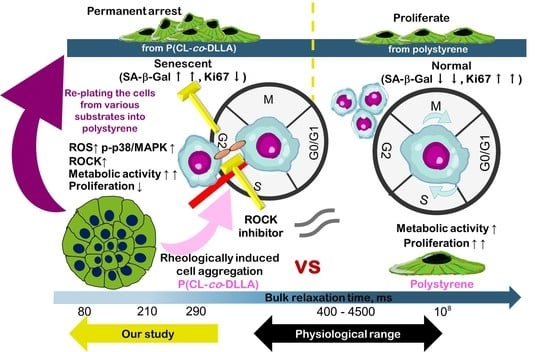
Viscoelastic Liquid Matrix with Faster Bulk Relaxation Time Reinforces the Cell Cycle Arrest Induction of the Breast Cancer Cells via Oxidative Stress
Abstract
1. Introduction
2. Results
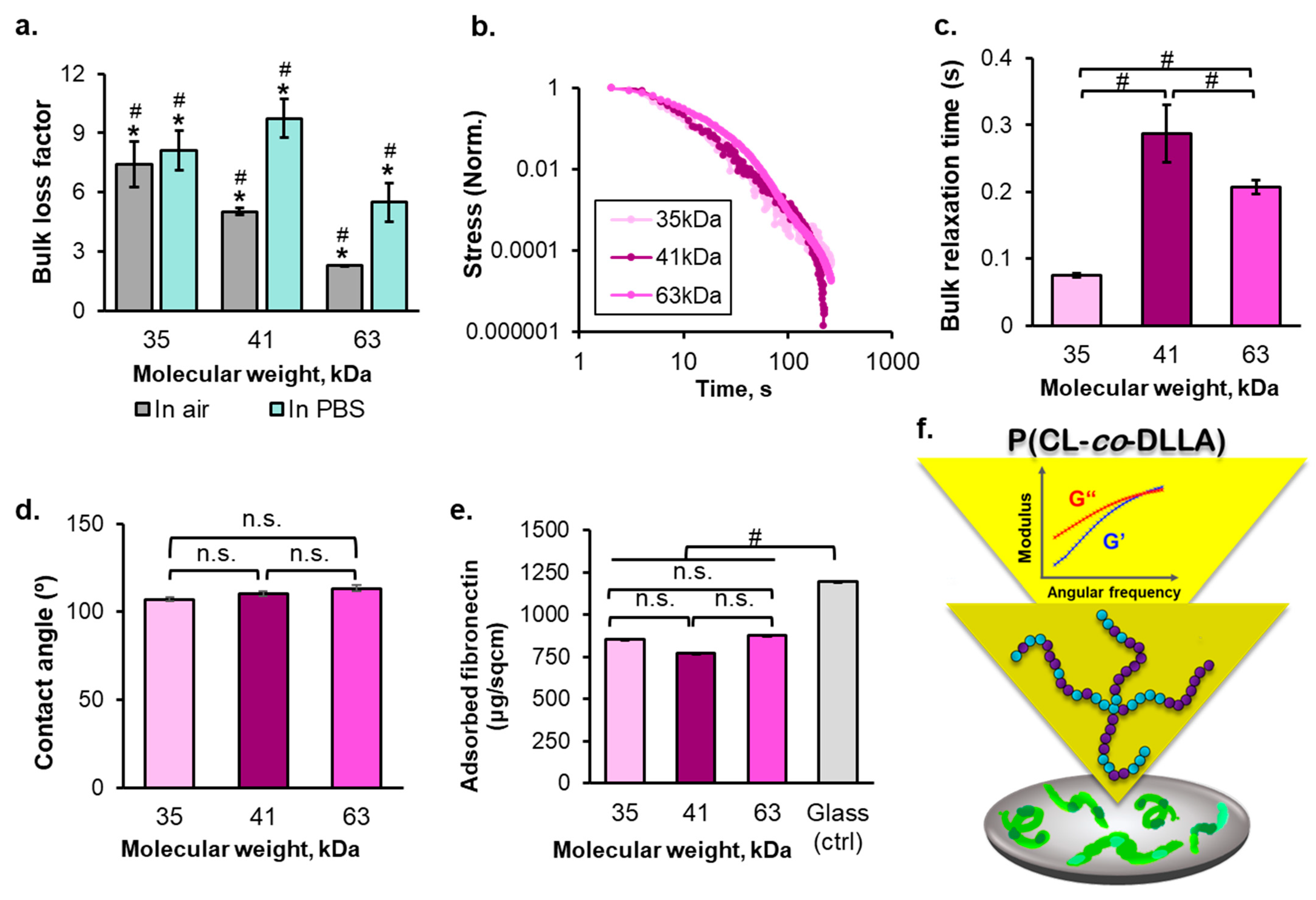
2.1. The Molecular Weight-Dependent Rheology and Surface Properties of P(CL-co-DLLA) Substrates
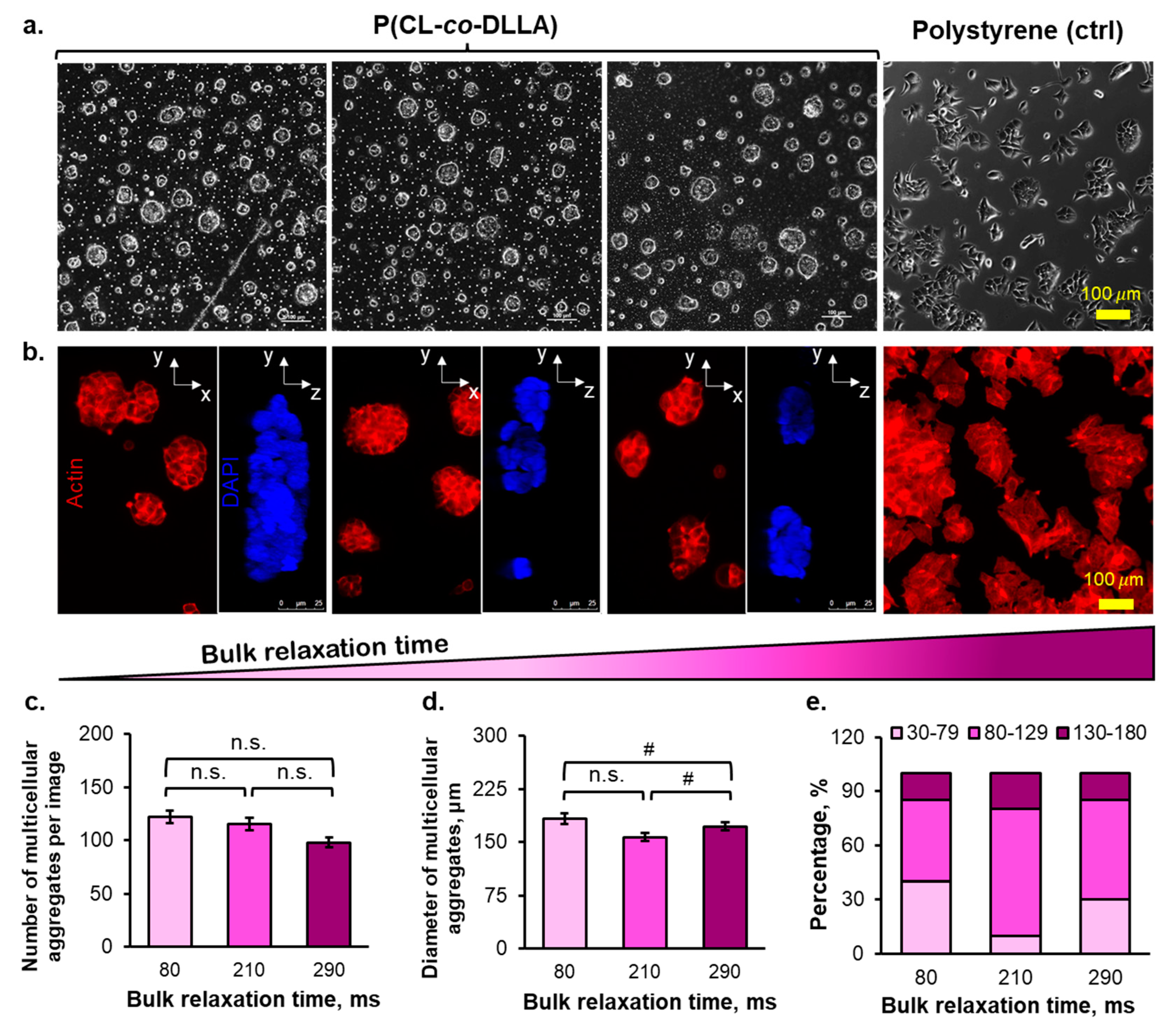
2.2. The Morphology of Multicellular Aggregates of Breast Cancer Cells in P(CL-co-DLLA) Substrates
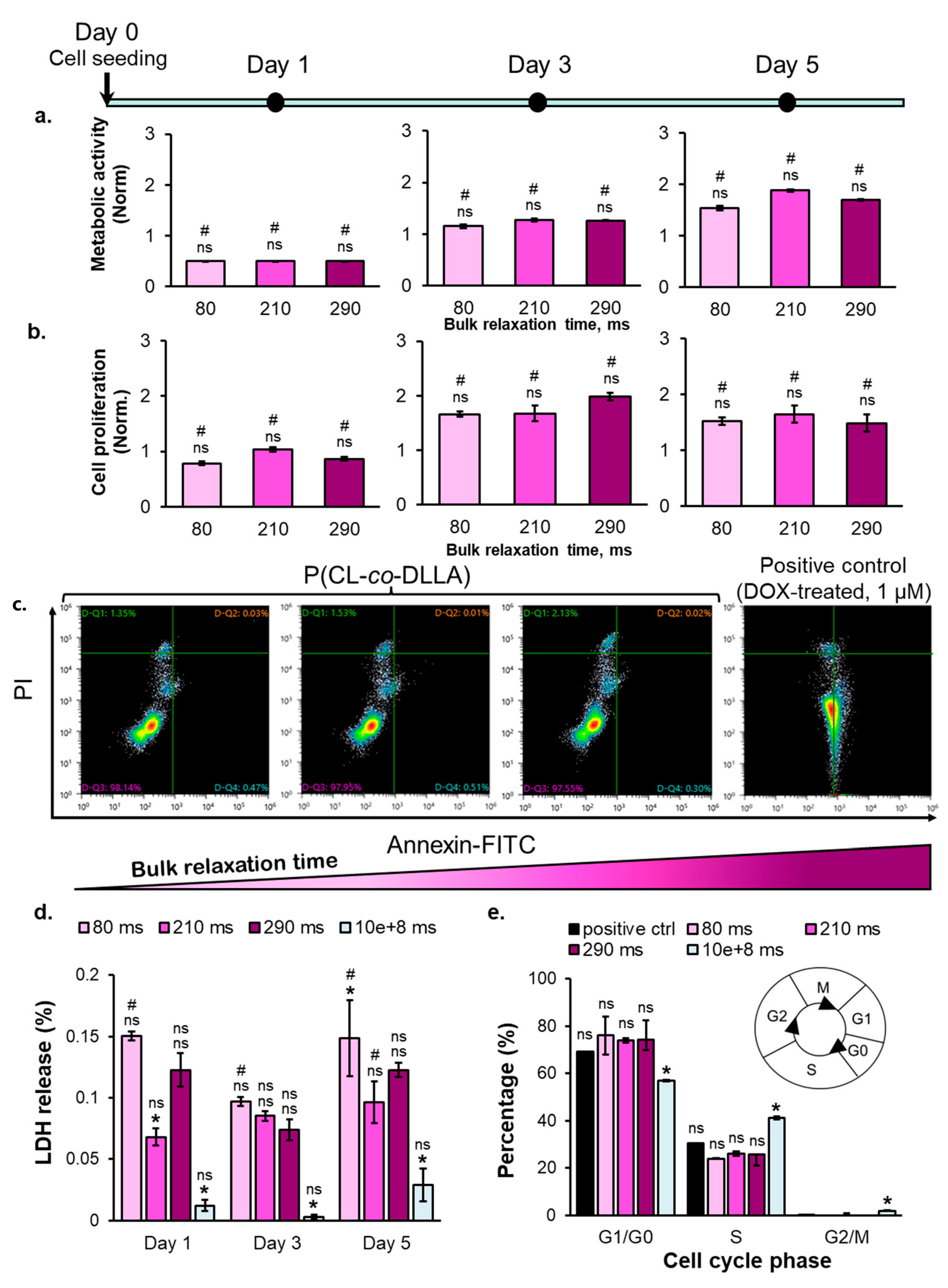
2.3. The Correlation between Substrate Bulk Stress Relaxation and the Metabolic Activity, Proliferation, Viability, and Cell Cycle of the Breast Cancer Cells
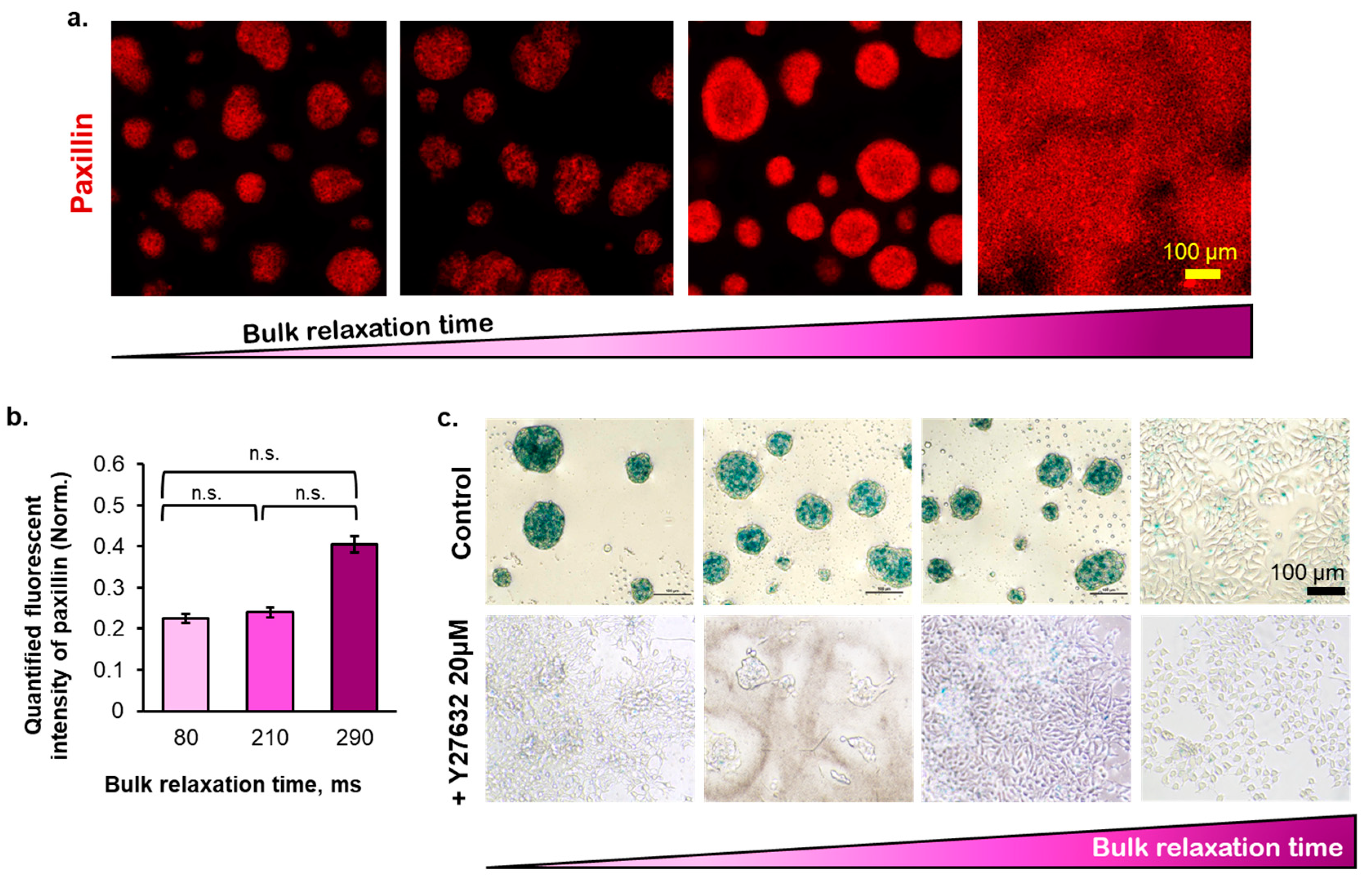
2.4. The Effect of Substrate Bulk Stress Relaxation on the Generation of Reactive Oxygen Species (ROS) in the Multicellular Aggregates of the Breast Cancer Cells
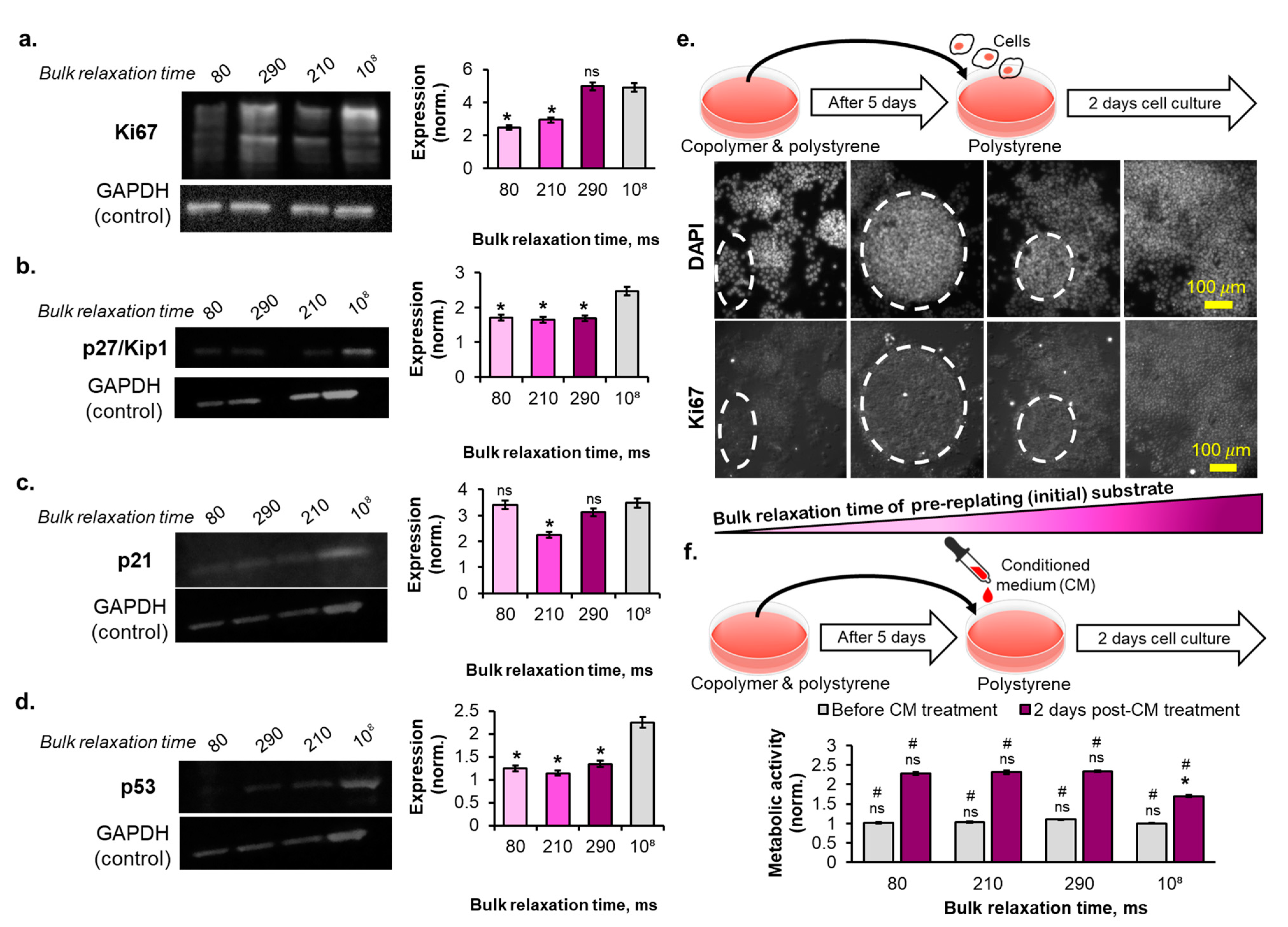
2.5. The Reversibility of the Breast Cancer Cells Senescence Fate Induced on the Copolymer Substrates
3. Discussion
4. Materials and Methods
4.1. Substrate Preparation
4.2. The Wettability and Surface Morphology of the Substrates
4.3. Protein Adsorption of the Substrates
4.4. Bulk Mechanical Characterization of the Substrates
4.5. Fluorescence Recovery after Photobleaching (FRAP) Assay
4.6. Surface Mechanical Properties of the Fluidic Substrates
4.7. Evaluation of the Fluidic Substrate Thickness
4.8. Cell Culture
4.9. Cell Metabolic Activity Assay
4.10. Cell Proliferation Assay
4.11. Cell Death Assay
4.12. Cell Cycle Assay
4.13. Immunofluorescence Analysis
4.14. Western Blot
4.15. Senescent-Associated-β-Galactosidase Staining and Quantification
4.16. Inhibition Study
4.17. Cell Replating Experiment
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The World Health Organization 2021. Breast Cancer World Health Organization Website. Available online: https://www.who.int/news-room/factsheets/detail/breast-cancer (accessed on 10 August 2022).
- Leal, J.; Smyth, H.D.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Nissan, N.; Furman-Haran, E.; Shapiro-Feinberg, M.; Grobgeld, D.; Degani, H. Diffusion-Tensor MR Imaging of the Breast: Hormonal Regulation. Radiology 2014, 271, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Akkus, O. Effects of age and shear rate on the rheological properties of human yellow bone marrow. Biorheology 2011, 48, 89–97. [Google Scholar] [CrossRef]
- Bloomfield, I.; Johnston, I.; Bilston, L. Effects of Proteins, Blood Cells and Glucose on the Viscosity of Cerebrospinal Fluid. Pediatr. Neurosurg. 1998, 28, 246–251. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Liu, Y.; Du, Y.; Yang, C.; Gao, F. Reduced hyaluronan cross-linking induces breast cancer malignancy in a CAF-dependent manner. Cell Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, S.; Werner, C.; Fratzl, P.; Cipitria, A. Microenvironment-mediated cancer dormancy: Insights from metastability theory. Proc. Natl. Acad. Sci. USA 2022, 119, e2111046118. [Google Scholar] [CrossRef] [PubMed]
- Perego, M.; Tyurin, V.A.; Tyurina, Y.Y.; Yellets, J.; Nacarelli, T.; Lin, C.; Nefedova, Y.; Kossenkov, A.; Liu, Q.; Sreedhar, S.; et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci. Transl. Med. 2020, 12, eabb5817. [Google Scholar] [CrossRef]
- Park, S.-Y.; Nam, J.-S. The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 2020, 52, 569–581. [Google Scholar] [CrossRef]
- Narkhede, A.A.; Crenshaw, J.H.; Crossman, D.K.; Shevde, L.A.; Rao, S.S. An in vitro hyaluronic acid hydrogel based platform to model dormancy in brain metastatic breast cancer cells. Acta Biomater. 2020, 107, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Short, S.; Fielder, E.; Miwa, S.; von Zglinicki, T. Senolytics and senostatics as adjuvant tumour therapy. eBioMedicine 2019, 41, 683–692. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Mano, S.S.; Uto, K.; Ebara, M. Material-induced Senescence (MIS): Fluidity induces senescent type cell death of lung cancer cells via insu-lin-like growth factor binding protein 5. Theranostics 2017, 7, 4658. [Google Scholar] [CrossRef] [PubMed]
- Najmina, M.; Uto, K.; Ebara, M. Fluidic substrate as a tool to probe breast cancer cell adaptive behavior in response to fluidity level. Polym. J. 2020, 52, 985–995. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2015, 15, 326–334. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Gupta, A.; Najibi, A.J.; Seo, B.R.; Garry, R.; Darnell, M.; Gu, W.; Zhou, Q.; Weitz, D.A.; Mahadevan, L.; et al. Matrix Viscoelasticity Controls Spatio-Temporal Tissue Organization. Available online: https://www.biorxiv.org/content/10.1101/2022.01.19.476771v1 (accessed on 1 October 2022).
- Lewis, D.M.; Pruitt, H.; Jain, N.; Ciccaglione, M.; McCaffery, J.M.; Xia, Z.; Weber, K.; Eisinger-Mathason, T.K.; Gerecht, S. A feedback loop between hypoxia and matrix stress relaxation increases oxygen-axis migration and me-tastasis in sarcoma. Cancer Res. 2019, 79, 1981–1995. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Sidi, A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J.; Francisco, S. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6364. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Gupta, V.K.; Lee, H.-P.; Lee, J.Y.; Wisdom, K.M.; Varma, S.; Flaum, E.M.; Davis, C.; West, R.B.; Chaudhuri, O. Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27 Kip1 signaling axis. Sci. Adv. 2019, 5, eaaw6171. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.; Uto, K.; Homma, K.; Nakanishi, J. Viscoelastically tunable substrates elucidate the interface-relaxation-dependent adhesion and assembly behaviors of epithelial cells. Biomaterials 2021, 274, 120861. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Bennett, M.; Cantini, M.; Reboud, J.; Cooper, J.M.; Roca-Cusachs, P.; Salmeron-Sanchez, M. Molecular clutch drives cell response to surface viscosity. Proc. Natl. Acad. Sci. USA 2018, 115, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Papalazarou, V.; Salmeron-Sanchez, M.; Machesky, L.M. Tissue engineering the cancer microenvironment—Challenges and opportunities. Biophys. Rev. 2018, 10, 1695–1711. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhang, M.; Hu, J.; Cao, C.; Gu, Q.; Liu, Y.; Li, X.; Xu, D.; Le Ying, L.; Yang, Y.; et al. The kinase activity of integrin-linked kinase regulates cellular senescence in gastric cancer. Cell Death Dis. 2022, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Almasabi, S.; Ahmed, A.U.; Boyd, R.; Williams, B.R. A potential role for integrin-linked kinase in colorectal cancer growth and progression via regulating se-nescence and immunity. Front. Genet. 2021, 12, 638558. [Google Scholar] [CrossRef]
- Pradhan, S.; Slater, J.H. Tunable hydrogels for controlling phenotypic cancer cell states to model breast cancer dormancy and reactivation. Biomaterials 2019, 215, 119177. [Google Scholar] [CrossRef] [PubMed]
- Dufort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef]
- Kyykallio, H.; Oikari, S.; Álvez, M.B.; Dodd, C.J.G.; Capra, J.; Rilla, K. The Density and Length of Filopodia Associate with the Activity of Hyaluronan Synthesis in Tumor Cells. Cancers 2020, 12, 1908. [Google Scholar] [CrossRef]
- Mierke, C.T. The Pertinent Role of Cell and Matrix Mechanics in Cell Adhesion and Migration. Front. Cell Dev. Biol. 2021, 9, 2822. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Andreassen, E. Stress relaxation of polypropylene fibres with various morphologies. Polymer 1999, 40, 3909–3918. [Google Scholar] [CrossRef]
- Tweedie, C.A.; Constantinides, G.; Lehman, K.E.; Brill, D.J.; Blackman, G.S.; Van Vliet, K.J. Enhanced Stiffness of Amorphous Polymer Surfaces under Confinement of Localized Contact Loads. Adv. Mater. 2007, 19, 2540–2546. [Google Scholar] [CrossRef]
- Murakami, K.; Ono, K.; Shiia, K.; Ueno, T.; Matsuo, M. Stress Relaxation of Blended Polymers of Monodisperse Polystyrenes. Polym. J. 1971, 2, 698–708. [Google Scholar] [CrossRef][Green Version]
- Tobolsky, A.V.; Murakami, K. Existence of a sharply defined maximum relaxation time for monodisperse polystyrene. J. Polym. Sci. 1959, 40, 443–456. [Google Scholar] [CrossRef]
- Caballero, D.; Brancato, V.; Lima, A.C.; Abreu, C.M.; Neves, N.M.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L.; Kundu, S.C. Tumor-Associated Protrusion Fluctuations as a Signature of Cancer Invasiveness. Adv. Biol. 2021, 5, e2101019. [Google Scholar] [CrossRef]
- Unni, A.B.; Chat, K.; Duarte, D.M.; Wojtyniak, M.; Geppert-Rybczyńska, M.; Kubacki, J.; Wrzalik, R.; Richert, R.; Adrjanowicz, K. Experimental evidence on the effect of substrate roughness on segmental dynamics of confined polymer films. Polymer 2020, 199, 122501. [Google Scholar] [CrossRef]
- Carraher, C.L.; Schwarzbauer, J.E. Regulation of Matrix Assembly through Rigidity-dependent Fibronectin Conformational Changes. J. Biol. Chem. 2013, 288, 14805–14814. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Klein, S.R.; Bonar, S.J.; Zielonka, J.; Mizuno, N.; Dickey, J.S.; Keller, P.W.; Joseph, J.; Kalyanaraman, B.; Shacter, E. The Antioxidant Transcription Factor Nrf2 Negatively Regulates Autophagy and Growth Arrest Induced by the Anticancer Redox Agent Mitoquinone. J. Biol. Chem. 2010, 285, 34447–34459. [Google Scholar] [CrossRef]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 122, pp. 1–67. [Google Scholar]
- Martínez-Zamudio, R.I.; Dewald, H.K.; Vasilopoulos, T.; Gittens-Williams, L.; Fitzgerald-Bocarsly, P.; Herbig, U. Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans. Aging Cell 2021, 20, e13344. [Google Scholar] [CrossRef]
- Ishibashi, M.; Miyanaga, Y.; Matsuoka, S.; Kozuka, J.; Togashi, Y.; Kinashi, T.; Ueda, M. Integrin LFA-1 regulates cell adhesion via transient clutch formation. Biochem. Biophys. Res. Commun. 2015, 464, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Jia, X.; Minami, K.; Uto, K.; Chang, A.C.; Hill, J.P.; Nakanishi, J.; Ariga, K. Adaptive Liquid Interfacially Assembled Protein Nanosheets for Guiding Mesenchymal Stem Cell Fate. Adv. Mater. 2019, 32, e1905942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, W.; Ling, J.; Yan, Y.; Hu, J.; Cheng, Y. The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat. Commun. 2018, 9, 1377. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.V.; Hopkins, A.M.; Chen, J.; Narumiya, S.; Parkos, C.A.; Nusrat, A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intes-tinal epithelia. Gastroenterology 2001, 121, 566–579. [Google Scholar] [CrossRef]
- Salvador, E.; Burek, M.; Löhr, M.; Nagai, M.; Hagemann, C.; Förster, C.Y. Senescence and associated blood–brain barrier alterations in vitro. Histochem. Cell Biol. 2021, 156, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Chatterjee, K.; Saini, D.K. Senescent cells in 3D culture show suppressed senescence signatures. Biomater. Sci. 2021, 9, 6461–6473. [Google Scholar] [CrossRef]
- Pereira, P.; Berisha, N.; Bhupathiraju, N.V.S.D.K.; Fernandes, R.; Tomé, J.P.C.; Drain, C.M. Cancer cell spheroids are a better screen for the photodynamic efficiency of glycosylated photosensitizers. PLoS ONE 2017, 12, e0177737. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Choi, Y.W.; Kim, J.-H.; Kim, H.S.; Park, T.J. Senescent tumor cells: An overlooked adversary in the battle against cancer. Exp. Mol. Med. 2021, 53, 1834–1841. [Google Scholar] [CrossRef]
- Pranzini, E.; Raugei, G.; Taddei, M.L. Metabolic Features of Tumor Dormancy: Possible Therapeutic Strategies. Cancers 2022, 14, 547. [Google Scholar] [CrossRef]
- Santos-De-Frutos, K.; Djouder, N. When dormancy fuels tumour relapse. Commun. Biol. 2021, 4, 747. [Google Scholar] [CrossRef]
- Carlson, P.; Dasgupta, A.; Grzelak, C.A.; Kim, J.; Barrett, A.; Coleman, I.M.; Shor, R.E.; Goddard, E.T.; Dai, J.; Schweitzer, E.M.; et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 2019, 21, 238–250. [Google Scholar] [CrossRef]
- Bui, T.; Gu, Y.; Ancot, F.; Sanguin-Gendreau, V.; Zuo, D.; Muller, W.J. Emergence of β1 integrin-deficient breast tumours from dormancy involves both inactivation of p53 and genera-tion of a permissive tumour microenvironment. Oncogene 2022, 41, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Price, T.T.; Burness, M.L.; Sivan, A.; Warner, M.J.; Cheng, R.; Lee, C.H.; Olivere, L.; Comatas, K.; Magnani, J.; Lyerly, H.K.; et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016, 8, 340ra73. [Google Scholar] [CrossRef]
- Justesen, J.; Stenderup, K.; Ebbesen, E.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Farino, C.J.; Pradhan, S.; Slater, J.H. The Influence of Matrix-Induced Dormancy on Metastatic Breast Cancer Chemoresistance. ACS Appl. Bio Mater. 2020, 3, 5832–5844. [Google Scholar] [CrossRef] [PubMed]
- Kondapaneni, R.V.; Rao, S. Matrix stiffness and cluster size collectively regulate dormancy versus proliferation in brain metastatic breast cancer cell clusters. Biomater. Sci. 2020, 8, 6637–6646. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Pillai, V.V.S.; Rodriguez, B.J.; Prencipe, M.; Benedetto, A. Sub-Toxic Concentrations of Ionic Liquids Enhance Cell Migration by Reducing the Elasticity of the Cellular Lipid Membrane. J. Phys. Chem. Lett. 2020, 11, 7327–7333. [Google Scholar] [CrossRef]
- Uto, K.; Mano, S.S.; Aoyagi, T.; Ebara, M. Substrate Fluidity Regulates Cell Adhesion and Morphology on Poly(ε-caprolactone)-Based Materials. ACS Biomater. Sci. Eng. 2016, 2, 446–453. [Google Scholar] [CrossRef]
- Uto, K.; Muroya, T.; Okamoto, M.; Tanaka, H.; Murase, T.; Ebara, M.; Aoyagi, T. Design of super-elastic biodegradable scaffolds with longitudinally oriented microchannels and optimization of the channel size for Schwann cell migration. Sci. Technol. Adv. Mater. 2012, 13, 064207. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Day, C.A.; Kenworthy, A.K.; DiBenedetto, E. Simplified Equation to Extract Diffusion Coefficients from Confocal FRAP Data. Traffic 2012, 13, 1589–1600. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najmina, M.; Ebara, M.; Ohmura, T.; Uto, K. Viscoelastic Liquid Matrix with Faster Bulk Relaxation Time Reinforces the Cell Cycle Arrest Induction of the Breast Cancer Cells via Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 14637. https://doi.org/10.3390/ijms232314637
Najmina M, Ebara M, Ohmura T, Uto K. Viscoelastic Liquid Matrix with Faster Bulk Relaxation Time Reinforces the Cell Cycle Arrest Induction of the Breast Cancer Cells via Oxidative Stress. International Journal of Molecular Sciences. 2022; 23(23):14637. https://doi.org/10.3390/ijms232314637
Chicago/Turabian StyleNajmina, Mazaya, Mitsuhiro Ebara, Takahito Ohmura, and Koichiro Uto. 2022. "Viscoelastic Liquid Matrix with Faster Bulk Relaxation Time Reinforces the Cell Cycle Arrest Induction of the Breast Cancer Cells via Oxidative Stress" International Journal of Molecular Sciences 23, no. 23: 14637. https://doi.org/10.3390/ijms232314637
APA StyleNajmina, M., Ebara, M., Ohmura, T., & Uto, K. (2022). Viscoelastic Liquid Matrix with Faster Bulk Relaxation Time Reinforces the Cell Cycle Arrest Induction of the Breast Cancer Cells via Oxidative Stress. International Journal of Molecular Sciences, 23(23), 14637. https://doi.org/10.3390/ijms232314637