Na+/K+- and Mg2+-ATPases and Their Interaction with AMPA, NMDA and D2 Dopamine Receptors in an Animal Model of Febrile Seizures
Abstract
:1. Introduction
2. Results
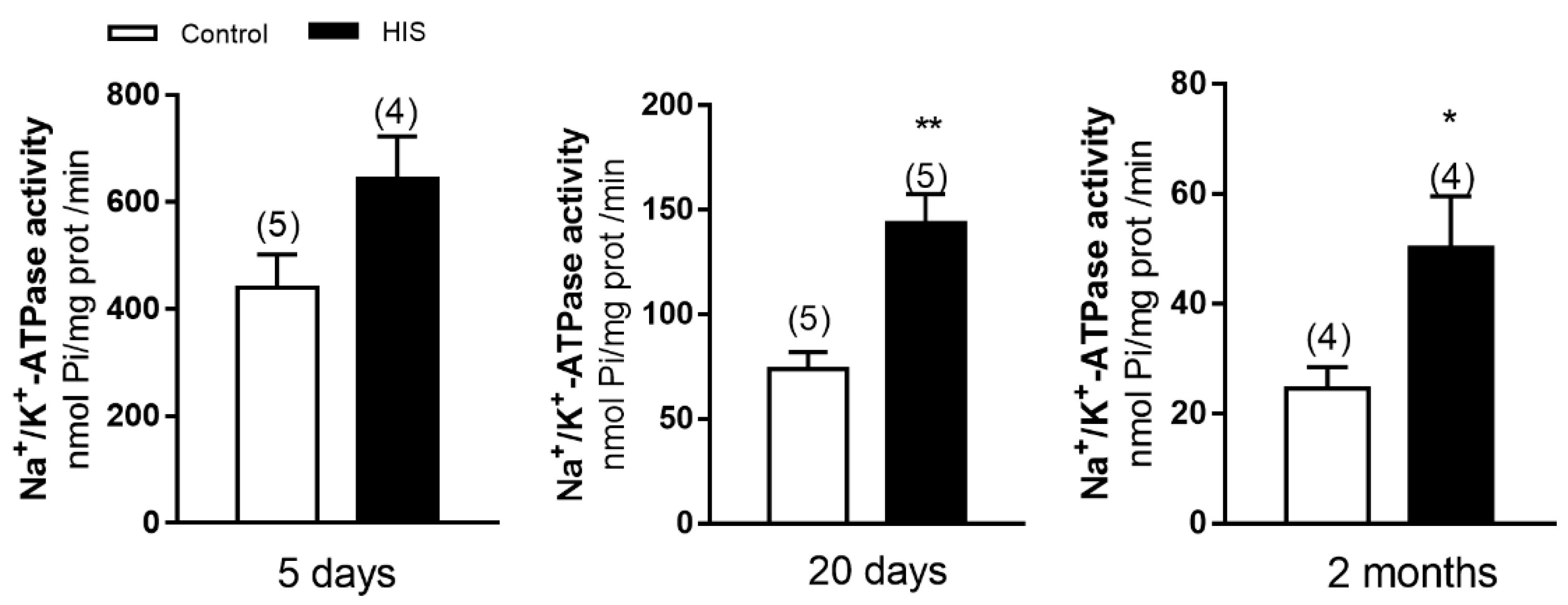
2.1. Na+/K+-ATPase Activity in Cortical Plasma Membranes after HIS
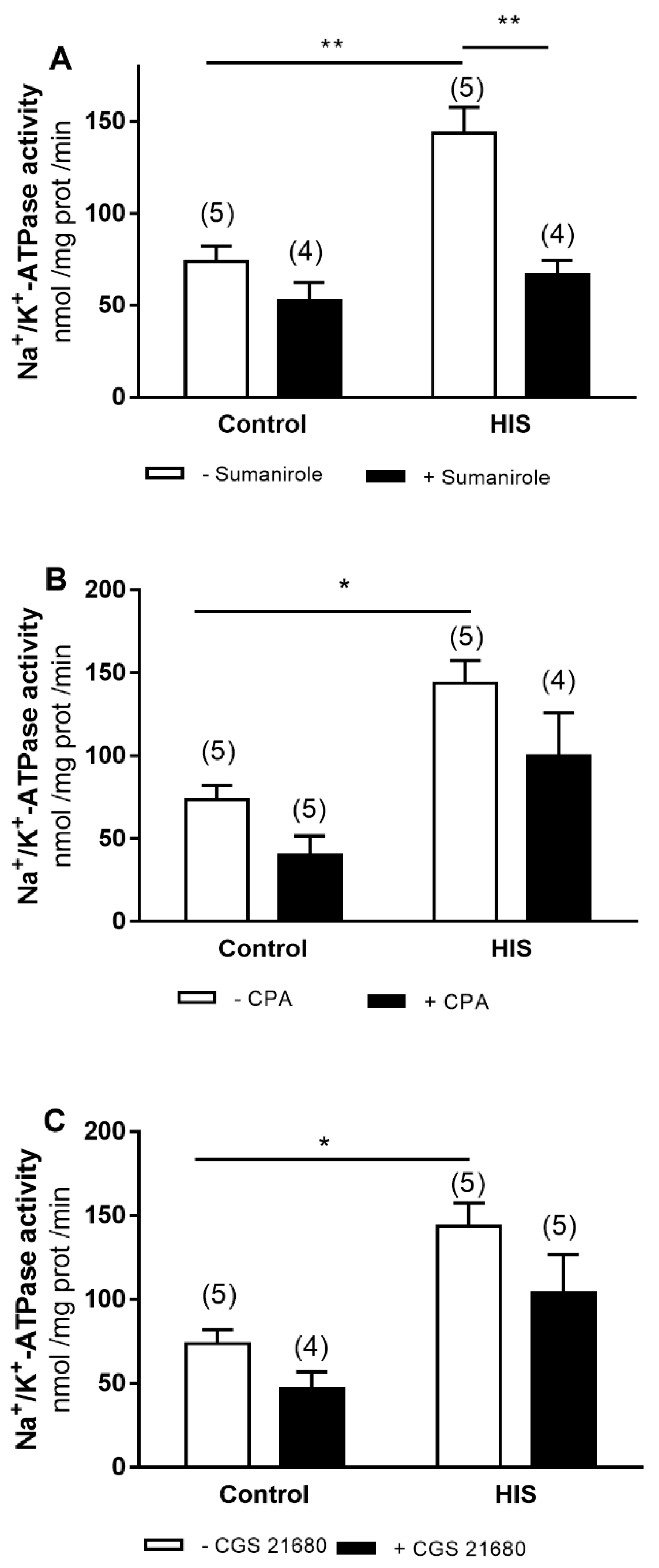
2.2. Modulation of Na+/K+-ATPase Activity by G-Protein-Coupled Receptor after HIS
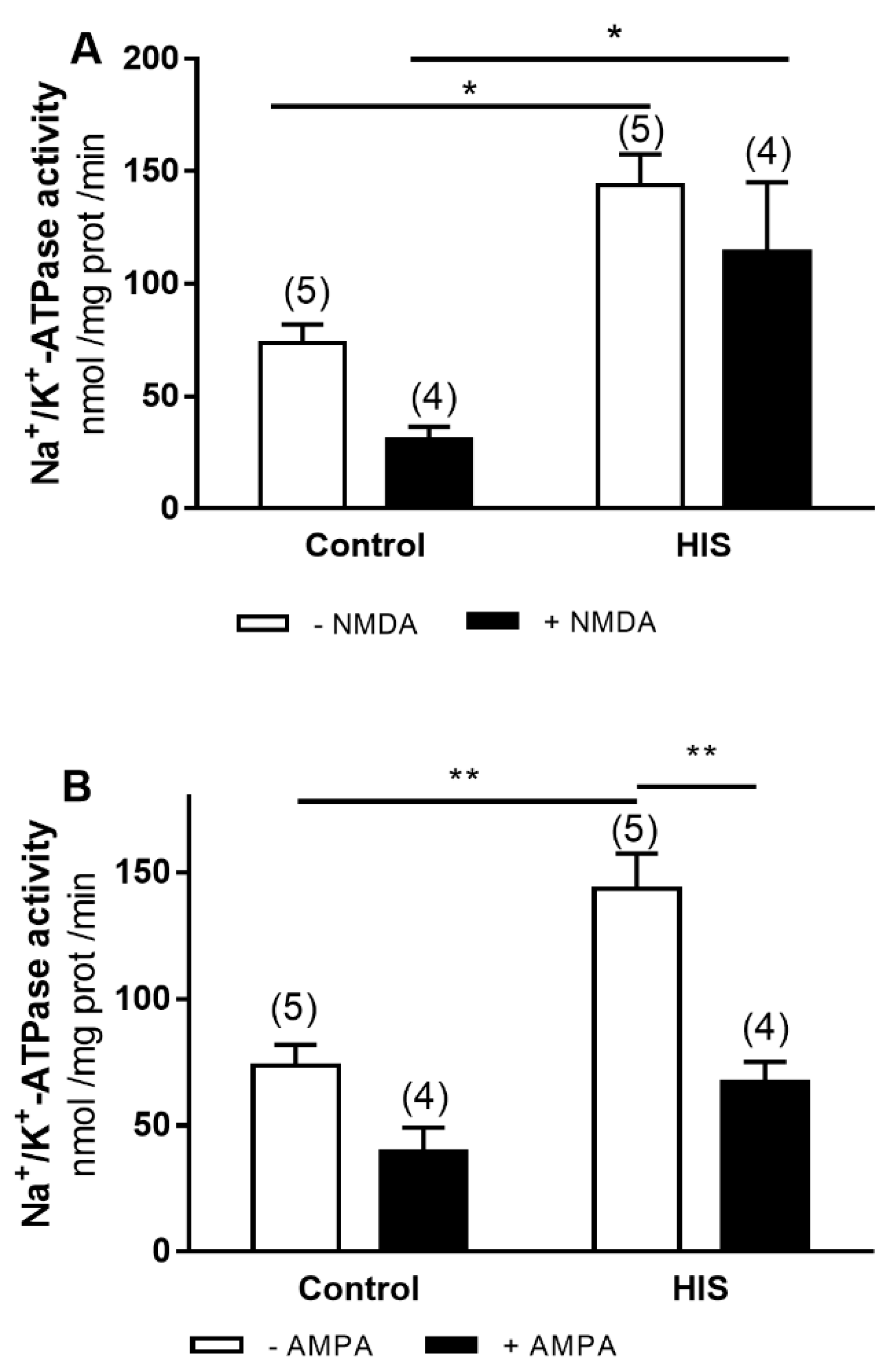
2.3. Modulation of Na+/K+-ATPase Activity by Ionotropic Glutamate Receptor after HIS
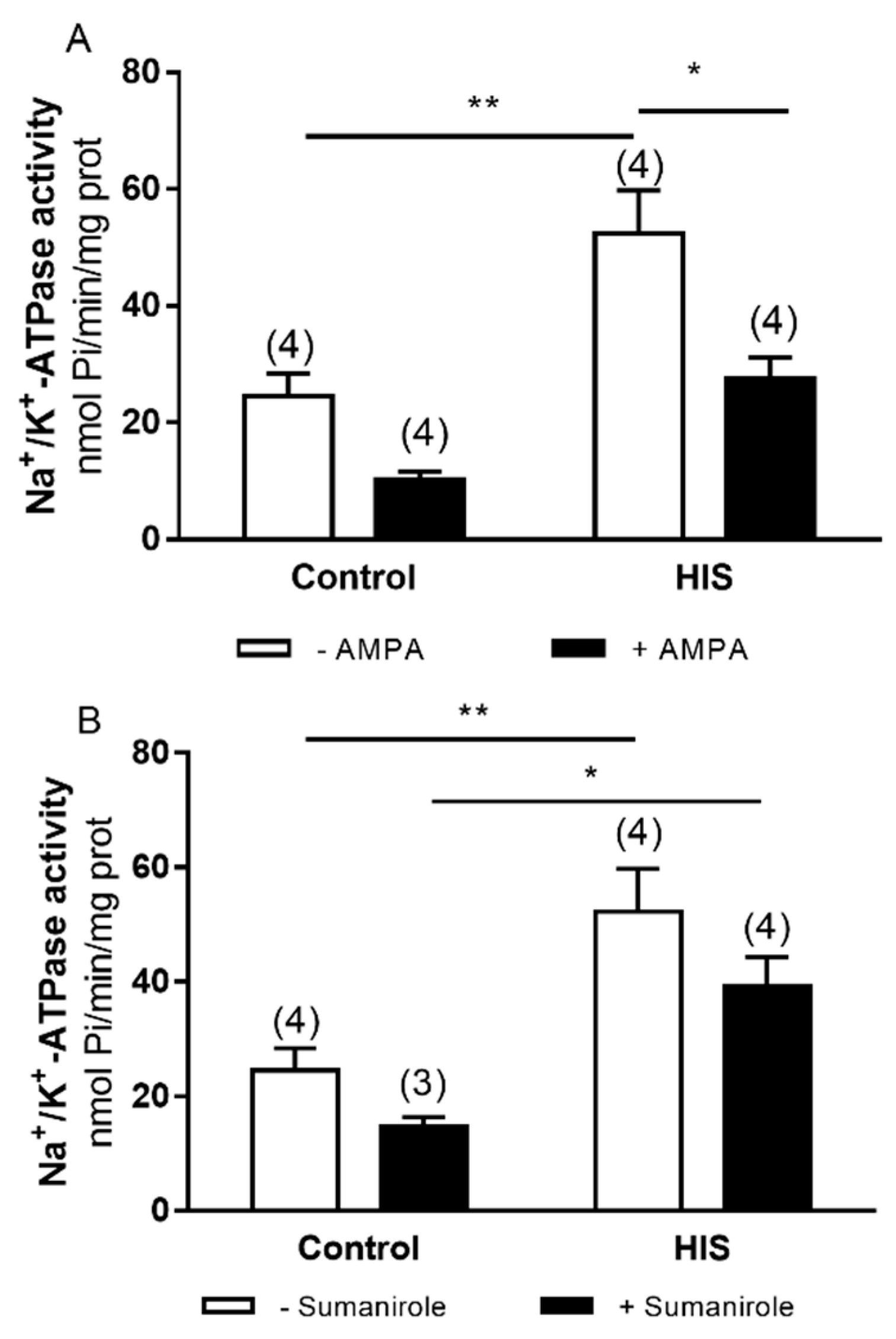
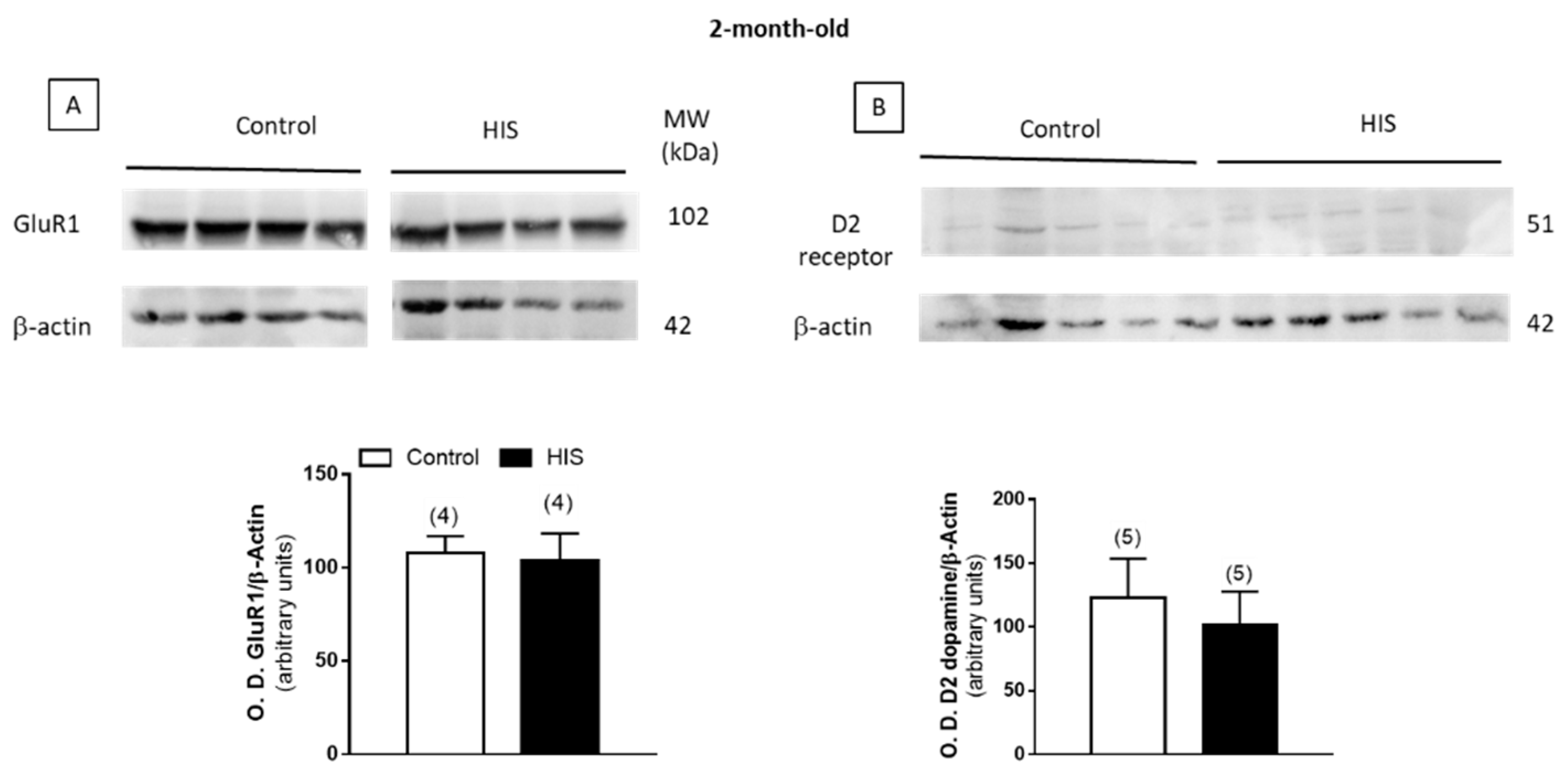
2.4. Modulation of Na+/K+-ATPase Activity after HIS by AMPA and Sumanirole in 2-Month-Old Animals
2.5. Mg2+-ATPase Activity in Cortical Plasma Membrane after HIS
2.6. Modulation of Mg2+-ATPase Activity by G-Protein-Coupled Receptor after HIS
2.7. Modulation of Mg2+-ATPase by Ionotropic Receptors after HIS
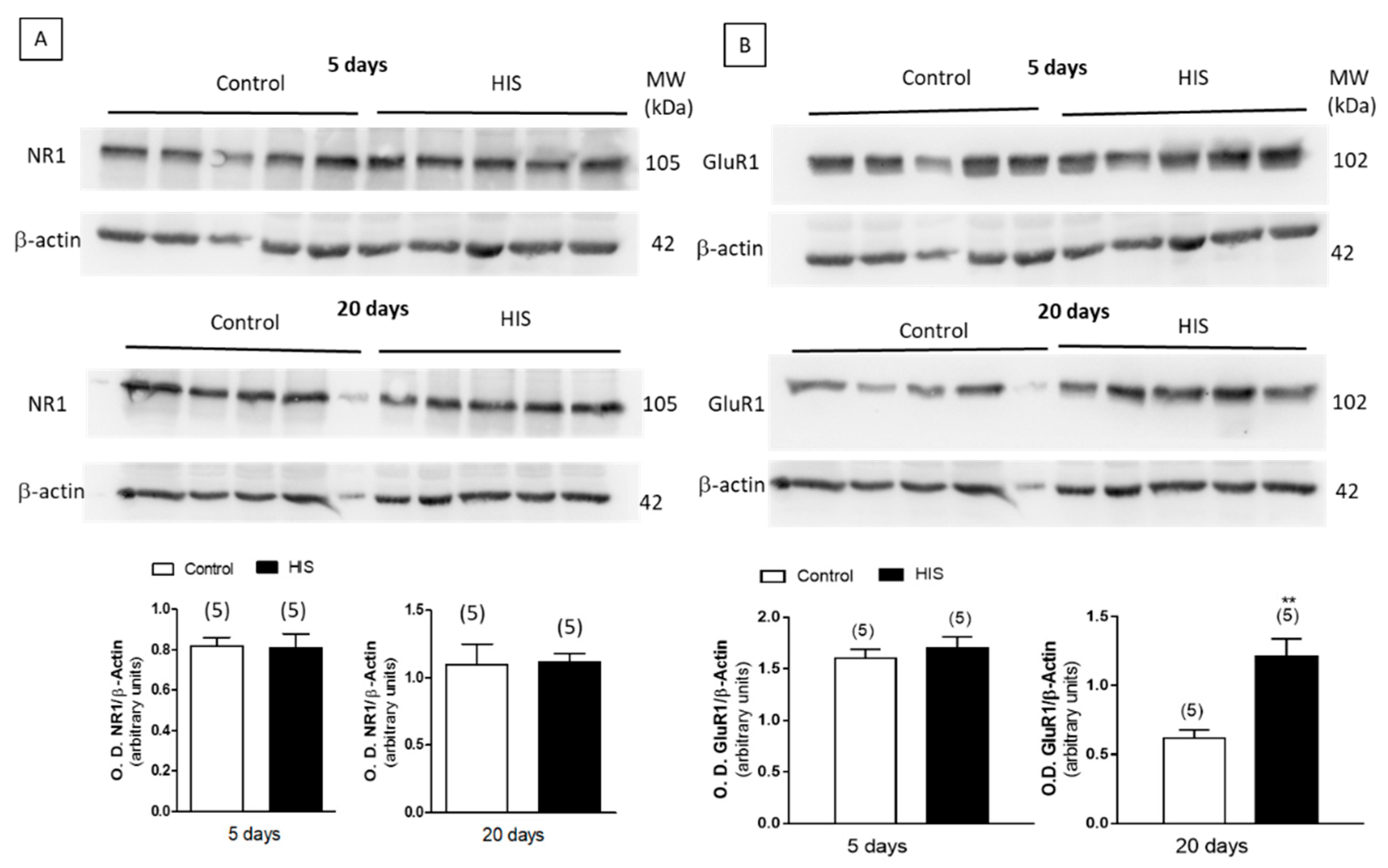
2.8. Protein Level of Na+/K+ATPase and Receptors after HIS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Hyperthermia-Induced Seizures
4.4. Cerebral Cortical Membrane Isolation
4.5. Na+/K+-ATPase and Mg2+-ATPase Activities Assay
4.6. Western-Blotting Assay
4.7. Statistical and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Shinnar, S.; Glauser, T.A. Febrile seizures. J. Child Neurol. 2002, 17 (Suppl. 1), S44–S52. [Google Scholar] [CrossRef]
- Stafstrom, C.E. Assessing the behavioral and cognitive effects of seizures on the developing brain. Prog. Brain Res. 2002, 135, 377–390. [Google Scholar]
- Dubé, C.M.; Brewster, A.L.; Baram, T.Z. Febrile seizures: Mechanisms and relationship to epilepsy. Brain Dev. 2009, 31, 366–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClelland, S.; Dubé, C.M.; Yang, J.; Baram, T.Z. Epileptogenesis after prolonged febrile seizures: Mechanisms, biomarkers and therapeutic opportunities. Neurosci. Lett. 2011, 497, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Baram, T.Z.; Gerth, A.; Schultz, L. Febrile seizures: An appropriate-aged model suitable for long-term studies. Brain Res. Dev. Brain Res. 1997, 98, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubé, C.; Richichi, C.; Bender, R.A.; Chung, G.; Litt, B.; Baram, T.Z. Temporal lobe epilepsy after experimental prolonged febrile seizures: Prospective analysis. Brain 2006, 129, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Dubé, C.M.; Brewster, A.L.; Richichi, C.; Zha, Q.; Baram, T.Z. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007, 30, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Bender, R.A.; Baram, T.Z. Epileptogenesis in the developing brain: What can we learn from animal models? Epilepsia 2007, 48 (Suppl. 5), 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-Navarro, D.A.; Albasanz, J.L.; Martín, M. Hyperthermia-induced seizures alter adenosine A1 and A2A receptors and 5′-nucleotidase activity in rat cerebral cortex. J. Neurochem. 2015, 134, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Cerebellar oxidative stress and fine motor impairment in adolescent rats exposed to hyperthermia-induced seizures is prevented by maternal caffeine intake during gestation and lactation. Eur. J. Pharmacol. 2018, 822, 186–198. [Google Scholar] [CrossRef]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Early-life hyperthermic seizures upregulate adenosine A2A receptors in the cortex and promote depressive-like behavior in adult rats. Epilepsy Behav. 2018, 86, 173–178. [Google Scholar] [CrossRef]
- Crespo, M.; León-Navarro, D.A.; Ruíz, M.Á.; Martín, M. Hyperthermia-induced seizures produce long-term effects on the functionality of adenosine A1 receptor in rat cerebral cortex. Int. J. Dev. Neurosci. 2020, 80, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Hyperthermia-induced seizures during neonatal period alter the functionality of A1 and A2A receptors in the cerebellum and evoke fine motor impairment and gait disturbances in adult rats. Physiol. Behav. 2021, 240, 113543. [Google Scholar] [CrossRef]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Glutamatergic System is Affected in Brain from an Hyperthermia-Induced Seizures Rat Model. Cell. Mol. Neurobiol. 2022, 42, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-L.; Leung, L.S. Decrease of hippocampal GABA B receptor-mediated inhibition after hyperthermia-induced seizures in immature rats. Epilepsia 2006, 47, 277–287. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Griflyuk, A.V.; Amakhin, D.V.; Kovalenko, A.A.; Soboleva, E.B.; Zubareva, O.E.; Zaitsev, A.V. Early Life Febrile Seizures Impair Hippocampal Synaptic Plasticity in Young Rats. Int. J. Mol. Sci. 2021, 22, 8218. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kuo, Y.-M.; Huang, A.-M.; Huang, C.-C. Repetitive febrile seizures in rat pups cause long-lasting deficits in synaptic plasticity and NR2A tyrosine phosphorylation. Neurobiol. Dis. 2005, 18, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Feng, B.; Tang, Y.; You, Y.; Wang, Y.; Hou, W.; Hu, W.; Chen, Z. Blocking GluN2B subunits reverses the enhanced seizure susceptibility after prolonged febrile seizures with a wide therapeutic time-window. Exp. Neurol. 2016, 283, 29–38. [Google Scholar] [CrossRef]
- González Ramírez, M.; Orozco Suárez, S.; Salgado Ceballos, H.; Feria Velasco, A.; Rocha, L. Hyperthermia-induced seizures modify the GABA(A) and benzodiazepine receptor binding in immature rat brain. Cell. Mol. Neurobiol. 2007, 27, 211–227. [Google Scholar] [CrossRef]
- Deprez, L.; Weckhuysen, S.; Peeters, K.; Deconinck, T.; Claeys, K.G.; Claes, L.R.F.; Suls, A.; Van Dyck, T.; Palmini, A.; Matthijs, G.; et al. Epilepsy as part of the phenotype associated with ATP1A2 mutations. Epilepsia 2008, 49, 500–508. [Google Scholar] [CrossRef]
- Clapcote, S.J.; Duffy, S.; Xie, G.; Kirshenbaum, G.; Bechard, A.R.; Rodacker Schack, V.; Petersen, J.; Sinai, L.; Saab, B.J.; Lerch, J.P.; et al. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc. Natl. Acad. Sci. USA 2009, 106, 14085–14090. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Furian, A.F.; Royes, L.F.F.; Fighera, M.R.; de Carvalho Myskiw, J.; Fiorenza, N.G.; Mello, C.F. Ascorbate modulates pentylenetetrazol-induced convulsions biphasically. Neuroscience 2004, 128, 721–728. [Google Scholar] [CrossRef]
- Marquezan, B.P.; Funck, V.R.; Oliveira, C.V.; Pereira, L.M.; Araújo, S.M.; Zarzecki, M.S.; Royes, L.F.F.; Furian, A.F.; Oliveira, M.S. Pentylenetetrazol-induced seizures are associated with Na+,K+-ATPase activity decrease and alpha subunit phosphorylation state in the mice cerebral cortex. Epilepsy Res. 2013, 105, 396–400. [Google Scholar] [CrossRef]
- Akkuratov, E.E.; Lopacheva, O.M.; Kruusmägi, M.; Lopachev, A.V.; Shah, Z.A.; Boldyrev, A.A.; Liu, L. Functional Interaction between Na/K-ATPase and NMDA Receptor in Cerebellar Neurons. Mol. Neurobiol. 2015, 52, 1726–1734. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, Q.; Wang, M.; Lin, A.; Jarzylo, L.; Navis, A.; Raissi, A.; Liu, F.; Man, H.-Y. Na, K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J. Neurosci. 2009, 29, 4498–4511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therien, A.G.; Blostein, R. Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 2000, 279, C541–C566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, H.; Morth, P.; Egebjerg, J.; Nissen, P. Phosphorylation of the Na+,K+-ATPase and the H+,K+-ATPase. FEBS Lett. 2010, 584, 2589–2595. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, M.J.; Naffah-Mazzacoratti, M.G.; Cavalheiro, E.A. Na+K+ ATPase activity in the rat hippocampus: A study in the pilocarpine model of epilepsy. Neurochem. Int. 1996, 28, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, E.R.; Arida, R.M.; de Oliveira, D.M.; da Silva Fernandes, M.J. The Na+/K+ ATPase activity is increased in the hippocampus after multiple status epilepticus induced by pilocarpine in developing rats. Brain Res. 2007, 1138, 203–207. [Google Scholar] [CrossRef]
- Du, M.; Li, J.; Ying, W.; Yu, Y. A dynamics model of neuron-astrocyte network accounting for febrile seizures. Cogn. Neurodyn. 2022, 16, 411–423. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Free, R.B.; Cabrera, D.M.; Skinbjerg, M.; Sibley, D.R. Reciprocal modulation of function between the D1 and D2 dopamine receptors and the Na+,K+-ATPase. J. Biol. Chem. 2008, 283, 36441–36453. [Google Scholar] [CrossRef] [Green Version]
- Monyer, H.; Sprengel, R.; Schoepfer, R.; Herb, A.; Higuchi, M.; Lomeli, H.; Burnashev, N.; Sakmann, B.; Seeburg, P.H. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science 1992, 256, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Babb, T.L.; Comair, Y.G.; Bushey, M.; Touhalisky, K. Increased densities of AMPA GluR1 subunit proteins and presynaptic mossy fiber sprouting in the fascia dentata of human hippocampal epilepsy. Brain Res. 1998, 798, 239–246. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Li, L.; Ma, L.; Liu, X. Effects of perampanel on cognitive behavior and GluR1 expression in immature mice of temporal lobe epilepsy. Biochem. Biophys. Res. Commun. 2022, 588, 68–74. [Google Scholar] [CrossRef]
- Stangherlin, A.; O’Neill, J.S. Signal Transduction: Magnesium Manifests as a Second Messenger. Curr. Biol. 2018, 28, R1403–R1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M.; Rubin, H. Evidence that intracellular magnesium is present in cells at a regulatory concentration for protein synthesis. Proc. Natl. Acad. Sci. USA 1985, 82, 7324–7326. [Google Scholar] [CrossRef] [Green Version]
- Knape, M.J.; Ahuja, L.G.; Bertinetti, D.; Burghardt, N.C.G.; Zimmermann, B.; Taylor, S.S.; Herberg, F.W. Divalent Metal Ions Mg2+ and Ca2+ Have Distinct Effects on Protein Kinase A Activity and Regulation. ACS Chem. Biol. 2015, 10, 2303–2315. [Google Scholar] [CrossRef] [Green Version]
- Mathew, A.A.; Panonnummal, R. ’Magnesium’—The master cation-as a drug-possibilities and evidences. Biometals 2021, 34, 955–986. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, R.M.; Feng, D.; Jordán, J. Neuropharmacological effects of lipoic acid and ubiquinone on δ-aminolevulinic dehydratase, Na+, K+-ATPase, and Mg2+-ATPase activities in rat hippocampus after pilocarpine-induced seizures. Fundam. Clin. Pharmacol. 2011, 25, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.A.L.; Crespo, M.; Martín, M. Oxidative stress in epileptogenesis: Febrile seizures, chemoconvulsant pilocarpine, and electrical stimulation. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 81–94. [Google Scholar]
- López-Zapata, A.; León-Navarro, D.A.; Crespo, M.; Martín, M. Gender-specific desensitization of group I metabotropic glutamate receptors after maternal l-glutamate intake during lactation. Int. J. Dev. Neurosci. 2018, 68, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Schweinberger, B.M.; Schwieder, L.; Scherer, E.; Sitta, A.; Vargas, C.R.; Wyse, A.T.S. Development of an animal model for gestational hypermethioninemia in rat and its effect on brain Na+,K+-ATPase/Mg2+-ATPase activity and oxidative status of the offspring. Metab. Brain Dis. 2014, 29, 153–160. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo, M.; León-Navarro, D.A.; Martín, M. Na+/K+- and Mg2+-ATPases and Their Interaction with AMPA, NMDA and D2 Dopamine Receptors in an Animal Model of Febrile Seizures. Int. J. Mol. Sci. 2022, 23, 14638. https://doi.org/10.3390/ijms232314638
Crespo M, León-Navarro DA, Martín M. Na+/K+- and Mg2+-ATPases and Their Interaction with AMPA, NMDA and D2 Dopamine Receptors in an Animal Model of Febrile Seizures. International Journal of Molecular Sciences. 2022; 23(23):14638. https://doi.org/10.3390/ijms232314638
Chicago/Turabian StyleCrespo, María, David Agustín León-Navarro, and Mairena Martín. 2022. "Na+/K+- and Mg2+-ATPases and Their Interaction with AMPA, NMDA and D2 Dopamine Receptors in an Animal Model of Febrile Seizures" International Journal of Molecular Sciences 23, no. 23: 14638. https://doi.org/10.3390/ijms232314638





