Cancer History Avoids the Increase of Senescence Markers in Peripheral Cells of Amnestic Mild Cognitive Impaired Patients
Abstract
1. Introduction
2. Results
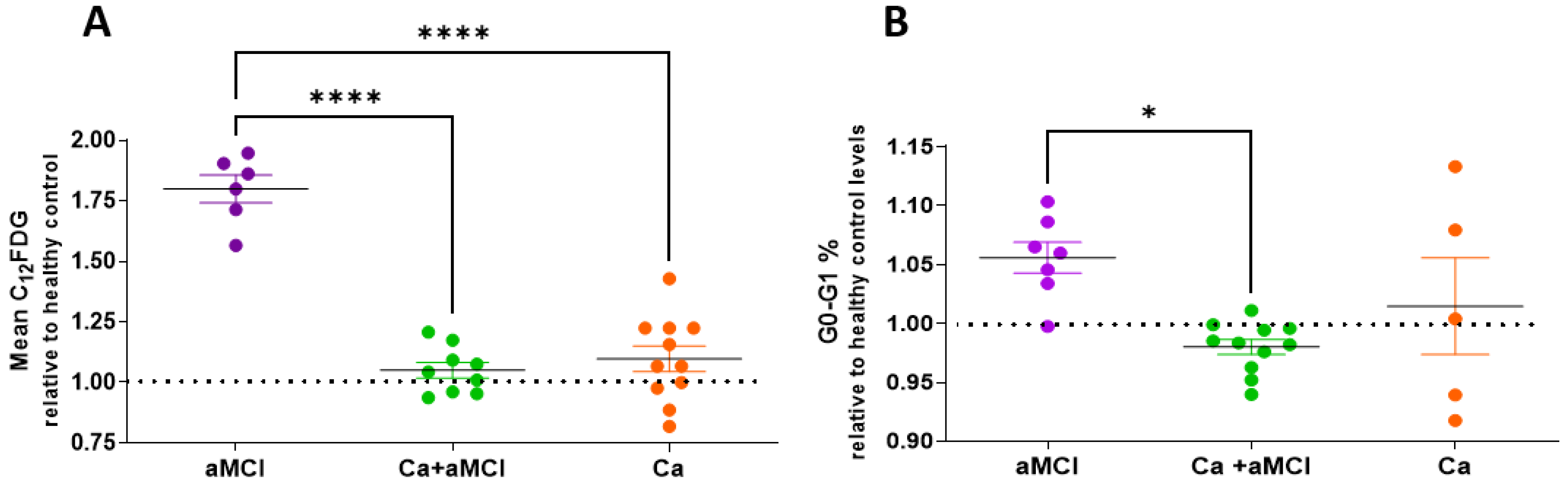
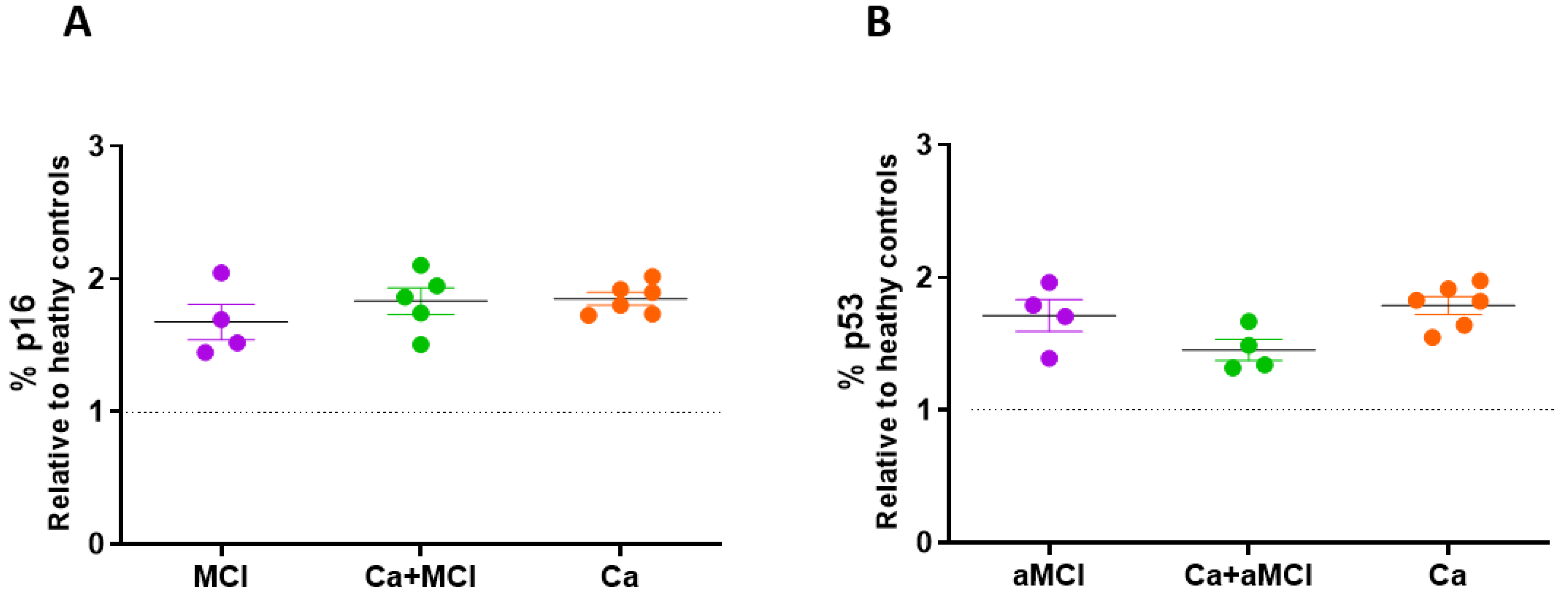
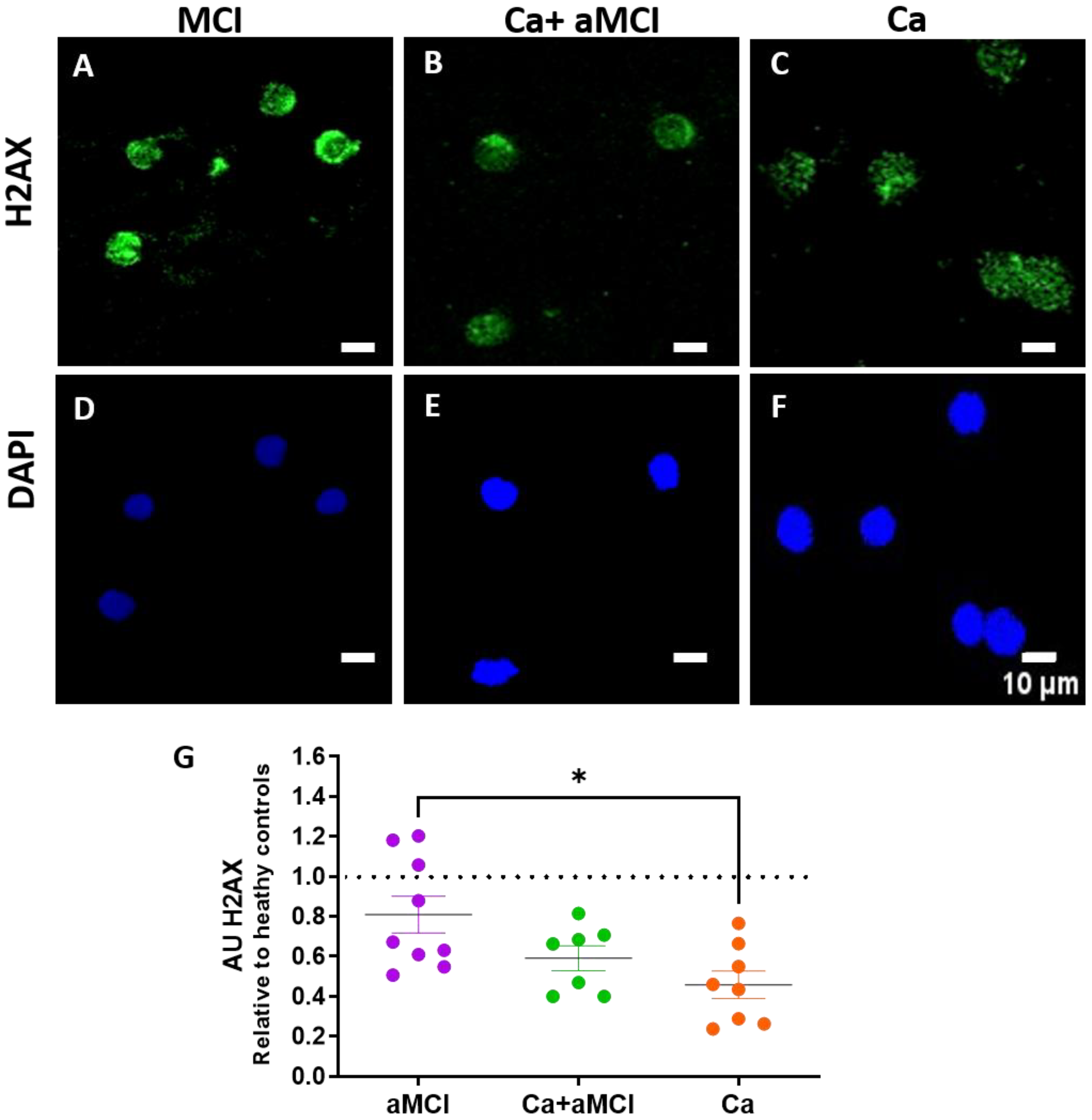
2.1. Senescent Markers in PBMCs of aMCI Patients with and without a History of Cancer and in Cognitively Normal Cancer Survivors as Compared to Healthy Controls
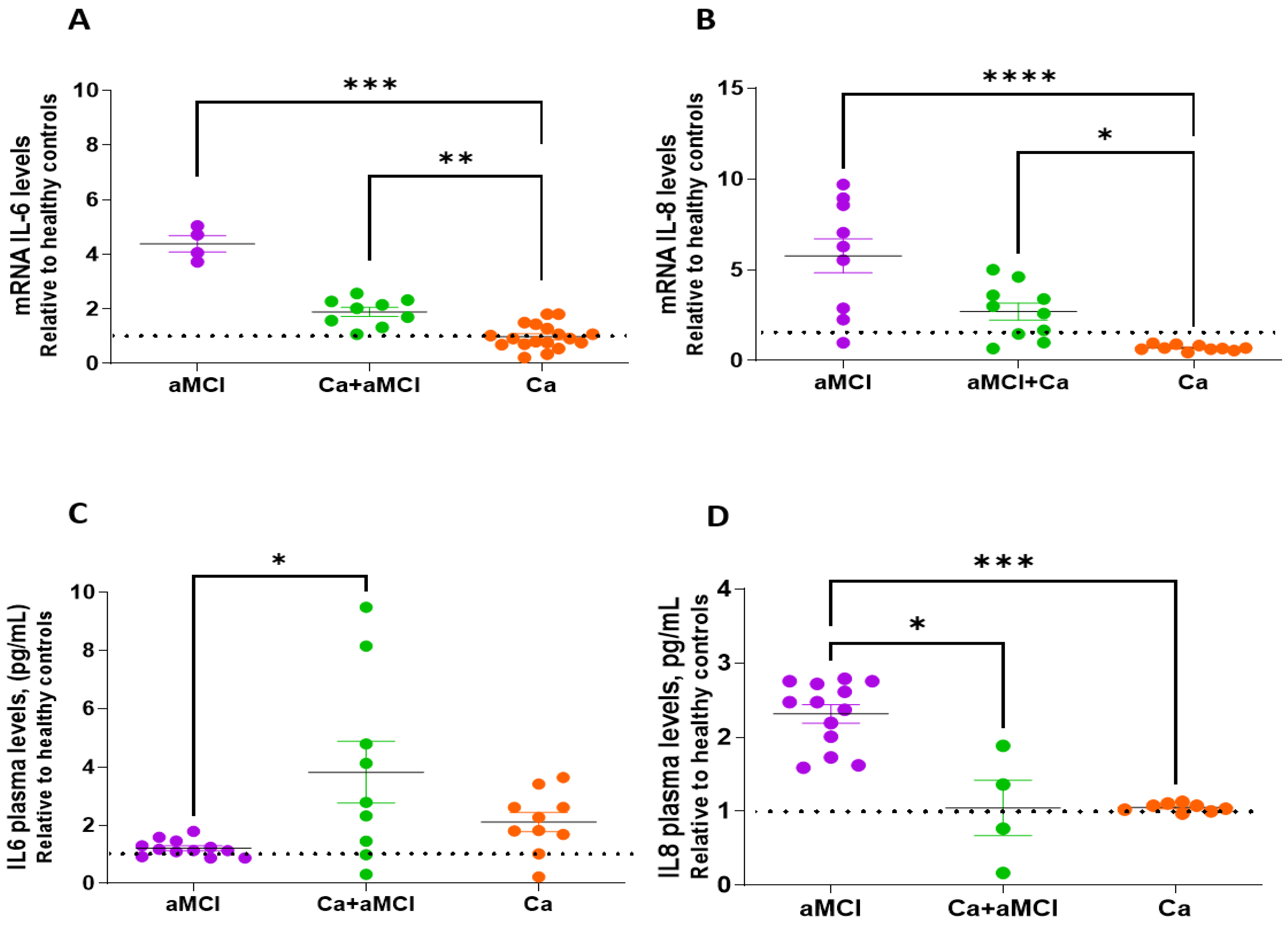
2.2. Profile of Senescence-Associated Secretory Phenotype of aMCI Patients with and without a History of Cancer and in Cognitively Normal Cancer Survivors as Compared to Healthy Controls
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Peripheral Blood Mononuclear Cells (PBMCs) Isolation
4.3. β-Galactosidase Activity
4.4. p16 and p53 Determination
4.5. G0G1 Phase Cell-Cycle Arrest
4.6. H2AX Immunocytochemistry
4.7. RNA Isolation and PCR Analysis
4.8. ELISA
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Roe, C.M.; Behrens, M.I.; Xiong, C.; Miller, J.P.; Morris, J.C. Alzheimer Disease and Cancer. Neurology 2005, 64, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.M.; Fitzpatrick, A.L.; Xiong, C.; Sieh, W.; Kuller, L.; Miller, J.P.; Williams, M.M.; Kopan, R.; Behrens, M.I.; Morris, J.C. Cancer Linked to Alzheimer Disease but Not Vascular Dementia. Neurology 2010, 74, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Catalá-López, F.; Hutton, B.; Driver, J.A.; Page, M.J.; Ridao, M.; Valderas, J.M.; Alonso-Arroyo, A.; Forés-Martos, J.; Martínez, S.; Gènova-Maleras, R.; et al. Cancer and Central Nervous System Disorders: Protocol for an Umbrella Review of Systematic Reviews and Updated Meta-Analyses of Observational Studies. Syst. Rev. 2017, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chiang, S.; Kalinowski, D.; Bae, D.; Sahni, S.; Richardson, D. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk Between Antioxidant Defense, Autophagy, and Apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef] [PubMed]
- Zabłocka, A.; Kazana, W.; Sochocka, M.; Stańczykiewicz, B.; Janusz, M.; Leszek, J.; Orzechowska, B. Inverse Correlation Between Alzheimer’s Disease and Cancer: Short Overview. Mol. Neurobiol. 2021, 58, 6335–6349. [Google Scholar] [CrossRef]
- Attner, B.; Lithman, T.; Noreen, D.; Olsson, H. Low Cancer Rates among Patients with Dementia in a Population-Based Register Study in Sweden. Dement. Geriatr. Cogn. Disord. 2010, 30, 39–42. [Google Scholar] [CrossRef]
- Frain, L.; Swanson, D.; Cho, K.; Gagnon, D.; Lu, K.P.; Betensky, R.A.; Driver, J. Association of Cancer and Alzheimer’s Disease Risk in a National Cohort of Veterans. Alzheimer’s Dement. 2017, 13, 1364–1370. [Google Scholar] [CrossRef]
- Musicco, M.; Adorni, F.; Di Santo, S.; Prinelli, F.; Pettenati, C.; Caltagirone, C.; Palmer, K.; Russo, A. Inverse Occurrence of Cancer and Alzheimer Disease: A Population-Based Incidence Study. Neurology 2013, 81, 322–328. [Google Scholar] [CrossRef]
- Nudelman, K.N.H.; Risacher, S.L.; West, J.D.; McDonald, B.C.; Gao, S.; Saykin, A.J.; The Alzheimer’s Disease Neuroimaging Initiative. Association of Cancer History with Alzheimer’s Disease Onset and Structural Brain Changes. Front. Physiol. 2014, 5, 423. [Google Scholar] [CrossRef]
- Ou, S.-M.; Lee, Y.-J.; Hu, Y.-W.; Liu, C.-J.; Chen, T.-J.; Fuh, J.-L.; Wang, S.-J. Does Alzheimer’s Disease Protect against Cancers? A Nationwide Population-Based Study. Neuroepidemiology 2013, 40, 42–49. [Google Scholar] [CrossRef]
- Romero, J.P.; Benito-León, J.; Louis, E.D.; Bermejo-Pareja, F. Alzheimer’s Disease Is Associated with Decreased Risk of Cancer-Specific Mortality: A Prospective Study (NEDICES). J. Alzheimer’s Dis. 2014, 40, 465–473. [Google Scholar] [CrossRef]
- Catalá-López, F.; Suárez-Pinilla, M.; Suárez-Pinilla, P.; Valderas, J.M.; Gómez-Beneyto, M.; Martinez, S.; Balanzá-Martínez, V.; Climent, J.; Valencia, A.; McGrath, J.; et al. Inverse and Direct Cancer Comorbidity in People with Central Nervous System Disorders: A Meta-Analysis of Cancer Incidence in 577,013 Participants of 50 Observational Studies. Psychother. Psychosom. 2014, 83, 89–105. [Google Scholar] [CrossRef]
- Ibáñez, K.; Boullosa, C.; Tabarés-Seisdedos, R.; Baudot, A.; Valencia, A. Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-Analyses. PLoS Genet. 2014, 10, e1004173. [Google Scholar] [CrossRef]
- Sánchez-Valle, J.; Tejero, H.; Ibáñez, K.; Portero, J.L.; Krallinger, M.; Al-Shahrour, F.; Tabarés-Seisdedos, R.; Baudot, A.; Valencia, A. A Molecular Hypothesis to Explain Direct and Inverse Co-Morbidities between Alzheimer’s Disease, Glioblastoma and Lung Cancer. Sci. Rep. 2017, 7, 4474. [Google Scholar] [CrossRef]
- Behrens, M.; Lendon, C.; Roe, C. A Common Biological Mechanism in Cancer and Alzheimers Disease? Curr. Alzheimer Res. 2009, 6, 196–204. [Google Scholar] [CrossRef]
- Rogers, N.K.; Romero, C.; Sanmartín, C.D.; Ponce, D.P.; Salech, F.; López, M.N.; Gleisner, A.; Tempio, F.; Behrens, M.I. Inverse Relationship between Alzheimer’s Disease and Cancer: How Immune Checkpoints Might Explain the Mechanisms Underlying Age-Related Diseases. J. Alzheimer’s Dis. 2020, 73, 443–454. [Google Scholar] [CrossRef]
- Behrens, M.I.; Silva, M.; Salech, F.; Ponce, D.P.; Merino, D.; Sinning, M.; Xiong, C.; Roe, C.M.; Quest, A.F.G. Inverse Susceptibility to Oxidative Death of Lymphocytes Obtained from Alzheimer’s Patients and Skin Cancer Survivors: Increased Apoptosis in Alzheimer’s and Reduced Necrosis in Cancer. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1036–1040. [Google Scholar] [CrossRef]
- Ponce, D.P.; Salech, F.; Sanmartin, C.D.; Silva, M.; Xiong, C.; Roe, C.M.; Henriquez, M.; Quest, A.F.; Behrens, M.I. Increased Susceptibility to Oxidative Death of Lymphocytes from Alzheimer Patients Correlates with Dementia Severity. Curr. Alzheimer Res. 2014, 11, 892–898. [Google Scholar] [CrossRef]
- Salech, F.; Ponce, D.P.; SanMartín, C.D.; Rogers, N.K.; Henríquez, M.; Behrens, M.I. Cancer Imprints an Increased PARP-1 and P53-Dependent Resistance to Oxidative Stress on Lymphocytes of Patients That Later Develop Alzheimer’s Disease. Front. Neurosci. 2018, 12, 58. [Google Scholar] [CrossRef]
- Castillo-Passi, R.I.; Vergara, R.C.; Rogers, N.K.; Ponce, D.P.; Bennett, M.; Behrens, M.I. Cancer History Is Associated with Slower Speed of Cognitive Decline in Patients with Amnestic Cognitive Impairment. J. Alzheimer’s Dis. 2022, 87, 1695–1711. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Kritsilis, M.; Rizou, S.V.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef] [PubMed]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cué, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic Therapy Alleviates Aβ-Associated Oligodendrocyte Progenitor Cell Senescence and Cognitive Deficits in an Alzheimer’s Disease Model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Salech, F.; SanMartín, C.D.; Concha-Cerda, J.; Romero-Hernández, E.; Ponce, D.P.; Liabeuf, G.; Rogers, N.K.; Murgas, P.; Bruna, B.; More, J.; et al. Senescence Markers in Peripheral Blood Mononuclear Cells in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 9387. [Google Scholar] [CrossRef]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Jill Saffrey, M.; Cameron, K.; et al. Postmitotic Neurons Develop a P21-Dependent Senescence-like Phenotype Driven by a DNA Damage Response. Aging Cell 2012, 11, 996–1004. [Google Scholar] [CrossRef]
- Musi, N.; Valentine, J.M.; Sickora, K.R.; Baeuerle, E.; Thompson, C.S.; Shen, Q.; Orr, M.E. Tau Protein Aggregation Is Associated with Cellular Senescence in the Brain. Aging Cell 2018, 17, e12840. [Google Scholar] [CrossRef]
- Flanary, B. The Role of Microglial Cellular Senescence in the Aging and Alzheimer Diseased Brain. Rejuvenation Res. 2005, 8, 82–85. [Google Scholar] [CrossRef]
- He, N.; Jin, W.L.; Lok, K.H.; Wang, Y.; Yin, M.; Wang, Z.J. Amyloid-β(1-42) Oligomer Accelerates Senescence in Adult Hippocampal Neural Stem/Progenitor Cells via Formylpeptide Receptor 2. Cell Death Dis. 2013, 4, e924. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; Lebrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of P16 Ink4a-Positive Senescent Cells Delays Ageing-Associated Disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally Occurring P16 Ink4a-Positive Cells Shorten Healthy Lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The Flavonoid Quercetin Ameliorates Alzheimer’s Disease Pathology and Protects Cognitive and Emotional Function in Aged Triple Transgenic Alzheimer’s Disease Model Mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting Senescence for the Treatment of Cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Zeng, S.; Shen, W.H.; Liu, L. Senescence and Cancer. Cancer Transl. Med. 2018, 4, 70–74. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and Cancer—Role and Therapeutic Opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Myung, N.H.; Zhu, X.; Kruman, I.I.; Castellani, R.J.; Petersen, R.B.; Siedlak, S.L.; Perry, G.; Smith, M.A.; Lee, H.G. Evidence of DNA Damage in Alzheimer Disease: Phosphorylation of Histone H2AX in Astrocytes. Age 2008, 30, 209–215. [Google Scholar] [CrossRef]
- Mariotti, L.G.; Pirovano, G.; Savage, K.I.; Ghita, M.; Ottolenghi, A.; Prise, K.M.; Schettino, G. Use of the γ-H2AX Assay to Investigate DNA Repair Dynamics Following Multiple Radiation Exposures. PLoS ONE 2013, 8, e79541. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of Cellular Senescence. Telomere Shortening as a Marker of Cellular Senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; Van Deursen, J.M. Senescent Cells: An Emerging Target for Diseases of Ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells Exhibiting Strong P16 INK4a Promoter Activation in Vivo Display Features of Senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.E.; Ince, P.G.; Haynes, L.J.; Theaker, R.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Lace, G.L.; Shaw, P.J.; Matthews, F.E.; et al. Population Variation in Oxidative Stress and Astrocyte DNA Damage in Relation to Alzheimer-Type Pathology in the Ageing Brain. Neuropathol. Appl. Neurobiol. 2010, 36, 25–40. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A New Gene Set Identifies Senescent Cells and Predicts Senescence-Associated Pathways across Tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- Pepin, M.E.; Infante, T.; Benincasa, G.; Schiano, C.; Miceli, M.; Ceccarelli, S.; Megiorni, F.; Anastasiadou, E.; Della Valle, G.; Fatone, G.; et al. Differential DNA Methylation Encodes Proliferation and Senescence Programs in Human Adipose-Derived Mesenchymal Stem Cells. Front. Genet. 2020, 11, 346. [Google Scholar] [CrossRef]
- Xie, W.; Kagiampakis, I.; Pan, L.; Zhang, Y.W.; Murphy, L.; Tao, Y.; Kong, X.; Kang, B.; Xia, L.; Carvalho, F.L.F.; et al. DNA Methylation Patterns Separate Senescence from Transformation Potential and Indicate Cancer Risk. Cancer Cell 2018, 33, 309–321.e5. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Berg, L.; Miller, J.P.; Storandt, M.; Duchek, J.; Morris, J.C.; Rubin, E.H.; Burke, W.J.; Coben, L.A. Mild Senile Dementia of the Alzheimer Type: 2. Longitudinal Assessment. Ann. Neurol. 1988, 23, 477–484. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Delgado, C.; Araneda, A.; Behrens, M.I. Validación Del Instrumento Montreal Cognitive Assessment En Español En Adultos Mayores de 60 Años. Neurología 2017, 34, 376–385. [Google Scholar] [CrossRef]
- Julayanont, P.; Brousseau, M.; Chertkow, H.; Phillips, N.; Nasreddine, Z.S. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a Predictor of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. J. Am. Geriatr. Soc. 2014, 62, 679–684. [Google Scholar] [CrossRef]
- Galvin, J.E.; Roe, C.M.; Powlishta, K.K.; Coats, M.A.; Muich, S.J.; Grant, E.; Miller, J.P.; Storandt, M.; Morris, J.C. The AD8: A Brief Informant Interview to Detect Dementia. Neurology 2005, 65, 559–564. [Google Scholar] [CrossRef]
- Behrens, M.I.; Silva, M.; Schmied, A.; Salech, F.; Manzur, H.; Rebolledo, R.; Bull, R.; Torres, V.; Henriquez, M.; Quest, A.F.G. Age-Dependent Increases in Apoptosis/Necrosis Ratios in Human Lymphocytes Exposed to Oxidative Stress. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 732–740. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to Detect Senescence-Associated Beta-Galactosidase (SA-Βgal) Activity, a Biomarker of Senescent Cells in Culture and in Vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]

| Controls * n = 26 | aMCI * n = 22 | Ca + aMCI n = 21 | Ca Survivors n = 18 | p Value | |
|---|---|---|---|---|---|
| Age, mean ± SE (range) | 75.6 ± 1.8 (65–85) | 77.2 ± 1.5 (63–93) | 79.5 ± 1.6 (69–90) | 75.8 ± 1.9 (61–91) | ns |
| Female sex, n (%) | 17 (68.0) | 16 (72.7) | 10 (47.6) | 13 (72.2) | ns |
| Education, years | 12.5 ± 1.0 | 10.1 ± 1.1 | 11.3 ± 1.8 | 14.3 ± 1.1 | ns |
| MoCA test score, mean ± SE | 28.2 ± 0.4 | 19.8 ± 0.9 | 22.5 ± 0.7 | 28.8 ± 0.2 | p < 0.0001 for control vs. aMCI; control vs. Ca + aMCI; aMCI vs. Ca; and Ca + aMCI vs. Ca. |
| MoCA-MIS test score, mean ± SE | 14.1 ± 0.2 | 8.7 ± 0.5 | 8.4 ± 0.9 | 14.3 ± 1.1 | p < 0.0001 for control vs. aMCI; control vs. Ca + aMCI; aMCI vs. Ca; and Ca + aMCI vs. Ca |
| AD8, mean ± SE | 0.4 ± 0.1 | 4.3 ± 0.5 | 3.6 ± 0.5 | 0.1 ± 0.08 | p < 0.0001 for control vs. aMCI; control vs. Ca + aMCI; aMCI vs. Ca; and Ca + aMCI vs. Ca. |
| CDR-SOB, mean ± SE | 0.1 ± 0.04 | 2.1 ± 0.2 | 2.6 ± 0.3 | 0.1 ± 0.04 | p < 0.0001 for control vs. aMCI; control vs. Ca + aMCI; aMCI vs. Ca; and Ca + aMCI vs. Ca. p < 0.05 for aMCI vs. Ca + aMCI |
| CDR 0, n | 26 | 0 | 0 | 18 | |
| CDR 0.5, n | 0 | 22 | 13 | 0 | |
| CDR 1, n | 0 | 0 | 8 | 0 | |
| CDR 2, n | 0 | 0 | 0 | 0 | |
| CDR 3, n | 0 | 0 | 0 | 0 | |
| Diabetes/Insulin Resistance, n (%) | 6 (22.2) | 6 (25.0) | 4 (19.1) | 7 (38.0) | ns |
| Hypertension, n (%) | 19 (72.2) | 10 (45.0) | 12 (57.1) | 8 (44.4) | ns |
| Hypercholesterolemia, n (%) | 7 (27.8) | 10 (47.4) | 10 (47.6) | 3 (16.6) | p < 0.05: Ca + aMCI vs. Ca |
| Anti-Dementia medication | |||||
| AChE inhibitor + Memantine, n (%) | - | 9 (40.9) | 9 (42.9) | - | ns |
| AChE inhibitor only, n (%) | - | 0 | 2 (9.5) | - | ns |
| Memantine only, n (%) | - | 5 (22.7) | 2 (9.5) | - | ns |
| Cancer type, n | |||||
| Skin | 9 # | 14 $ | |||
| Breast | 5 | 5 | |||
| Prostate | 2 | 3 | |||
| Colon | 4 | - | |||
| Ovary | 1 | 1 | |||
| Pancreas | - | 1 | |||
| Cervix uterus | - | 1 | |||
| Kidney | - | 1 | |||
| Thyroid | - | 1 | |||
| Gastric | - | 1 | |||
| Bladder | 1 | 1 | |||
| Lymphoma | 1 | - | |||
| Head and neck | 1 | - | |||
| Lung | 1 | - | |||
| Rectum | 1 | - | |||
| Endometrium | 1 | - | |||
| Appendix | 1 | ||||
| Total Cancer (n) | 0 | 0 | 27 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
SanMartín, C.D.; Salech, F.; Ponce, D.P.; Concha-Cerda, J.; Romero-Hernández, E.; Liabeuf, G.; Rogers, N.K.; Murgas, P.; Bruna, B.; More, J.; et al. Cancer History Avoids the Increase of Senescence Markers in Peripheral Cells of Amnestic Mild Cognitive Impaired Patients. Int. J. Mol. Sci. 2023, 24, 7364. https://doi.org/10.3390/ijms24087364
SanMartín CD, Salech F, Ponce DP, Concha-Cerda J, Romero-Hernández E, Liabeuf G, Rogers NK, Murgas P, Bruna B, More J, et al. Cancer History Avoids the Increase of Senescence Markers in Peripheral Cells of Amnestic Mild Cognitive Impaired Patients. International Journal of Molecular Sciences. 2023; 24(8):7364. https://doi.org/10.3390/ijms24087364
Chicago/Turabian StyleSanMartín, Carol D., Felipe Salech, Daniela Paz Ponce, Jorge Concha-Cerda, Esteban Romero-Hernández, Gianella Liabeuf, Nicole K. Rogers, Paola Murgas, Bárbara Bruna, Jamileth More, and et al. 2023. "Cancer History Avoids the Increase of Senescence Markers in Peripheral Cells of Amnestic Mild Cognitive Impaired Patients" International Journal of Molecular Sciences 24, no. 8: 7364. https://doi.org/10.3390/ijms24087364
APA StyleSanMartín, C. D., Salech, F., Ponce, D. P., Concha-Cerda, J., Romero-Hernández, E., Liabeuf, G., Rogers, N. K., Murgas, P., Bruna, B., More, J., & Behrens, M. I. (2023). Cancer History Avoids the Increase of Senescence Markers in Peripheral Cells of Amnestic Mild Cognitive Impaired Patients. International Journal of Molecular Sciences, 24(8), 7364. https://doi.org/10.3390/ijms24087364






