or without TEOS are stable when stored in water and EtOH.
3. Materials and Methods
3.1. Materials
Tetraethylorthosilicate (TEOS), ethyl alcohol (EtOH, 99.5% and 95%), 3-mercaptopropyl trimethoxysilane (MPTS), silver nitrate (AgNO3), polyvinylpyrrolidone (PVP, MW 40,000), ethylene glycol (EG), octylamine (OA), hydrochloric acid (HCl), sodium hydroxide (NaOH), and acetone were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. Aqueous ammonium hydroxide (NH4OH, 27%) was purchased from Daejung (Siheung, Gyeonggi-do, Korea). Water was purified using a Direct-Q Millipore water purification system (SAM WOO S&T Co., Ltd., Seoul, Korea).
3.2. Methods
3.2.1. Synthesis of Ag-Embedded Silica Nanoparticles (SiO2@Ag NPs)
SiO2 NPs (~153 nm) were synthesized using the modified Stöber method. TEOS (1.6 mL) was added to EtOH (40 mL) in a round flask. Then, NH4OH (3 mL) was added quickly to this solution. The mixture was vigorously stirred at 700 rpm for 20 h at room temperature (RT). Then, the mixture was centrifuged (8500 rpm, 15 min) and washed 3 times with EtOH to remove the excess reagents. After washing, the SiO2 NPs were dispersed in EtOH, and the SiO2 concentration was adjusted to 50 mg·mL−1.
To embed Ag NPs on the SiO2 surface, the SiO2 NP suspension was incubated with MPTS to transfer the hydroxyl groups on its surface to the thiol groups. In particular, the SiO2 NPs (50 mg·mL−1, 4 mL) were added to EtOH (4 mL). Then, MPTS (200 μL) and NH4OH (40 μL) were added to the solution. The suspension was vigorously stirred at 700 rpm for 12 h at RT. After the reaction, the suspension was centrifuged and washed 3 times with EtOH. The final concentration of thiolated SiO2 (SiO2-SH) was adjusted to 50 mg·mL−1.
Ag NPs were attached on the SiO2-SH by reducing AgNO3 with octylamine in EG. PVP (5 mg) was dissolved in EG (25 mL). AgNO3 (26 mg) dissolved in EG (25 mL) was suspended in this PVP solution. SiO2-SH (30 mg) was then added to this suspension Octylamine (41.4 μL) was sequentially added and the suspension was vigorously stirred at 700 rpm for 1 h at RT. Then, the suspension was centrifuged and washed 5 times with EtOH.
3.2.2. Synthesis of SiO2@Ag NPs with Silica Shells of Different Thicknesses
- Effect of the amount of the SiO2@Ag NPs on the silica shell thickness
Various amounts of the SiO2@Ag NPs (5, 10, and 20 mg) were dispersed in EtOH (1 mL). The SiO2@Ag NPs suspension was added in distilled water (15 mL) containing Na2SiO3 solution (14.4 μL). The suspension was stirred at 700 rpm for 1 h, and EtOH (60 mL) was added in the suspension, followed by stirring for 3 h. After stirring, the suspension was centrifuged at 8500 rpm for 15 min and washed by EtOH 3 times.
- Effect of the TEOS volume on silica shell thickness
SiO2@Ag NPs (10 mg) were dispersed in 1 mL of EtOH. The SiO2@Ag NPs suspension was added in distilled water (15 mL) containing Na2SiO3 solution (14.4 μL). The suspension was stirred at 700 rpm for 1 h, and EtOH (60 mL) was added in the suspension, followed by stirring for 3 h. Then, various volumes of TEOS (20, 40, and 60 μL) were added to the suspension under stirring. NH4OH (250 μL) was also added to the suspension, and the suspension was continuously stirred for 24 h. After stirring, the suspension was centrifuged at 8500 rpm for 15 min and washed 3 times with EtOH to remove excess reagents.
3.2.3. Measurement of UV-Vis Absorption Spectra
The particles were dispersed in EtOH (2 mg·mL−1) and transferred to a cuvette. UV-Vis absorption of the sample was performed using a UV-Vis spectrophotometer (Mecasys OPTIZEN POP, Daejeon, Korea).
3.2.4. Transmission Electron Microscopy (TEM) Imaging
The particles were dispersed in EtOH (2 mg·mL−1). Then, 10 μL of the sample was dropped on a copper grid (400 Mesh Cu, Pelco, Presno, CA, USA) and dried at RT. The size and morphology of the samples were observed by TEM (Libra 120, Carl Zeiss, Oberkochen, Germany).
3.2.5. Measurement of the Size of the SiO2 NPs
The size of the nanoparticles and the thickness of the SiO2 shell were analyzed by digitalized measurement using Image J software (Bethesda, MD, USA). The average size of the NPs and the thickness of the SiO2 shell were calculated after analyzing at least 60 NPs.
4. Conclusions
In summary, the silica shell thickness of SiO2@Ag NPs was finely tuned in the range 5–40 nm by adjusting the number of SiO2@Ag NPs and the TEOS concentration. The silica shell thickness of SiO2@Ag NPs was found to be inversely proportional to the number of SiO2@Ag NPs and proportional to the volume of TEOS. Thin silica shells with thickness in the range 5.8–16.5 nm were formed on the surface of SiO2@Ag NP when the number of SiO2@Ag NPs decreased from 4.02 × 1012 NPs to 1.01 × 1012 NPs. In addition, the silica shell thickness increased from 23.5 to 40.1 nm when the final TEOS concentration was increased from 1.16 to 3.49 mM. In general, we obtained SiO2@Ag@SiO2 NPs with silica shell thicknesses of 5.8 ± 0.9, 9.7 ± 1.2, 16.5 ± 1.3, 23.5 ± 1.8, 32.6 ± 1.3, and 40.1 ± 2.1 nm by adjusting the number of SiO2@Ag NPs and the silica precursor volume. As expected, a thin silica shell coating on the surface of SiO2@Ag NPs does not seriously affect the plasmonic resonance properties of SiO2@Ag NPs. The successful coating of the thin and homogenous silica shell on the surface of SiO2@Ag NP was found to provide colloidal stability to the nanocomposite. The proposed technique is expected to be useful for understanding the distance-dependent electromagnetic field effects of SERS enhancement, MEF effect, and the quenching of complex NPs in sol-phase.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222111983/s1.
Author Contributions
E.H. and B.-H.J. conceived and designed the experiments. E.H., A.J. and E.J.K. performed the experiments. E.H., A.J., E.J.K., S.B. and X.-H.P. analyzed the data. E.H., A.J., E.J.K. and X.-H.P. wrote the manuscript. B.-H.J. and H.C. edited manuscript. B.-H.J. and H.C. supervised the overall work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation (NRF) of Korea (NRF-2017H1A2A1044051-Fostering Core Leaders of the Future Basic Science Program/Global Ph.D. Fellowship Program) and by the Korean Government (MSIT) (No.2019R1G1A1100734). This study was also supported by Konkuk University (2018A0190462).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Acknowledgments
The authors are grateful for the financial support from the NRF of Korea, Korean Government (MIST) and from the Konkuk University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalambate, P.K.; Huang, Z.; Li, Y.; Shen, Y.; Xie, M.; Huang, Y.; Srivastava, A.K. Core@ shell nanomaterials based sensing devices: A review. TrAC Trends Anal. Chem. 2019, 115, 147–161. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core–shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Mahdavi, Z.; Rezvani, H.; Moraveji, M.K. Core–shell nanoparticles used in drug delivery-microfluidics: A review. RSC Adv. 2020, 10, 18280–18295. [Google Scholar] [CrossRef]
- Schärtl, W. Current directions in core–shell nanoparticle design. Nanoscale 2010, 2, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Q.; Gu, L.; Xu, S.; Wang, P.; Liu, J.; Ding, Y.; Yao, Y.F.; Nan, C.; Zhao, M. Ruthenium–platinum core–shell nanocatalysts with substantially enhanced activity and durability towards methanol oxidation. Nano Energy 2016, 21, 247–257. [Google Scholar] [CrossRef]
- Chen, T.Y.; Lin, T.L.; Luo, T.J.M.; Choi, Y.; Lee, J.F. Effects of Pt shell thicknesses on the atomic structure of Ru–Pt core–shell nanoparticles for methanol electrooxidation applications. ChemPhysChem 2010, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yang, G.; Fu, C.; Liu, Y.; Zhao, C.-X. Biomimetic core–shell silica nanoparticles using a dual-functional peptide. J. Colloid Interface Sci. 2021, 581, 185–194. [Google Scholar]
- Yang, F.; Chu, Y.; Ma, S.; Zhang, Y.; Liu, J. Preparation of uniform silica/polypyrrole core/shell microspheres and polypyrrole hollow microspheres by the template of modified silica particles using different modified agents. J. Colloid Interface Sci. 2006, 301, 470–478. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Chen, S.-Y.; Song, J.-M.; Chen, I.-G.; Lee, H.-Y. Thermal stability of Cu@Ag core–shell nanoparticles. Corros. Sci. 2013, 74, 123–129. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Huang, R.; Shao, G.-F.; Sun, S.-G. Thermal stability of Co–Pt and Co–Au core–shell structured nanoparticles: Insights from molecular dynamics simulations. J. Phys. Chem. Lett. 2017, 8, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gu, J.; Qiao, X.; Wu, H.; Fu, H.; Wang, L.; Li, H.; Ma, L. Double protected lanthanide fluorescence core@ shell colloidal hybrid for the selective and sensitive detection of ClO−. Sens. Actuators B Chem. 2019, 282, 437–442. [Google Scholar] [CrossRef]
- Szekeres, M.; Tóth, I.Y.; Illés, E.; Hajdú, A.; Zupkó, I.; Farkas, K.; Oszlánczi, G.; Tiszlavicz, L.; Tombácz, E. Chemical and colloidal stability of carboxylated core-shell magnetite nanoparticles designed for biomedical applications. Int. J. Mol. Sci. 2013, 14, 14550–14574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhao, D. Extension of the Stöber Method to Construct Mesoporous SiO2 and TiO2 Shells for Uniform Multifunctional Core–Shell Structures; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar]
- Chen, S.; Bedia, J.; Li, H.; Ren, L.Y.; Naluswata, F.; Belver, C. Nanoscale zero-valent iron@ mesoporous hydrated silica core-shell particles with enhanced dispersibility, transportability and degradation of chlorinated aliphatic hydrocarbons. Chem. Eng. J. 2018, 343, 619–628. [Google Scholar] [CrossRef]
- Isa, L.; Amstad, E.; Schwenke, K.; Del Gado, E.; Ilg, P.; Kröger, M.; Reimhult, E. Adsorption of core-shell nanoparticles at liquid–liquid interfaces. Soft Matter 2011, 7, 7663–7675. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhu, H.; Liu, G.; Tan, L.; Hu, X.; Chen, C.; Alharbi, N.S.; Hayat, T.; Tan, X. Fabrication of core–shell CMNP@ PmPD nanocomposite for efficient As (V) adsorption and reduction. ACS Sustain. Chem. Eng. 2017, 5, 4399–4407. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.-X.; Yan, X.-P. Controllable preparation of core–shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution. Chem. Commun. 2017, 53, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Langlois, C.; Li, Z.L.; Yuan, J.; Alloyeau, D.; Nelayah, J.; Bochicchio, D.; Ferrando, R.; Ricolleau, C. Transition from core–shell to Janus chemical configuration for bimetallic nanoparticles. Nanoscale 2012, 4, 3381–3388. [Google Scholar] [CrossRef] [Green Version]
- Nelli, D.; Ferrando, R. Core–shell vs. multi-shell formation in nanoalloy evolution from disordered configurations. Nanoscale 2019, 11, 13040–13050. [Google Scholar] [CrossRef]
- Narayanan, S.; Pavithran, M.; Viswanath, A.; Narayanan, D.; Mohan, C.C.; Manzoor, K.; Menon, D. Sequentially releasing dual-drug-loaded PLGA–casein core/shell nanomedicine: Design, synthesis, biocompatibility and pharmacokinetics. Acta Biomater. 2014, 10, 2112–2124. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Tan, D.W.; Yang, Y.Y. pH-triggered thermally responsive polymer core–shell nanoparticles for drug delivery. Adv. Mater. 2005, 17, 318–323. [Google Scholar] [CrossRef]
- Cha, M.G.; Kang, H.; Choi, Y.-S.; Cho, Y.; Lee, M.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D.H. Effect of alkylamines on morphology control of silver nanoshells for highly enhanced Raman scattering. ACS Appl. Mater. Interfaces 2019, 11, 8374–8381. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.; Cha, M.G.; Kang, E.J.; Pham, X.-H.; Lee, S.H.; Kim, H.-M.; Kim, D.-E.; Lee, Y.-S.; Jeong, D.-H.; Jun, B.-H. Multilayer Ag-embedded silica nanostructure as a surface-enhanced raman scattering-based chemical sensor with dual-function internal standards. ACS Appl. Mater. Interfaces 2018, 10, 40748–40755. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kang, E.; Ha, Y.N.; Lee, S.H.; Rho, W.-Y.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Gold-silver bimetallic nanoparticles with a Raman labeling chemical assembled on silica nanoparticles as an internal-standard-containing nanoprobe. J. Alloys Compd. 2019, 779, 360–366. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kim, H.-M.; Son, B.S.; Jo, A.; An, J.; Tran Thi, T.A.; Nguyen, D.Q.; Jun, B.-H. Silica-coated magnetic iron oxide nanoparticles grafted onto graphene oxide for protein isolation. Nanomaterials 2020, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Poovarodom, S.; Bass, J.D.; Hwang, S.-J.; Katz, A. Investigation of the Core− Shell Interface in Gold@ Silica Nanoparticles: A Silica Imprinting Approach. Langmuir 2005, 21, 12348–12356. [Google Scholar] [CrossRef] [Green Version]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Ren, B. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, M.; Yoon, J.A.; Gao, H.; Jin, R.; Matyjaszewski, K. One-pot synthesis of robust core/shell gold nanoparticles. J. Am. Chem. Soc. 2008, 130, 12852–12853. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Xu, Y.; Ren, Q.-G.; Xia, Y.-Y. Stable aqueous ZnO@ polymer core−shell nanoparticles with tunable photoluminescence and their application in cell imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, S.; Li, Y.; Liu, X.; Li, X.; Lin, S.; Cui, Z.-K.; Zhuang, Q. NH2-functionalized carbon-coated Fe3O4 core–shell nanoparticles for in situ preparation of robust polyimide composite films with high dielectric constant, low dielectric loss, and high breakdown strength. RSC Adv. 2016, 6, 107533–107541. [Google Scholar] [CrossRef]
- Venturelli, L.; Nappini, S.; Bulfoni, M.; Gianfranceschi, G.; Dal Zilio, S.; Coceano, G.; Del Ben, F.; Turetta, M.; Scoles, G.; Vaccari, L. Glucose is a key driver for GLUT1-mediated nanoparticles internalization in breast cancer cells. Sci. Rep. 2016, 6, 21629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef]
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core–shell quantum dots: Properties and applications. J. Alloys Compd. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Yin, S.; Sato, T. Control of silica shell thickness and microporosity of titania–silica core–shell type nanoparticles to depress the photocatalytic activity of titania. J. Colloid Interface Sci. 2006, 300, 123–130. [Google Scholar] [CrossRef]
- Tian, X.D.; Liu, B.J.; Li, J.F.; Yang, Z.L.; Ren, B.; Tian, Z.Q. SHINERS and plasmonic properties of Au Core SiO2 shell nanoparticles with optimal core size and shell thickness. J. Raman Spectrosc. 2013, 44, 994–998. [Google Scholar] [CrossRef]
- Narita, A.; Naka, K.; Chujo, Y. Facile control of silica shell layer thickness on hydrophilic iron oxide nanoparticles via reverse micelle method. Colloids Surf. A Physicochem. Eng. Asp. 2009, 336, 46–56. [Google Scholar] [CrossRef]
- Vanderkooy, A.; Chen, Y.; Gonzaga, F.; Brook, M.A. Silica shell/gold core nanoparticles: Correlating shell thickness with the plasmonic red shift upon aggregation. ACS Appl. Mater. Interfaces 2011, 3, 3942–3947. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.H.; Kim, G.; Jeong, S.; Noh, M.S.; Pham, X.H.; Kang, H.; Cho, M.H.; Kim, J.H.; Lee, Y.S.; Jeong, D.H. Silica Core-based Surface-enhanced Raman Scattering (SERS) Tag: Advances in Multifunctional SERS Nanoprobes for Bioimaging and Targeting of Biomarkers#. Bull. Korean Chem. Soc. 2015, 36, 963–978. [Google Scholar]
- Kim, H.-M.; Kim, D.-M.; Jeong, C.; Park, S.Y.; Cha, M.G.; Ha, Y.; Jang, D.; Kyeong, S.; Pham, X.-H.; Hahm, E. Assembly of plasmonic and magnetic nanoparticles with fluorescent silica shell layer for tri-functional SERS-magnetic-fluorescence probes and its bioapplications. Sci. Rep. 2018, 8, 13938. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, Y.-i.; Kang, H.; Kim, G.; Cha, M.G.; Chang, H.; Jung, K.O.; Kim, Y.-H.; Jun, B.-H.; Lee, Y.-S. Fluorescence-Raman dual modal endoscopic system for multiplexed molecular diagnostics. Sci. Rep. 2015, 5, 9455. [Google Scholar] [CrossRef] [Green Version]
- Jun, B.-H.; Kim, G.; Noh, M.S.; Kang, H.; Kim, Y.-K.; Cho, M.-H.; Jeong, D.H.; Lee, Y.-S. Surface-enhanced Raman scattering-active nanostructures and strategies for bioassays. Nanomedicine 2011, 6, 1463–1480. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Jeong, S.; Hahm, E.; Kim, J.; Cha, M.G.; Kim, K.-M.; Kang, H.; Kyeong, S.; Pham, X.-H.; Lee, Y.-S. Large scale synthesis of surface-enhanced Raman scattering nanoprobes with high reproducibility and long-term stability. J. Ind. Eng. Chem. 2016, 33, 22–27. [Google Scholar] [CrossRef]
- Hahm, E.; Kim, Y.-H.; Pham, X.-H.; Jun, B.-H. Highly reproducible surface-enhanced Raman scattering detection of alternariol using silver-embedded silica nanoparticles. Sensors 2020, 20, 3523. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Buining, P.A.; Liz-Marzán, L.M.; Philipse, A.P. A simple preparation of small, smooth silica spheres in a seed alcosol for Stöber synthesis. J. Colloid Interface Sci. 1996, 179, 318–321. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Giersig, M.; Liz-Marzán, L.M. Stabilization of CdS semiconductor nanoparticles against photodegradation by a silica coating procedure. Chem. Phys. Lett. 1998, 286, 497–501. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).