Anti-Inflammatory Effects Exerted by 14-Methoxyalternate C from Antarctic Fungal Strain Pleosporales sp. SF-7343 via the Regulation of NF-κB and JAK2/STAT3 in HaCaT Human Keratinocytes
Abstract
1. Introduction
2. Results
2.1. Determination of the Molecular Structure of the Isolated Compounds from Metabolites
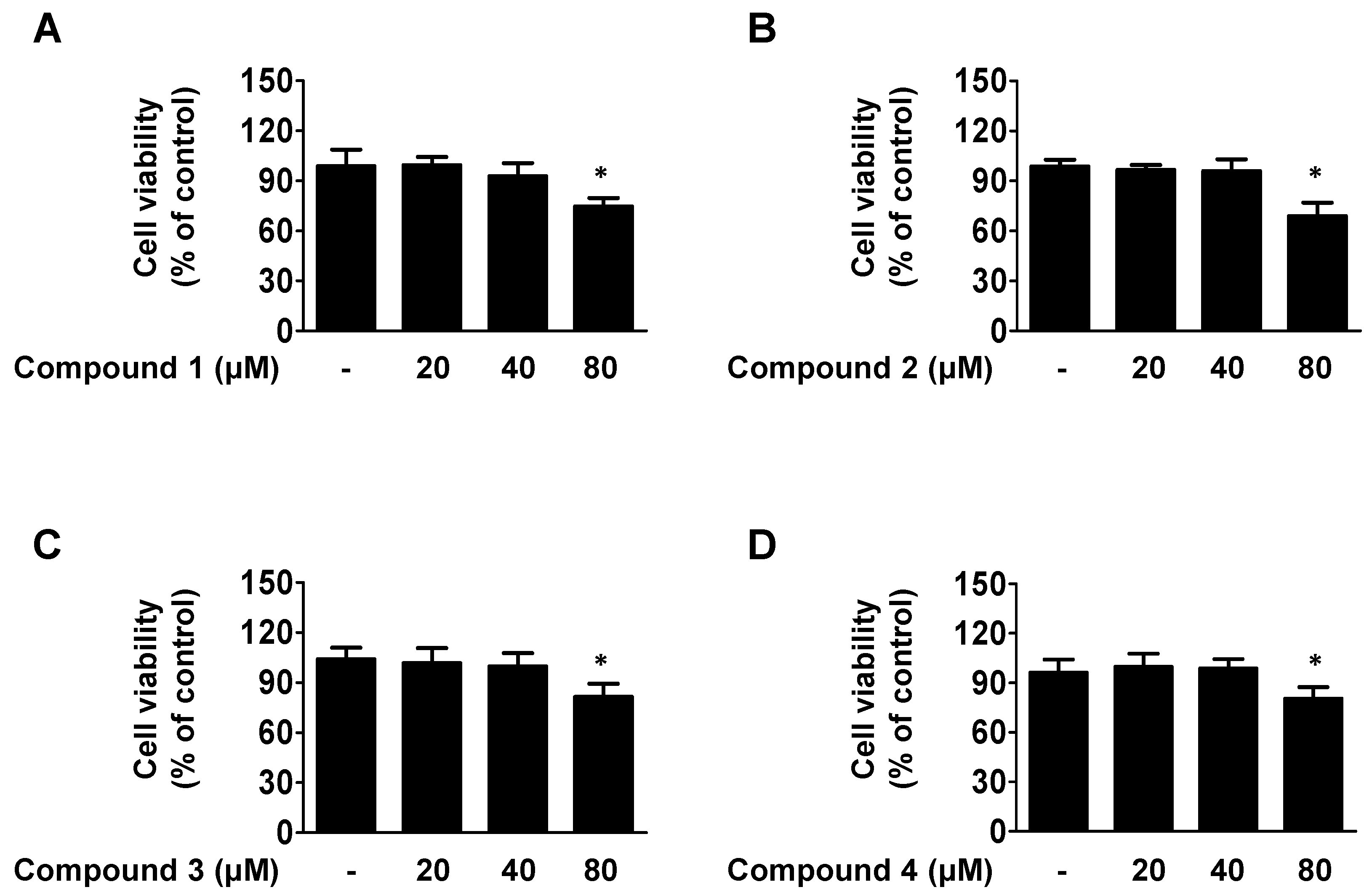
2.2. Cell Viability of Isolated Compounds 1, 2, 3, and 4 in HaCaT Cells
2.3. Inhibitory Effects of the Four Compounds on IL-6 and IL-8 Production in TNF-α/IFN-γ-Treated HaCaT Cells
2.4. Effects of Compound 1 on the Level of MDC and RANTES in TNF-α/IFN-γ-Stimulated HaCaT Cells
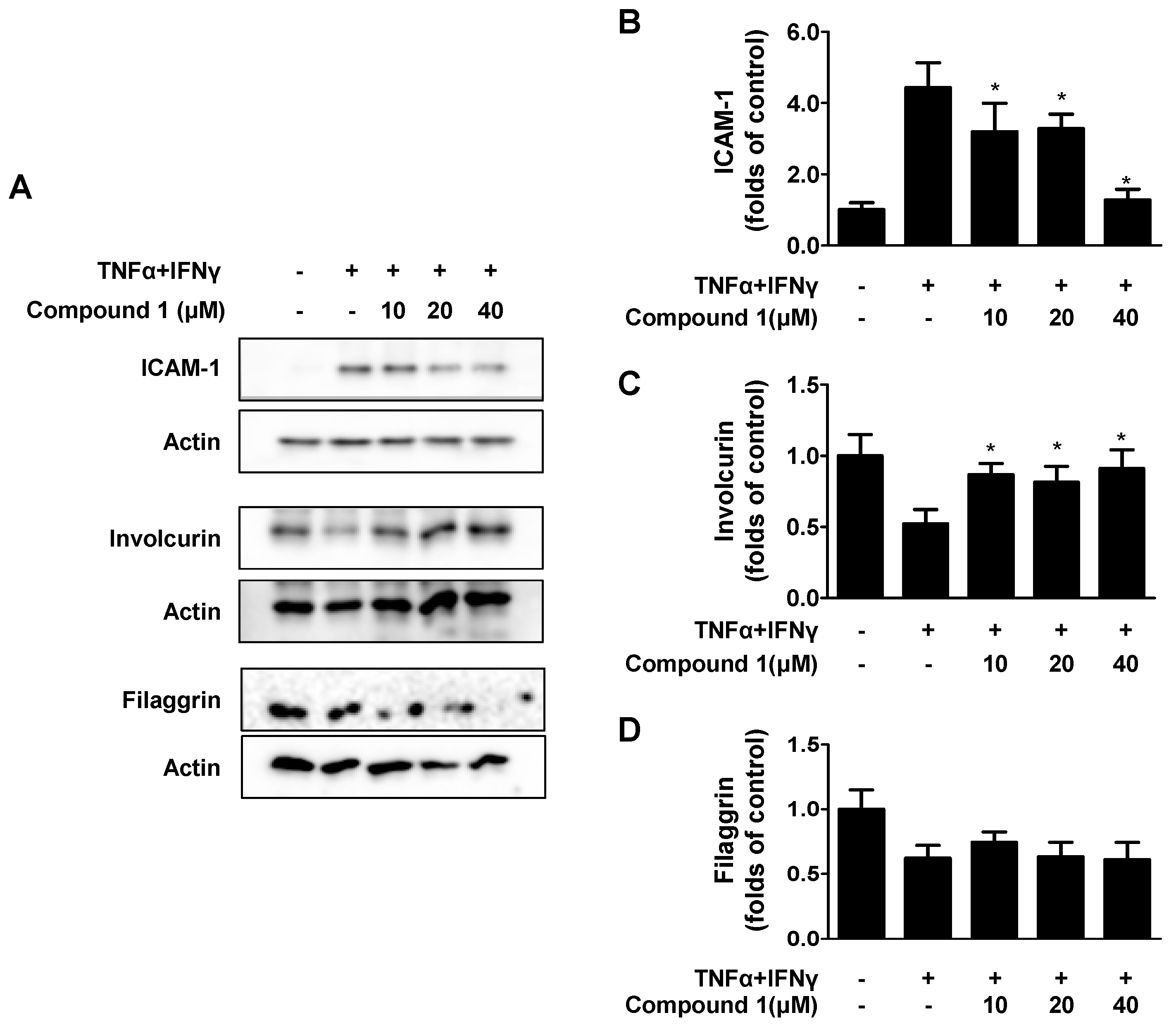
2.5. Effects of Compound 1 on TNF-α/IFN-γ-Induced ICAM-1 and on Barrier-Related Molecules, FLG/IVL in HaCaT Cells
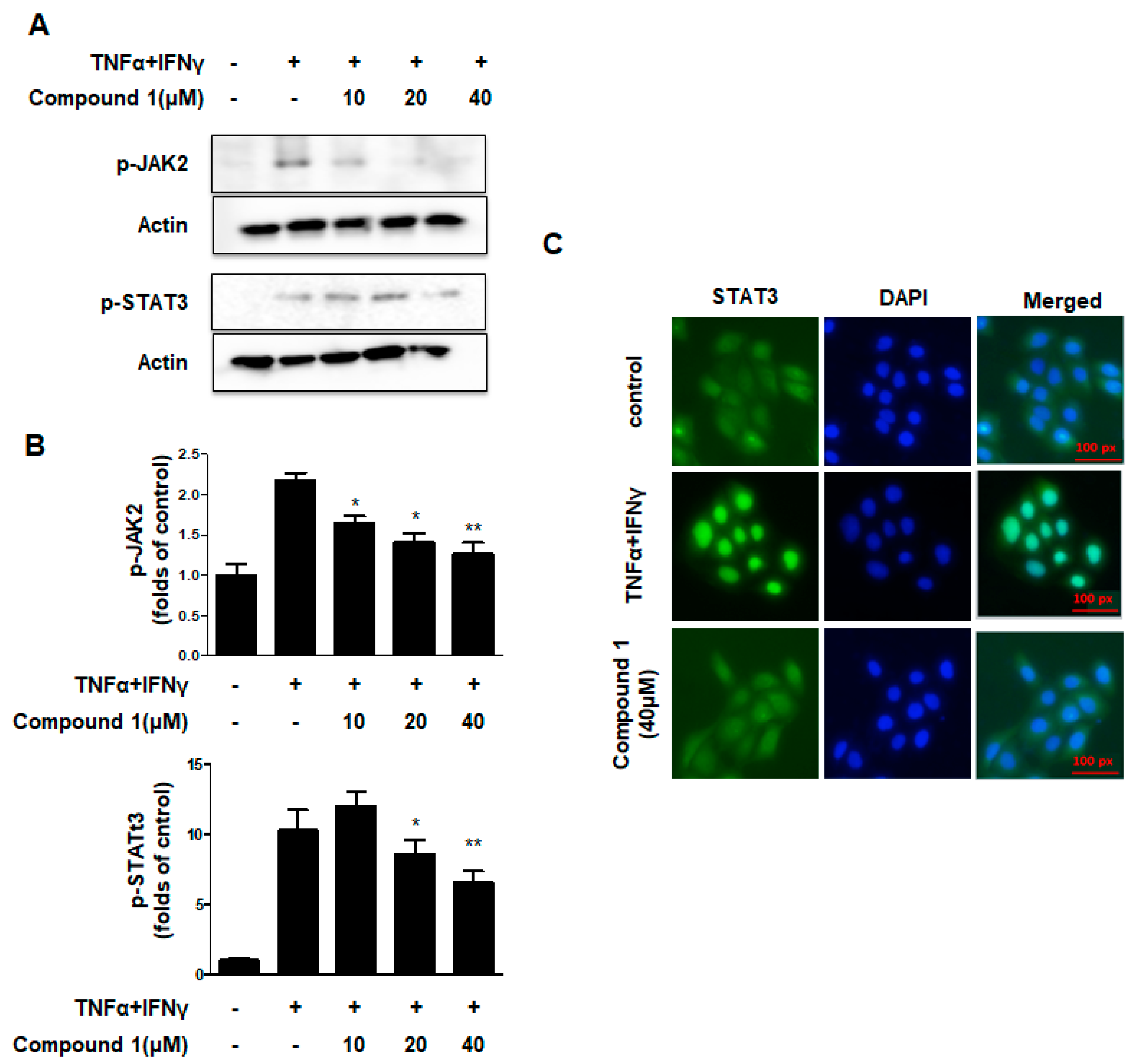
2.6. Effects of Compound 1 on the JAK2/STAT3 Signaling Pathways in HaCaT Cells
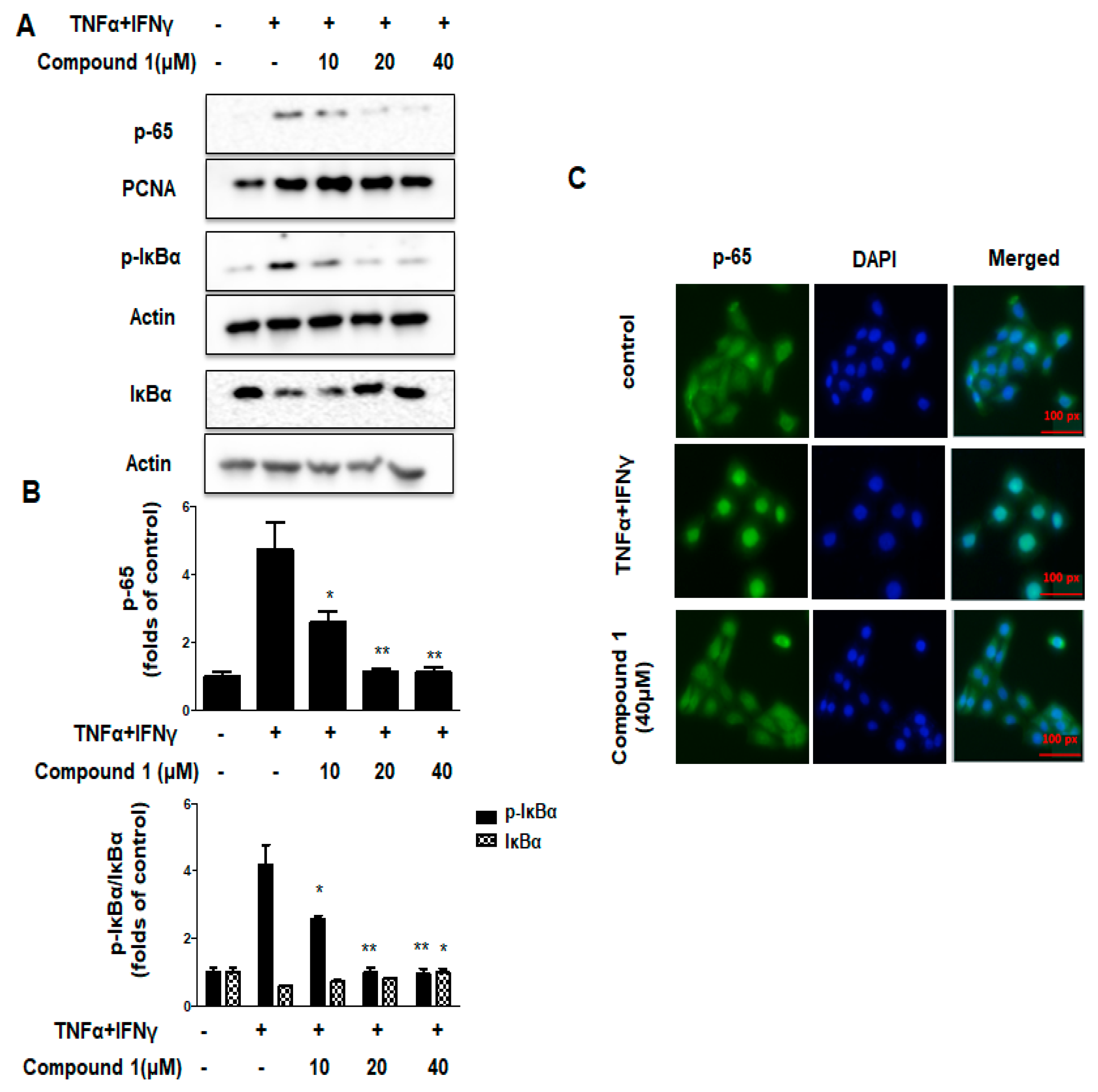
2.7. Effects of Compound 1 on NF-κB Signaling Pathways in HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation of Compound 1 and 2 from Pleosporales sp. SF-7343
4.2. Extraction and Isolation of Compound 3 from Pleosporales sp. SF-7343
4.3. Extraction and Isolation of Compound 4 from Pleosporales sp. SF-7343
4.4. Cell Culture and Reagents
4.5. MTT Assay
4.6. Measurement of Cytokines and Chemokines
4.7. Extraction of Total, Nuclear, and Cytosolic Protein
4.8. Western Blot Analysis
4.9. Immunofluorescence
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berke, R.; Singh, A.; Guralnick, M. Atopic dermatitis: An overview. Am. Fam. Physician 2016, 86, 35–42. [Google Scholar]
- Torres, T.; Ferreira, E.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on atopic dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Patruno, C.; Potestio, L.; Napolitano, M. Clinical phenotypes of adult atopic dermatitis and related therapies. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mounika, P.; Yellurkar, M.L.; Prasanna, V.S.; Sarkar, S.; Velayutham, R.; Arumugam, S. Keratinocytes: An Enigmatic Factor in Atopic Dermatitis American family physician. Cells 2022, 11, 1683. [Google Scholar] [CrossRef]
- Kleinman, E.; Laborada, J.; Metterle, L.; Eichenfield, L.F. What’s New in Topicals for Atopic Dermatitis? Am. J. Clin. Dermatol. 2022, 23, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.H.; Lee, S.; Choi, Y.A.; Song, K.S.; Kim, S.H. Inhibitory effects of euscaphic acid in the atopic dermatitis model by reducing skin inflammation and intense pruritus. Inflammation 2022, 45, 1680–1691. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Z.; Yang, W.; Loo, S.K.F.; Ip, S.P.; Xian, Y.F.; Lin, Z.X. Anti-atopic dermatitis effect of a modified Huang-Lian-Jie-Du decoction and its active fraction on 2, 4-dinitrobenzene and MC903-induced mouse models. Phytomedicine 2022, 104, 154346. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Liou, C.J.; Shen, S.C.; Hu, S.; Chao, J.C.; Huang, C.H.; Wu, S.J. Oleuropein attenuates inflammation and regulates immune responses in a 2, 4-dinitrochlorobenzene-induced atopic dermatitis mouse model. Asian Pac. J. Allergy Immunol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, R.; Wang, M.; Zhai, L.; Liu, J.; Xu, X.; Zhao, D. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J. Ginseng Res. 2022, 46, 115–125. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic dermatitis: Pathophysiology. Adv Exp Med Biol. 2017, 1027, 21–37. [Google Scholar]
- Sugumaran, A.; Pandiyan, R.; Kandasamy, P.; Antoniraj, M.G.; Navabshan, I.; Sakthivel, B.; Ngamcharussrivichai, C. Marine biome-derived secondary metabolites, a class of promising antineoplastic agents: A systematic review on their classification, mechanism of action and future perspectives. Sci. Total Environ. 2022, 155445. [Google Scholar] [CrossRef]
- Sureshkumar, S.; Merlin, I.; Prasai, J.R.; Rajapriya, P.; Pandi, M. Antioxidant, antibacterial, cytotoxicity, and phytochemical potentials of fungal bioactive secondary metabolites. J. Basic Microbiol. 2022, 9. [Google Scholar] [CrossRef]
- Xu, T.C.; Song, Z.Q.; Hou, Y.; Liu, S.S.; Li, X.P.; Yang, Q.R.; Wu, S.H. Secondary metabolites of the genus Nigrospora from terrestrial and marine habitats: Chemical diversity and biological activity. Fitoterapia 2022, 9, 161. [Google Scholar] [CrossRef]
- Wang, J.T.; Ma, Z.H.; Wang, G.K.; Xu, F.Q.; Yu, Y.; Wang, G.; Peng, D.Y.; Liu, J.S. Chemical constituents from plant endophytic fungus Alternaria alternate. Nat. Prod. Res. 2021, 35, 1199–1206. [Google Scholar] [CrossRef]
- Wang, Q.X.; Bao, L.; Yang, X.L.; Guo, H.; Yang, R.N.; Ren, B.; Zhang, L.X.; Dai, H.Q.; Guo, L.D.; Liu, H.W. Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp. Fitoterapia 2012, 83, 209–214. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, G.; Wang, H.Y.; Liu, J.; Li, X.B.; Zhang, L.L.; Zhao, Z.T.; Lou, H.X. New metabolites from endolichenic fungus Pleosporales sp. Chem. Biodivers. 2015, 12, 1095–1104. [Google Scholar] [CrossRef]
- Souza, G.D.; Mithöfer, A.; Daolio, C.; Schneider, B.; Rodrigues-Filho, R. Identification of Alternaria alternate mycotoxins by LC-SPE-NMR and their cytotoxic effects to soybean (Glycine max) cell suspension culture. Molecules 2013, 18, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Linse, F.; Heller, R.; Moths, C.; Goebel, R.; Neumann, C.H. Adhesion molecules in atopic dermatitis: VCAM-1 and ICAM-1 expression is increased in healthy-appearing skin. Allergy 1996, 51, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Qinwufeng, G.; Jiacheng, L.; Xiaoling, L.; Tingru, C.; Yunyang, W.; Yanlong, Y. Jiu-Wei-Yong-An Formula suppresses JAK1/STAT3 and MAPK signaling alleviates atopic dermatitis-like skin lesions. J. Ethnopharmacol. 2022, 295, 115428. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.; Goleva, E.; Bissonnette, R.; Bronova, I.; Bronoff, A.S.; Richers, B.N.; Leung, D.Y. Dupilumab significantly improves skin barrier function in patients with moderate-to-severe atopic dermatitis. Allergy 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B. Dupilumab for Atopic Dermatitis—From Clinical Trials to Molecular and Cellular Mechanisms. Dermatitis 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, E.H.; Lee, B.; Noh, H.M.; Park, S.; Lee, Y.M.; Park, M.C. Soshiho-Tang, a traditional herbal medicine, alleviates atopic dermatitis symptoms via regulation of inflammatory mediators. Front. Pharmacol. 2019, 10, 742. [Google Scholar] [CrossRef]
- Pietra, F. Secondary metabolites from marine microorganisms: Bacteria, protozoa, algae and fungi. Achievements and prospects. Nat. Prod. Rep. 1997, 14, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Tsiogka, A.; Kyriazopoulou, M.; Kontochristopoulos, G.; Nicolaidou, E.; Stratigos, A.; Rigopoulos, D.; Gregoriou, S. The JAK/STAT Pathway and Its Selective Inhibition in the Treatment of Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2022, 11, 4431. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jung, E.; Yeo, H.; Ahn, S.S.; Lim, Y.; Lee, Y.H. The Natural Janus Kinase Inhibitor Agerarin Downregulates Interleukin-4-Induced PER2 Expression in HaCaT Keratinocytes. Molecules 2022, 27, 4205. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Yeo, H.; Jung, E.; Lim, Y.; Lee, Y.H.; Shin, S.Y. FRA1: C-JUN: HDAC1 complex down-regulates filaggrin expression upon TNFα and IFNγ stimulation in keratinocytes. Proc. Natl. Acad. Sci. USA 2022, 119, e2123451119. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 Inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov. 2022, 2, 100131. [Google Scholar] [CrossRef]
- Liu, G.T.; Li, Y.L.; Wang, J.; Dong, C.Z.; Deng, M.; Tai, M.; Chen, H.X. Improvement of Skin Barrier Dysfunction by Phenolic-containing Extracts of Lycium barbarum via Nrf2/HO-1 Regulation. Photochem. Photobiol. 2022, 98, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Song, H.K.; Park, S.H.; Jang, S.; Park, K.S.; Song, K.H.; Kim, T. Terminalia chebula Retz. extract ameliorates the symptoms of atopic dermatitis by regulating anti-inflammatory factors in vivo and suppressing STAT1/3 and NF-κB signaling in vitro. Phytomedicine 2022, 104, 154318. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.J.; Huang, J.Y.; Li, S.P.; Gong, X.P.; Sun, J.B.; Mao, W.; Guo, S.N. Portulaca oleracea L. extracts alleviate 2, 4-dinitrochlorobenzene-induced atopic dermatitis in mice. Front. Nutr. 2022, 9, 1873. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Lee, W.H.; Kim, J.K.; Jeon, H.K.; Lee, J.; Kim, Y.J. Polyopes affinis Suppressed IFN-γ-and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways. Molecules 2022, 27, 1836. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Han, B.; Chen, Y.; Bai, X.; Yu, S.; Liu, M. Huanglian ointment alleviates eczema by maintaining the balance of c-Jun and JunB and inhibiting AGE-RAGE-mediated pro-inflammation signaling pathway. Phytomedicine 2022, 105, 154372. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.S.; Kim, H.J.; Cao, T.Q.; Liu, Z.H.; Lee, H.; Ko, W.M.; Kim, Y.C.; Sohn, J.H.; Kim, T.K.; Yim, J.H.; et al. Anti-Inflammatory Effects of Metabolites from Antarctic Fungal Strain Pleosporales sp. SF-7343 in HaCaT Human Keratinocytes. Int. J. Mol. Sci. 2021, 22, 9674. [Google Scholar] [CrossRef] [PubMed]
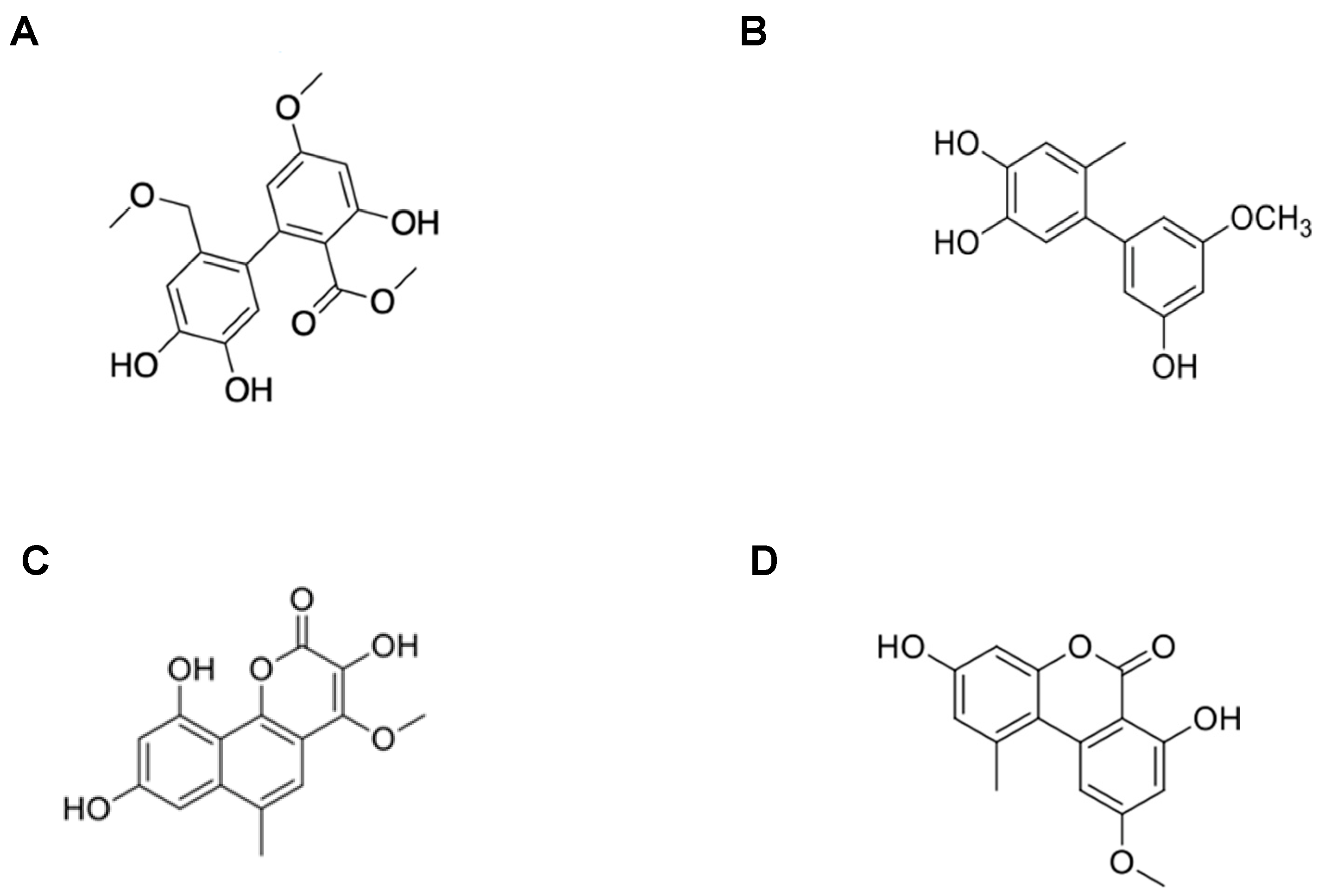

| Compound | Name | Rate of Inhibition (%) | |
|---|---|---|---|
| IL-8 | IL-6 | ||
| Compound 1 Compound 2 Compound 3 Compound 4 | 14-methoxyalternate C 5′-methoxy-6-methyl-biphenyl-3,4,3’-triol 3,8,10-trihydroxy-4-methoxy-6-methylbenzocoumarin Altenuene | 76.74 ± 5.38 a 45.90 ± 3.66 a 44.59 ± 9.58 a - | 55.19 ± 4.89 a - - - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Cao, T.Q.; Liu, Z.; Tuan, N.Q.; Kim, Y.-C.; Sohn, J.H.; Yim, J.H.; Lee, D.-S.; Oh, H. Anti-Inflammatory Effects Exerted by 14-Methoxyalternate C from Antarctic Fungal Strain Pleosporales sp. SF-7343 via the Regulation of NF-κB and JAK2/STAT3 in HaCaT Human Keratinocytes. Int. J. Mol. Sci. 2022, 23, 14642. https://doi.org/10.3390/ijms232314642
Dong L, Cao TQ, Liu Z, Tuan NQ, Kim Y-C, Sohn JH, Yim JH, Lee D-S, Oh H. Anti-Inflammatory Effects Exerted by 14-Methoxyalternate C from Antarctic Fungal Strain Pleosporales sp. SF-7343 via the Regulation of NF-κB and JAK2/STAT3 in HaCaT Human Keratinocytes. International Journal of Molecular Sciences. 2022; 23(23):14642. https://doi.org/10.3390/ijms232314642
Chicago/Turabian StyleDong, Linsha, Thao Quyen Cao, Zhiming Liu, Nguyen Quoc Tuan, Youn-Chul Kim, Jae Hak Sohn, Joung Han Yim, Dong-Sung Lee, and Hyuncheol Oh. 2022. "Anti-Inflammatory Effects Exerted by 14-Methoxyalternate C from Antarctic Fungal Strain Pleosporales sp. SF-7343 via the Regulation of NF-κB and JAK2/STAT3 in HaCaT Human Keratinocytes" International Journal of Molecular Sciences 23, no. 23: 14642. https://doi.org/10.3390/ijms232314642
APA StyleDong, L., Cao, T. Q., Liu, Z., Tuan, N. Q., Kim, Y.-C., Sohn, J. H., Yim, J. H., Lee, D.-S., & Oh, H. (2022). Anti-Inflammatory Effects Exerted by 14-Methoxyalternate C from Antarctic Fungal Strain Pleosporales sp. SF-7343 via the Regulation of NF-κB and JAK2/STAT3 in HaCaT Human Keratinocytes. International Journal of Molecular Sciences, 23(23), 14642. https://doi.org/10.3390/ijms232314642






