Therapeutic Potential of Plant Oxylipins
Abstract
:1. Introduction
2. Variety of Plant Oxylipins
3. Potential Cellular Targets for Inflammation, Allergy, and Cancer Treatment
3.1. Nuclear Factor NF-κB
3.2. Peroxisome Proliferator-Activated Receptor (PPAR)
3.3. Other Cellular Targets
4. Therapeutic Properties of Plant Oxylipins
4.1. Acetylenic Fatty Acid Derivatives
4.2. Jasmonates
4.3. Hydroperoxide Lyase Branch Oxylipins
4.4. Oxy-, Hydroxy-, and Epoxy-Derivatives of Fatty Acids
4.5. Phytoprostanes and Phytofurans
4.6. Unusual and Unidentified Oxylipins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LOX | Lipoxygenase |
| α-DOG | α-Dioxygenase |
| AOS | Allene oxide synthase |
| HPL | Hydroperoxide lyase |
| DES | Divinyl ether synthase |
| EAS | Epoxy alcohol synthase |
| JA | Jasmonic acid |
| MeJA | Methyl jasmonate |
| 12-OPDA | 12-Oxo-phytodienoic acid |
| dn-OPDA | Dinor-12-oxophytodienoic acid |
| PP | Phytoprostane |
| RES | Reactive electrophile species |
| ILs | Interleukins |
| TNF-α | Tumor necrosis factor alpha |
| PKC-β | Protein kinase C-beta |
| NF-κB | Nuclear factor kappa B |
| PPAR | Peroxisome proliferation activator receptor |
| TA | Traumatic acid |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| NRF2 | Nuclear factor associated with erythroid factor 2 |
| iNOS | Inducible nitric oxide synthase |
| GABA | γ-Aminobutyric acid |
| SOD | Superoxide dismutase |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCloud, T.G. High Throughput Extraction of Plant, Marine and Fungal Specimens for Preservation of Biologically Active Molecules. Molecules 2010, 15, 4526–4563. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants Metabolites: Possibility of Natural Therapeutics Against the COVID-19 Pandemic. Front. Med. 2020, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Wolfender, J.-L. Innovative Strategies in the Search for Bioactive Plant Constituents. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2014; pp. 1–20. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: From discovery to clinic. J. Ethnopharmacol. 1996, 51, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Global_Biomedical_Materials_Market_Research_Report. Available online: https://www.marketgrowthreports.com/global-biomedical-materials-market-21051012 (accessed on 29 August 2022).
- Apaya, M.K.; Lin, C.-Y.; Chiou, C.-Y.; Yang, C.-C.; Ting, C.-Y.; Shyur, L.-F. Simvastatin and a Plant Galactolipid Protect Animals from Septic Shock by Regulating Oxylipin Mediator Dynamics through the MAPK-cPLA2 Signaling Pathway. Mol. Med. 2015, 21, 988–1001. [Google Scholar] [CrossRef]
- Nappo, M.; Berkov, S.; Massucco, C.; Di Maria, V.; Bastida, J.; Codina, C.; Avila, C.; Messina, P.; Zupo, V.; Zupo, S. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm. Biol. 2012, 50, 529–535. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Heuvel, J.P.V.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 2005, 336, 909–917. [Google Scholar] [CrossRef]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef]
- Straus, D.S.; Glass, C.K. Cyclopentenone prostaglandins: New insights on biological activities and cellular targets. Med. Res. Rev. 2001, 21, 185–210. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- van Ryn, J.; Trummlitz, G.; Pairet, M. COX-2 selectivity and inflammatory processes. Curr. Med. Chem. 2000, 7, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.C. Shared Signals and the Potential for Phylogenetic Espionage Between Plants and Animals. Integr. Comp. Biol. 2002, 42, 454–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savchenko, T.V.; Zastrijnaja, O.M.; Klimov, V.V. Oxylipins and plant abiotic stress resistance. Biochemistry 2014, 79, 362–375. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Blee, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Ricker, K.E.; Bostock, R.M. Evidence for release of the elicitor arachidonic acid and its metabolites from sporangia of Phytophthora infestans during infection of potato. Physiol. Mol. Plant Pathol. 1992, 41, 61–72. [Google Scholar] [CrossRef]
- Savchenko, T.; Pearse, I.S.; Ignatia, L.; Karban, R.; Dehesh, K. Insect herbivores selectively suppress the HPL branch of the oxylipin pathway in host plants. Plant J. 2013, 73, 653–662. [Google Scholar] [CrossRef]
- Savchenko, T.; Dehesh, K. Insect herbivores selectively mute GLV production in plants. Plant Signal. Behav. 2013, 8, e24136. [Google Scholar] [CrossRef]
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Lee, G.I.; Itoh, A.; Li, L.; DeRocher, A.E. Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiol. 2000, 123, 711–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fammartino, A.; Cardinale, F.; Gobel, C.; Mene-Saffrane, L.; Fournier, J.; Feussner, I.; Esquerre-Tugaye, M.T. Characterization of a divinyl ether biosynthetic pathway specifically associated with pathogenesis in tobacco. Plant Physiol. 2007, 143, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grechkin, A.N. Hydroperoxide lyase and divinyl ether synthase. Prostaglandins Other Lipid Mediat. 2002, 68–69, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Saffert, A.; Hartmann-Schreier, J.; Schon, A.; Schreier, P. A dual function alpha-dioxygenase-peroxidase and NAD(+) oxidoreductase active enzyme from germinating pea rationalizing alpha-oxidation of fatty acids in plants. Plant Physiol. 2000, 123, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamberg, M. Biosynthesis of new divinyl ether oxylipins in Ranunculus plants. Lipids 2002, 37, 427–433. [Google Scholar] [CrossRef]
- Hamberg, M. Hidden stereospecificity in the biosynthesis of divinyl ether fatty acids. FEBS J. 2005, 272, 736–743. [Google Scholar] [CrossRef]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. PPB 2009, 47, 511–517. [Google Scholar] [CrossRef]
- Liavonchanka, A.; Feussner, I. Lipoxygenases: Occurrence, functions and catalysis. J. Plant Physiol. 2006, 163, 348–357. [Google Scholar] [CrossRef]
- Andreou, A.; Feussner, I. Lipoxygenases—Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef]
- Kihara, H.; Tanaka, M.; Yamato, K.T.; Horibata, A.; Yamada, A.; Kita, S.; Ishizaki, K.; Kajikawa, M.; Fukuzawa, H.; Kohchi, T.; et al. Arachidonic acid-dependent carbon-eight volatile synthesis from wounded liverwort (Marchantia polymorpha). Phytochemistry 2014, 107, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feussner, I.; Balkenhohl, T.J.; Porzel, A.; Kühn, H.; Wasternack, C. Structural Elucidation of Oxygenated Storage Lipids in Cucumber Cotyledons: Implication of lipid body lipoxygenase in lipid mobilization during germination. J. Biol. Chem. 1997, 272, 21635–21641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamberg, M.; Sanz, A.; Castresana, C. α-Oxidation of Fatty Acids in Higher Plants: Identification of a pathogen-inducible oxygenase (piox) as an α-dioxygenase and biosynthesis of 2-hydroperoxylinolenic acid. J. Biol. Chem. 1999, 274, 24503–24513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaber, A.; Tamoi, M.; Takeda, T.; Nakano, Y.; Shigeoka, S. NADPH-dependent glutathione peroxidase-like proteins (Gpx-1, Gpx-2) reduce unsaturated fatty acid hydroperoxides in Synechocystis PCC 6803. FEBS Lett. 2001, 499, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Blee, E. Phytooxylipins and plant defense reactions. Prog. Lipid Res. 1998, 37, 33–72. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Kolomiets, M.V. Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev. 2009, 28, 79–88. [Google Scholar] [CrossRef]
- Lee, D.S.; Nioche, P.; Hamberg, M.; Raman, C.S. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 2008, 455, 363–368. [Google Scholar] [CrossRef]
- Matsui, K.; Minami, A.; Hornung, E.; Shibata, H.; Kishimoto, K.; Ahnert, V.; Kindl, H.; Kajiwara, T.; Feussner, I. Biosynthesis of fatty acid derived aldehydes is induced upon mechanical wounding and its products show fungicidal activities in cucumber. Phytochemistry 2006, 67, 649–657. [Google Scholar] [CrossRef]
- Toporkova, Y.Y.; Askarova, E.K.; Gorina, S.S.; Ogorodnikova, A.V.; Mukhtarova, L.S.; Grechkin, A.N. Epoxyalcohol synthase activity of the CYP74B enzymes of higher plants. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158743. [Google Scholar] [CrossRef] [PubMed]
- Toporkova, Y.Y.; Fatykhova, V.S.; Gogolev, Y.V.; Khairutdinov, B.I.; Mukhtarova, L.S.; Grechkin, A.N. Epoxyalcohol synthase of Ectocarpus siliculosus. First CYP74-related enzyme of oxylipin biosynthesis in brown algae. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Hamberg, G. Hydroperoxide-dependent epoxidation of unsaturated fatty acids in the broad bean (Vicia faba L.). Arch. Biochem. Biophys. 1990, 283, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Hamberg, G. Peroxygenase-Catalyzed Fatty Acid Epoxidation in Cereal Seeds (Sequential Oxidation of Linoleic Acid into 9(S),12(S),13(S)-Trihydroxy-10(E)-Octadecenoic Acid). Plant Physiol. 1996, 110, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Blee, E.; Schuber, F. Efficient epoxidation of unsaturated fatty acids by a hydroperoxide-dependent oxygenase. J. Biol. Chem. 1990, 265, 12887–12894. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Song, J.T.; Cheong, J.J.; Lee, Y.H.; Lee, Y.W.; Hwang, I.; Lee, J.S.; Choi, Y.D. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. USA 2001, 98, 4788–4793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parchmann, S.; Gundlach, H.; Mueller, M.J. Induction of 12-oxo-phytodienoic acid in wounded plants and elicited plant cell cultures. Plant Physiol. 1997, 115, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Stintzi, A.; Weber, H.; Reymond, P.; Browse, J.; Farmer, E.E. Plant defense in the absence of jasmonic acid: The role of cyclopentenones. Proc. Natl. Acad. Sci. USA 2001, 98, 12837–12842. [Google Scholar] [CrossRef] [Green Version]
- Krumm, T.; Bandemer, K.; Boland, W. Induction of volatile biosynthesis in the lima bean (Phaseolus lunatus) by leucine- and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: Evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signalling pathway. FEBS Lett. 1995, 377, 523–529. [Google Scholar] [CrossRef]
- Kramell, R.; Miersch, O.; Hause, B.; Ortel, B.; Parthier, B.; Wasternack, C. Amino acid conjugates of jasmonic acid induce jasmonate-responsive gene expression in barley (Hordeum vulgare L.) leaves. FEBS Lett. 1997, 414, 197–202. [Google Scholar]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar] [CrossRef]
- Von Dahl, C.C.; Baldwin, I.T. Methyl jasmonate and cis-jasmone do not dispose of the herbivore-induced jasmonate burst in Nicotiana attenuata. Physiol. Plant 2004, 120, 474–481. [Google Scholar] [CrossRef]
- Gidda, S.K.; Miersch, O.; Levitin, A.; Schmidt, J.; Wasternack, C.; Varin, L. Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 17895–17900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, A.J.; Howe, G.A. Catabolism and deactivation of the lipid-derived hormone jasmonoyl-isoleucine. Front. Plant. Sci. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miersch, O.; Neumerkel, J.; Dippe, M.; Stenzel, I.; Wasternack, C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol. 2008, 177, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Baldwin, I.T.; Galis, I. NaJAZh Regulates a Subset of Defense Responses against Herbivores and Spontaneous Leaf Necrosis in Nicotiana attenuata Plants. Plant Physiol. 2012, 159, 769–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.G.; Ellis, C.; Devoto, A. The jasmonate signal pathway. Plant Cell 2002, 14, S153–S164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noordermeer, M.A.; Van Dijken, A.J.; Smeekens, S.C.; Veldink, G.A.; Vliegenthart, J.F. Characterization of three cloned and expressed 13-hydroperoxide lyase isoenzymes from alfalfa with unusual N-terminal sequences and different enzyme kinetics. Eur. J. Biochem. 2000, 267, 2473–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, D.C.; Coudron, C.A. Identification of Traumatin, a Wound Hormone, as 12-Oxo-trans-10-dodecenoic Acid. Plant Physiol. 1979, 63, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vick, B.A.; Zimmerman, D.C. Levels of oxygenated Fatty acids in young corn and sunflower plants. Plant Physiol. 1982, 69, 1103–1108. [Google Scholar] [CrossRef]
- Grechkin, A.N.; Bruhlmann, F.; Mukhtarova, L.S.; Gogolev, Y.V.; Hamberg, M. Hydroperoxide lyases (CYP74C and CYP74B) catalyze the homolytic isomerization of fatty acid hydroperoxides into hemiacetals. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2006, 1761, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Grechkin, A.N.; Mukhtarova, L.S.; Hamberg, M. Detection of an enol intermediate in the hydroperoxide lyase chain cleavage reaction. FEBS Lett. 2003, 549, 31–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vancanneyt, G.; Sanz, C.; Farmaki, T.; Paneque, M.; Ortego, F.; Castanera, P.; Sanchez-Serrano, J.J. Hydroperoxide lyase depletion in transgenic potato plants leads to an increase in aphid performance. Proc. Natl. Acad. Sci. USA 2001, 98, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, H.; Chetelat, A.; Caldelari, D.; Farmer, E.E. Divinyl ether fatty acid synthesis in late blight-diseased potato leaves. Plant Cell 1999, 11, 485–494. [Google Scholar]
- Itoh, A.; Howe, G.A. Molecular cloning of a divinyl ether synthase. Identification as a CYP74 cytochrome P-450. J. Biol. Chem. 2001, 276, 3620–3627. [Google Scholar] [CrossRef] [Green Version]
- Grechkin, A.N.; Hamberg, M. Divinyl ether synthase from garlic (Allium sativum L.) bulbs: Sub-cellular localization and substrate regio-and stereospecificity. FEBS Lett. 1996, 388, 112–114. [Google Scholar] [CrossRef] [Green Version]
- Toporkova, Y.Y.; Gorina, S.S.; Bessolitsyna, E.K.; Smirnova, E.O.; Fatykhova, V.S.; Brühlmann, F.; Ilyina, T.M.; Mukhtarova, L.S.; Grechkin, A.N. Double function hydroperoxide lyases/epoxyalcohol synthases (CYP74C) of higher plants: Identification and conversion into allene oxide synthases by site-directed mutagenesis. Biochim. Biophys. Acta. (BBA) Mol. Cell Biol. Lipids 2018, 1863, 369–378. [Google Scholar] [CrossRef]
- Toporkova, Y.Y.; Smirnova, E.O.; Iljina, T.M.; Mukhtarova, L.S.; Gorina, S.S.; Grechkin, A.N. The CYP74B and CYP74D divinyl ether synthases possess a side hydroperoxide lyase and epoxyalcohol synthase activities that are enhanced by the site-directed mutagenesis. Phytochemistry 2020, 179, 112512. [Google Scholar] [CrossRef]
- Hughes, R.K.; Yousafzai, F.K.; Ashton, R.; Chechetkin, I.R.; Fairhurst, S.A.; Hamberg, M.; Casey, R. Evidence for communality in the primary determinants of CYP74 catalysis and of structural similarities between CYP74 and classical mammalian P450 enzymes. Proteins 2008, 72, 1199–1211. [Google Scholar] [CrossRef]
- Gorshkov, V.Y.; Toporkova, Y.Y.; Tsers, I.D.; Smirnova, E.O.; Ogorodnikova, A.V.; Gogoleva, N.E.; Parfirova, O.I.; Petrova, O.E.; Gogolev, Y.V. Differential modulation of the lipoxygenase cascade during typical and latent Pectobacterium atrosepticum infections. Ann. Bot. 2022, 129, 271–286. [Google Scholar] [CrossRef]
- Hamberg, M. An epoxy alcohol synthase pathway in higher plants: Biosynthesis of antifungal trihydroxy oxylipins in leaves of potato. Lipids 1999, 34, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Hanano, A.; Burcklen, M.; Flenet, M.; Ivancich, A.; Louwagie, M.; Garin, J.; Blee, E. Plant seed peroxygenase is an original heme-oxygenase with an EF-hand calcium binding motif. J. Biol. Chem. 2006, 281, 33140–33151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blée, E.; Flenet, M.; Boachon, B.; Fauconnier, M.L. A non-canonical caleosin from Arabidopsis efficiently epoxidizes physiological unsaturated fatty acids with complete stereoselectivity. FEBS J. 2012, 279, 3981–3995. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic. Biol. Med. 1989, 7, 65–86. [Google Scholar] [CrossRef]
- Partridge, M.; Murphy, D.J. Roles of a membrane-bound caleosin and putative peroxygenase in biotic and abiotic stress responses in Arabidopsis. Plant Physiol. Biochem. PPB 2009, 47, 796–806. [Google Scholar] [CrossRef]
- Göbel, C.; Feussner, I.; Schmidt, A.; Scheel, D.; Sanchez-Serrano, J.; Hamberg, M.; Rosahl, S. Oxylipin Profiling Reveals the Preferential Stimulation of the 9-Lipoxygenase Pathway in Elicitor-treated Potato Cells. J. Biol. Chem. 2001, 276, 6267–6273. [Google Scholar] [CrossRef] [Green Version]
- Blée, E. Biosynthesis of phytooxylipins: The Peroxygenase pathway. Lipid Fett. 1998, 100, 121–127. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, P.; Sharma, B.; Guerrero-Perilla, C.; Coy-Barrera, E. Chapter 23—Analysis of polyacetylenes. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 707–722. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Prior, R.M.; Lundgaard, N.H.; Light, M.E.; Stafford, G.I.; van Staden, J.; Jäger, A.K. The polyacetylene falcarindiol with COX-1 activity isolated from Aegopodium podagraria L. J. Ethnopharmacol. 2007, 113, 176–178. [Google Scholar] [CrossRef]
- Santos, P.; Busta, L.; Yim, W.C.; Cahoon, E.B.; Kosma, D.K. Structural diversity, biosynthesis, and function of plant falcarin-type polyacetylenic lipids. J. Exp. Bot. 2022, 73, 2889–2904. [Google Scholar] [CrossRef] [PubMed]
- Cunsolo, F.; Ruberto, G.; Amico, V.; Piattelli, M. Bioactive Metabolites from Sicilian Marine Fennel, Crithmum maritimum. J. Nat. Prod. 1993, 56, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Durand, T.; Bultel-Ponce, V.; Guy, A.; Berger, S.; Mueller, M.J.; Galano, J.M. New bioactive oxylipins formed by non-enzymatic free-radical-catalyzed pathways: The phytoprostanes. Lipids 2009, 44, 875–888. [Google Scholar] [CrossRef]
- Imbusch, R.; Mueller, M.J. Analysis of oxidative stress and wound-inducible dinor isoprostanes F1 (phytoprostanes F1) in plants. Plant Physiol. 2000, 124, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbusch, R.; Mueller, M.J. Formation of isoprostane F2-like compounds (phytoprostanes F1) from α-linolenic acid in plants. Free Radic. Biol. Med. 2000, 28, 720–726. [Google Scholar] [CrossRef]
- Yonny, M.E.; Rodríguez Torresi, A.; Cuyamendous, C.; Réversat, G.; Oger, C.; Galano, J.-M.; Durand, T.; Vigor, C.; Nazareno, M.A. Thermal Stress in Melon Plants: Phytoprostanes and Phytofurans as Oxidative Stress Biomarkers and the Effect of Antioxidant Supplementation. J. Agric. Food Chem. 2016, 64, 8296–8304. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Gil-Izquierdo, Á.; Durand, T.; Ferreres, F.; Domínguez-Perles, R. Structural/Functional Matches and Divergences of Phytoprostanes and Phytofurans with Bioactive Human Oxylipins. Antioxidants 2018, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Sattler, S.E.; Mene-Saffrane, L.; Farmer, E.E.; Krischke, M.; Mueller, M.J.; DellaPenna, D. Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 2006, 18, 3706–3720. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.J. Radically novel prostaglandins in animals and plants: The isoprostanes. Chem. Biol. 1998, 5, R323–R333. [Google Scholar] [CrossRef] [Green Version]
- Cuyamendous, C.; de la Torre, A.; Lee, Y.Y.; Leung, K.S.; Guy, A.; Bultel-Poncé, V.; Galano, J.-M.; Lee, J.C.-Y.; Oger, C.; Durand, T. The novelty of phytofurans, isofurans, dihomo-isofurans and neurofurans: Discovery, synthesis and potential application. Biochimie 2016, 130, 49–62. [Google Scholar] [CrossRef]
- Gobel, C.; Feussner, I.; Hamberg, M.; Rosahl, S. Oxylipin profiling in pathogen-infected potato leaves. Biochim. Et Biophys. Acta 2002, 1584, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Thoma, I.; Krischke, M.; Loeffler, C.; Mueller, M.J. The isoprostanoid pathway in plants. Chem. Phys. Lipids 2004, 128, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Deboever, E.; Deleu, M.; Mongrand, S.; Lins, L.; Fauconnier, M.L. Plant-Pathogen Interactions: Underestimated Roles of Phyto-oxylipins. Trends Plant Sci. 2020, 25, 22–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karcher, S.C.; Laufer, S.A. Successful structure-based design of recent p38 MAP kinase inhibitors. Curr. Top. Med. Chem. 2009, 9, 655–676. [Google Scholar] [CrossRef]
- Voronov, E.; Apte, R.; Sofer, S. The systemic inflammatory response syndrome related to the release of cytokines following severe envenomation. J. Venom. Anim. Toxins 1999, 5, 5–33. [Google Scholar] [CrossRef]
- Crockett-Torabi, E.; Ward, P.A. The role of leukocytes in tissue injury. Eur. J. Anaesthesiol. 1996, 13, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Gunjegaonkar, S.M.; Shanmugarajan, T.S. Molecular mechanism of plant stress hormone methyl jasmonate for its anti-inflammatory activity. Plant Signal. Behav. 2019, 14, e1642038. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Sun, S.C.; Chang, J.H.; Jin, J. Regulation of nuclear factor-κB in autoimmunity. Trends Immunol. 2013, 34, 282–289. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, N.; Wagner, K.D. PPAR Beta/Delta and the Hallmarks of Cancer. Cells 2020, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Elbrecht, A.; Chen, Y.; Cullinan, C.A.; Hayes, N.; Leibowitz, M.; Moller, D.E.; Berger, J. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors gamma 1 and gamma 2. Biochem. Biophys. Res. Commun. 1996, 224, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Naruhn, S.; Meissner, W.; Adhikary, T.; Kaddatz, K.; Klein, T.; Watzer, B.; Müller-Brüsselbach, S.; Müller, R. 15-hydroxyeicosatetraenoic acid is a preferential peroxisome proliferator-activated receptor β/δ agonist. Mol. Pharmacol. 2010, 77, 171–184. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Tarling, E.J.; Bennett, A.J.; Kendall, D.A.; Randall, M.D. Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem. Biophys. Res. Commun. 2005, 337, 824–831. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Burstein, S.H.; Zurier, R.B.; Chen, J.D. Activation and binding of peroxisome proliferator-activated receptor gamma by synthetic cannabinoid ajulemic acid. Mol. Pharmacol. 2003, 63, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Ezzeddini, R.; Taghikhani, M.; Salek Farrokhi, A.; Somi, M.H.; Samadi, N.; Esfahani, A.; Rasaee, M.J. Downregulation of fatty acid oxidation by involvement of HIF-1α and PPARγ in human gastric adenocarcinoma and related clinical significance. J. Physiol. Biochem. 2021, 77, 249–260. [Google Scholar] [CrossRef]
- Philippi, A.; Roschmann, E.; Tores, F.; Lindenbaum, P.; Benajou, A.; Germain-Leclerc, L.; Marcaillou, C.; Fontaine, K.; Vanpeene, M.; Roy, S.; et al. Haplotypes in the gene encoding protein kinase c-beta (PRKCB1) on chromosome 16 are associated with autism. Mol. Psychiatry 2005, 10, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.P. Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef]
- Li, C.; Cheng, L.; Wu, H.; He, P.; Zhang, Y.; Yang, Y.; Chen, J.; Chen, M. Activation of the KEAP1-NRF2-ARE signaling pathway reduces oxidative stress in Hep2 cells. Mol. Med. Rep. 2018, 18, 2541–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.L.; Murphy, R.C. Chapter 9—The Eicosanoids: Cyclooxygenase, Lipoxygenase and Epoxygenase Pathways. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 259–296. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo-Watanabe, A.; Okuno, T.; Yokomizo, T. The Role of Leukotrienes as Potential Therapeutic Targets in Allergic Disorders. Int. J. Mol. Sci. 2019, 20, 3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, M.S. Falcarindiol: An antifungal polyacetylene from Aegopodium podagraria. Phytochemistry 1978, 17, 1002. [Google Scholar] [CrossRef]
- Schinkovitz, A.; Stavri, M.; Gibbons, S.; Bucar, F. Antimycobacterial polyacetylenes from Levisticum officinale. Phytother. Res. PTR 2008, 22, 681–684. [Google Scholar] [CrossRef] [Green Version]
- Kobaisy, M.; Abramowski, Z.; Lermer, L.; Saxena, G.; Hancock, R.E.; Towers, G.H.; Doxsee, D.; Stokes, R.W. Antimycobacterial polyynes of Devil’s Club (Oplopanax horridus), a North American native medicinal plant. J. Nat. Prod. 1997, 60, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.C.; Everngam, M.C.; Sturtz, G.; Beck, J.J. Antibacterial activity of components from Lomatium californicum. Phytother. Res. PTR 2006, 20, 153–156. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Martín-Quintal, Z.; del Rosario García-Miss, M.; Mut-Martín, M.; Matus-Moo, A.; Torres-Tapia, L.W.; Peraza-Sánchez, S.R. The leishmanicidal effect of (3S)-16,17-didehydrofalcarinol, an oxylipin isolated from Tridax procumbens, is independent of NO production. Phytother. Res. PTR 2010, 24, 1004–1008. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Furumi, K.; Fujioka, T.; Fujii, H.; Okabe, H.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M.; Mihashi, K. Novel antiproliferative falcarindiol furanocoumarin ethers from the root of Angelica japonica. Bioorg. Med. Chem. Lett. 1998, 8, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential Effects of Falcarinol and Related Aliphatic C17-Polyacetylenes on Intestinal Cell Proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djebara, A.; Ciavatta, M.L.; Mathieu, V.; Colin, M.; Bitam, F.; Carbone, M.; Gavagnin, M. Oxygenated C17 polyacetylene metabolites from Algerian Eryngium tricuspidatum L. roots: Structure and biological activity. Fitoterapia 2019, 138, 104355. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Zschocke, S.; Reininger, E.; Bauer, R. Comparison of Radix Angelicae pubescentis and Substitutes—Constituents and Inhibitory Effect on 5-lipoxygenase and Cyclooxygenase. Pharm. Biol. 1998, 36, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 2: Medicinal plants with inhibitory activity on arachidonate 12-lipoxygenase, 15-lipoxygenase and leukotriene receptor antagonists. Phytother. Res. PTR 2005, 19, 263–272. [Google Scholar] [CrossRef]
- Liu, J.-H.; Zschocke, S.; Bauer, R. A Polyacetylenic acetate and a coumarin from Angelica pubescens f. biserrata. Phytochemistry 1998, 49, 211–213. [Google Scholar] [CrossRef]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Alanko, J.; Kurahashi, Y.; Yoshimoto, T.; Yamamoto, S.; Baba, K. Panaxynol, a polyacetylene compound isolated from oriental medicines, inhibits mammalian lipoxygenases. Biochem. Pharm. 1994, 48, 1979–1981. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Sakuma, S.; Komatsu, S.; Sato, D.; Nishida, H.; Xiao, Y.-Q.; Baba, K.; Fujita, T. Inhibition of 15-Hydroxyprostaglandin Dehydrogenase Activity in Rabbit Gastric Antral Mucosa by Panaxynol Isolated from Oriental Medicines. J. Pharm. Pharmacol. 2011, 50, 1075–1078. [Google Scholar] [CrossRef]
- Yamazaki, M.; Hirakura, K.; Miyaichi, Y.; Imakura, K.; Kita, M.; Chiba, K.; Mohri, T. Effect of Polyacetylenes on the Neurite Outgrowth of Neuronal Culture Cells and Scopolamine-Induced Memory Impairment in Mice. Biol. Pharm. Bull. 2001, 24, 1434–1436. [Google Scholar] [CrossRef] [Green Version]
- Nie, B.-M.; Jiang, X.-Y.; Cai, J.-X.; Fu, S.-L.; Yang, L.-M.; Lin, L.; Hang, Q.; Lu, P.-L.; Lu, Y. Panaxydol and panaxynol protect cultured cortical neurons against Aβ25–35-induced toxicity. Neuropharmacology 2008, 54, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Chen, S.N.; Yao, P.; Nikolic, D.; van Breemen, R.B.; Bolton, J.L.; Fong, H.H.; Farnsworth, N.R.; Pauli, G.F. Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. J. Nat. Prod. 2006, 69, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenichi, K.; Hiroyuki, E.; Kuniyoshi, M. Calcium2+ Signal Transduction Inhibitor. JP2005000044775, 2006. [Google Scholar]
- Gow, R.T.; Sypert, G.W.; Li, D.; Yan, X. Methods and Compositions Comprising Panax Species. U.S. Patent 2007/0065526A1, 22 March 2007. [Google Scholar]
- Nadler, J.L.; Wen, Y. Method of Treating or Preventing Abnormal Cardiac Cell Growth by Inhibiting the 12-Lipoxygenase Pathway. WO1999059562, 25 November 1999. [Google Scholar]
- Dizer, D.; Alon, R.; Kanner, Y. Gram Negative Biocide Obtainable from Apiaceae Vegetables. WO2009IB0007516, 2010. [Google Scholar]
- Yoshimitsu, A.; Takeshi, U. Agent for Treatment of Disease Caused by Virus Such As Herpes. JP1993000041667, 1994. [Google Scholar]
- Baldi, A.; Dixit, V.K. Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour. Technol. 2008, 99, 4609–4614. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Chan, A.T. Fruit, vegetables, and folate: Cultivating the evidence for cancer prevention. Gastroenterology 2011, 141, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Leonardi, M. Treatment of fibrocystic disease of the breast with myrtillus anthocyanins. Our experience. Minerva Ginecol. 1993, 45, 617–621. [Google Scholar]
- Morazzoni, P.; Bombardelli, E. Vaccinium myrtillus. Fitoterapia 1996, 67, 3–29. [Google Scholar]
- Schreiner, M.; Krumbein, A.; Knorr, D.; Smetanska, I. Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J. Agric. Food Chem. 2011, 59, 1400–1405. [Google Scholar] [CrossRef]
- Wu, Q.J.; Xie, L.; Zheng, W.; Vogtmann, E.; Li, H.L.; Yang, G.; Ji, B.T.; Gao, Y.T.; Shu, X.O.; Xiang, Y.B. Cruciferous vegetables consumption and the risk of female lung cancer: A prospective study and a meta-analysis. Ann. Oncol. 2013, 24, 1918–1924. [Google Scholar] [CrossRef]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous vegetables intake and the risk of colorectal cancer: A meta-analysis of observational studies. Ann. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Yang, Y.; Wang, J.; Han, L.H.; Xiang, Y.B. Cruciferous vegetable consumption and gastric cancer risk: A meta-analysis of epidemiological studies. Cancer Sci. 2013, 104, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Baasanjav-Gerber, C.; Hollnagel, H.M.; Brauchmann, J.; Iori, R.; Glatt, H. Detection of genotoxicants in Brassicales using endogenous DNA as a surrogate target and adducts determined by 32P-postlabelling as an experimental end point. Mutagenesis 2011, 26, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarocka-Karpowicz, I.; Markowska, A. Therapeutic Potential of Jasmonic Acid and Its Derivatives. Int. J. Mol. Sci. 2021, 22, 8437. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Krasauskas, J.; Gates, S.; Díaz-Sánchez, E.K.; Devoto, A. An Update on Core Jasmonate Signalling Networks, Physiological Scenarios, and Health Applications. In Annual Plant Reviews Online; Wiley: Hoboken, NJ, USA, 2019; pp. 387–452. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Sajjadi, S.E.; Parang, K. A review (research and patents) on jasmonic acid and its derivatives. Arch. Pharm. 2014, 347, 229–239. [Google Scholar] [CrossRef]
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonça Bdos, S.; Amôedo, N.D.; Rumjanek, F.D. Methyl jasmonate: Putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis. Int. J. Cell Biol. 2014, 2014, 572097. [Google Scholar] [CrossRef] [Green Version]
- Fingrut, O.; Flescher, E. Plant stress hormones suppress the proliferation and induce apoptosis in human cancer cells. Leukemia 2002, 16, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Flescher, E. Jasmonates in cancer therapy. Cancer Lett. 2007, 245, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, M.W.; Zhang, L.; Zhang, L. Methyl jasmonate and its potential in cancer therapy. Plant Signal Behav. 2015, 10, e1062199. [Google Scholar] [CrossRef] [Green Version]
- Raviv, Z.; Cohen, S.; Reischer-Pelech, D. The anti-cancer activities of jasmonates. Cancer Chemother. Pharm. 2013, 71, 275–285. [Google Scholar] [CrossRef]
- Kniazhanski, T.; Jackman, A.; Heyfets, A.; Gonen, P.; Flescher, E.; Sherman, L. Methyl jasmonate induces cell death with mixed characteristics of apoptosis and necrosis in cervical cancer cells. Cancer Lett. 2008, 271, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.S.; Jiang, G.S.; Zheng, L.D.; Tang, S.T.; Cai, J.B.; Liu, Y.; Zeng, F.Q.; Dong, J.H. Natural jasmonates of different structures suppress the growth of human neuroblastoma cell line SH-SY5Y and its mechanisms. Acta Pharmacol. Sin. 2008, 29, 861–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altiok, N.; Mezzadra, H.; Patel, P.; Koyuturk, M.; Altiok, S. A plant oxylipin, 12-oxo-phytodienoic acid, inhibits proliferation of human breast cancer cells by targeting cyclin D1. Breast Cancer Res. Treat. 2008, 109, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Masamha, C.P.; Benbrook, D.M. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009, 69, 6565–6572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldin, N.; Heyfets, A.; Reischer, D.; Flescher, E. Mitochondria-mediated ATP depletion by anti-cancer agents of the jasmonate family. J. Bioenerg. Biomembr. 2007, 39, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Kiyota, H.; Sakai, S.; Honma, Y. Induction of differentiation of human myeloid leukemia cells by jasmonates, plant hormones. Leukemia 2004, 18, 1413–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.; Flescher, E. Methyl jasmonate: A plant stress hormone as an anti-cancer drug. Phytochemistry 2009, 70, 1600–1609. [Google Scholar] [CrossRef]
- Fingrut, O.; Reischer, D.; Rotem, R.; Goldin, N.; Altboum, I.; Zan-Bar, I.; Flescher, E. Jasmonates induce nonapoptotic death in high-resistance mutant p53-expressing B-lymphoma cells. Br. J. Pharmacol. 2005, 146, 800–808. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, W.; Wang, M.; Huang, T.; Xiao, X.; Wang, L.; Tao, D.; Dong, L.; Zeng, F.; Jiang, G. Methyl jasmonate sensitizes human bladder cancer cells to gambogic acid-induced apoptosis through down-regulation of EZH2 expression by miR-101. Br. J. Pharm. 2014, 171, 618–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Hong, J.; Bao, B.; Choi, J.S.; Jung, J.H. New jasmonate analogues as potential anti-inflammatory agents. Bioorg. Med. Chem. 2008, 16, 10228–10235. [Google Scholar] [CrossRef]
- Eduviere, A.T.; Umukoro, S.; Aderibigbe, A.O.; Ajayi, A.M.; Adewole, F.A. Methyl jasmonate enhances memory performance through inhibition of oxidative stress and acetylcholinesterase activity in mice. Life Sci. 2015, 132, 20–26. [Google Scholar] [CrossRef]
- Lee, H.J.; Maeng, K.; Dang, H.T.; Kang, G.J.; Ryou, C.; Jung, J.H.; Kang, H.K.; Prchal, J.T.; Yoo, E.S.; Yoon, D. Anti-inflammatory effect of methyl dehydrojasmonate (J2) is mediated by the NF-κB pathway. J. Mol. Med. 2011, 89, 83–90. [Google Scholar] [CrossRef]
- Besson, J.C.F.; de Carvalho Picoli, C.; Matioli, G.; Natali, M.R.M. Methyl jasmonate: A phytohormone with potential for the treatment of inflammatory bowel diseases. J. Pharm. Pharmacol. 2018, 70, 178–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sá-Nakanishi, A.; Soni-Neto, J.; Moreira, L.; Gonçalves, G.; Silva, F.; Bracht, L.; Bersani-Amado, C.; Peralta, R.; Bracht, A.; Comar, J. Anti-Inflammatory and Antioxidant Actions of Methyl Jasmonate Are Associated with Metabolic Modifications in the Liver of Arthritic Rats. Oxidative Med. Cell. Longev. 2018, 2018, 2056250. [Google Scholar] [CrossRef] [PubMed]
- Gunjegaonkar, S.; Wankhede, S.B.; Shanmugarajan, T.S.; Shinde, S.D. Bioactive role of plant stress hormone methyl jasmonate against lipopolysaccharide induced arthritis. Heliyon 2020, 6, e05432. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Castro; Gonçalves; Silva; Bracht, L.; Bersani-Amado, C.; Peralta, R.; Comar; Sá-Nakanishi, A. Methyl Jasmonate Reduces Inflammation and Oxidative Stress in the Brain of Arthritic Rats. Antioxidants 2019, 8, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Lv, L.; Wu, C.; Qi, J.; Shi, B. Methyl Jasmonate Protects Microglial Cells Against β-Amyloid-Induced Oxidative Stress and Inflammation via Nrf2-Dependent HO-1 Pathway. Neuropsychiatr. Dis. Treat. 2020, 16, 1399–1410. [Google Scholar] [CrossRef]
- Solomon, U.; Taghogho, E.A. Methyl jasmonate attenuates memory dysfunction and decreases brain levels of biomarkers of neuroinflammation induced by lipopolysaccharide in mice. Brain Res. Bull. 2017, 131, 133–141. [Google Scholar] [CrossRef]
- Adebesin, A.; Adeoluwa, O.A.; Eduviere, A.T.; Umukoro, S. Methyl jasmonate attenuated lipopolysaccharide-induced depressive-like behaviour in mice. J. Psychiatr. Res. 2017, 94, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Taki-Nakano, N.; Kotera, J.; Ohta, H. 12-oxo-phytodienoic acid, a plant-derived oxylipin, attenuates lipopolysaccharide-induced inflammation in microglia. Biochem. Biophys. Res. Commun. 2016, 473, 1288–1294. [Google Scholar] [CrossRef]
- Lee, H.-J.; Dang, H.-T.; Kang, G.-J.; Jung, J.H.; Kang, H.-K.; Shin, H.; Prchal, J.T.; Yoo, E.-S.; Yoon, D. Methyl Dehydro-Jasmonate Has Anti-Inflammatory Effect Cells and Its Molecular Targets Mir-155 and NF-Kb Pathway against LPS Stimulation On RAW264.7. Blood 2009, 114, 1357. [Google Scholar] [CrossRef]
- Dang, H.T.; Lee, Y.M.; Kang, G.J.; Yoo, E.S.; Hong, J.; Lee, S.M.; Lee, S.K.; Pyee, Y.; Chung, H.-J.; Moon, H.R. In vitro stability and in vivo anti-inflammatory efficacy of synthetic jasmonates. Bioorg. Med. Chem. 2012, 20, 4109–4116. [Google Scholar] [CrossRef] [PubMed]
- Aluko, O.M.; Iroegbu, J.D.; Ijomone, O.M.; Umukoro, S. Methyl Jasmonate: Behavioral and Molecular Implications in Neurological Disorders. Clin. Psychopharmacol. Neurosci. 2021, 19, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.J.; Aoshima, H.; Koda, H.; Kiso, Y. Fragrances in oolong tea that enhance the response of GABAA receptors. Biosci. Biotechnol. Biochem. 2004, 68, 1842–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava-Mesa, M.O.; Jiménez-Díaz, L.; Yajeya, J.; Navarro-Lopez, J.D. GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer’s disease. Front. Cell. Neurosci. 2014, 8, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabi, A.O.; Ajayi, A.M.; Ben-Azu, B.; Bakre, A.G.; Umukoro, S. Methyl jasmonate abrogates rotenone-induced parkinsonian-like symptoms through inhibition of oxidative stress, release of pro-inflammatory cytokines, and down-regulation of immnopositive cells of NF-κB and α-synuclein expressions in mice. NeuroToxicology 2019, 74, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Umukoro, S.; Akinyinka, A.O.; Aladeokin, A.C. Antidepressant activity of methyl jasmonate, a plant stress hormone in mice. Pharmacol. Biochem. Behav. 2011, 98, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Umukoro, S.; Aluko, O.M.; Eduviere, A.T.; Owoeye, O. Evaluation of adaptogenic-like property of methyl jasmonate in mice exposed to unpredictable chronic mild stress. Brain Res. Bull. 2016, 121, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Umukoro, S.; Eduviere, A.T.; Aladeokin, A.C. Anti-aggressive activity of methyl jasmonate and the probable mechanism of its action in mice. Pharmacol. Biochem. Behav. 2012, 101, 271–277. [Google Scholar] [CrossRef]
- Aluko, O.M.; Umukoro, S. Methyl jasmonate reverses chronic stress-induced memory dysfunctions through modulation of monoaminergic neurotransmission, antioxidant defense system, and Nrf2 expressions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2339–2353. [Google Scholar] [CrossRef]
- Shanmugarajan, T.S. Potential of plant stress hormone methyl Jasmonate against lipopolysaccharide attenuated oxidative stress and arthritis in experimental animals. Int. J. Green Pharm. 2018, 12, S561. [Google Scholar]
- Malick, J.B. Potentiation of yohimbine-induced lethality in mice: Predictor of antidepressant potential. Drug Dev. Res. 1983, 3, 357–363. [Google Scholar] [CrossRef]
- Annafi, O.S.; Aluko, O.M.; Eduviere, A.T.; Omorogbe, O.; Umukoro, S. Probable mechanisms involved in the antipsychotic-like activity of methyl jasmonate in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 883–892. [Google Scholar] [CrossRef]
- Alabi, A.O.; Ajayi, A.M.; Ben-Azu, B.; Omorobge, O.; Umukoro, S. Methyl jasmonate ameliorates rotenone-induced motor deficits in rats through its neuroprotective activity and increased expression of tyrosine hydroxylase immunopositive cells. Metab. Brain Dis. 2019, 34, 1723–1736. [Google Scholar] [CrossRef]
- Henriet, E.; Jäger, S.; Tran, C.; Bastien, P.; Michelet, J.-F.; Minondo, A.-M.; Formanek, F.; Dalko-Csiba, M.; Lortat-Jacob, H.; Breton, L.; et al. A jasmonic acid derivative improves skin healing and induces changes in proteoglycan expression and glycosaminoglycan structure. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 2250–2260. [Google Scholar] [CrossRef]
- Asghari, M. Impact of jasmonates on safety, productivity and physiology of food crops. Trends Food Sci. Technol. 2019, 91, 169–183. [Google Scholar] [CrossRef]
- EPA. Methyl Jasmonate Exemption from the Requirement of a Tolerance; Environmental Protection Agency: Washington, DC, USA, 2013; pp. 22789–22794. [Google Scholar]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on methyl dihydrojasmonate. Food Chem. Toxicol. 2012, 50 (Suppl. 3), S562–S571. [Google Scholar] [CrossRef]
- FAO; WHO; Expert Committee on Food Additives; World Health Organization; Food and Agriculture Organization of the United Nations. Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Kallenbach, M.; Gilardoni, P.A.; Allmann, S.; Baldwin, I.T.; Bonaventure, G. C12 derivatives of the hydroperoxide lyase pathway are produced by product recycling through lipoxygenase-2 in Nicotiana attenuata leaves. New Phytol. 2011, 191, 1054–1068. [Google Scholar] [CrossRef]
- Zhou, J.; Song, S.; Jiang, F.; Fu, C.; Ma, S. Efficient Syntheses of Traumatic Lactone and Rhizobialide. Chem. Eur. J. 2019, 25, 9948–9958. [Google Scholar] [CrossRef]
- Deodhar, V.B.; Dalavoy, V.S.; Nayak, U.R. A simple preparation of traumatic lactone. a prostanoid synthon. Org. Prep. Proced. Int. 1977, 9, 155–157. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Pankiewicz, W.; Czerpak, R. Traumatic Acid Reduces Oxidative Stress and Enhances Collagen Biosynthesis in Cultured Human Skin Fibroblasts. Lipids 2016, 51, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, H.; Liu, Q.; Zhao, Y.; Cui, X.; Guo, S.; Zhang, L.; Ho, C.-T.; Bai, N. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016, 7, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Rodziewicz, J.; Butarewicz, A. Possible Protective Effects of TA on the Cancerous Effect of Mesotrione. Nutrients 2020, 12, 1343. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Krętowski, R.; Wołejko, E.; Wydro, U.; Butarewicz, A. Traumatic acid toxicity mechanisms in human breast cancer MCF-7 cells. Regul. Toxicol. Pharmacol. 2019, 106, 137–146. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Butarewicz, A. Toxicological Effects of Traumatic Acid and Selected Herbicides on Human Breast Cancer Cells: In Vitro Cytotoxicity Assessment of Analyzed Compounds. Molecules 2019, 24, 1710. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, V.; Rhee, K.Y.; Wang, W.; Yu, Y.; Khafizov, K.; Fiser, A.; Wu, P.; Ndir, O.; Mboup, S.; Ndiaye, D.; et al. Metabolomic analysis of patient plasma yields evidence of plant-like α-linolenic acid metabolism in Plasmodium falciparum. J. Infect. Dis. 2012, 206, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Creek, D.J.; Chua, H.H.; Cobbold, S.A.; Nijagal, B.; MacRae, J.I.; Dickerman, B.K.; Gilson, P.R.; Ralph, S.A.; McConville, M.J. Metabolomics-Based Screening of the Malaria Box Reveals both Novel and Established Mechanisms of Action. Antimicrob. Agents Chemother. 2016, 60, 6650–6663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivek-Ananth, R.P.; Sahoo, A.K.; Srivastava, A.; Samal, A. Virtual screening of phytochemicals from Indian medicinal plants against the endonuclease domain of SFTS virus L polymerase. RSC Adv. 2022, 12, 6234–6247. [Google Scholar] [CrossRef] [PubMed]
- ul Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Nakamura, S.; Hatanaka, A. Green-leaf-derived C6-aroma compounds with potent antibacterial action that act on both Gram-negative and Gram-positive bacteria. J. Agric. Food Chem. 2002, 50, 7639–7644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, S.; Lu, H.; Zhang, D.; Liu, F.; Lin, J.; Zhou, C.; Mu, W. Effects of the plant volatile trans-2-hexenal on the dispersal ability, nutrient metabolism and enzymatic activities of Bursaphelenchus xylophilus. Pestic. Biochem. Physiol. 2017, 143, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Münzbergová, Z.; Nesvorná, M.; Poltronieri, P.; Santino, A. Acaricidal effects of natural six-carbon and nine-carbon aldehydes on stored-product mites. Exp. Appl. Acarol. 2008, 44, 315–321. [Google Scholar] [CrossRef]
- van Iersel, M.L.P.S.; Ploemen, J.-P.H.T.M.; Struik, I.; van Amersfoort, C.; Keyzer, A.E.; Schefferlie, J.G.; van Bladeren, P.J. Inhibition of glutathione S-transferase activity in human melanoma cells by α,β-unsaturated carbonyl derivatives. Effects of acrolein, cinnamaldehyde, citral, crotonaldehyde, curcumin, ethacrynic acid, and trans-2-hexenal. Chem. Biol. Interact. 1996, 102, 117–132. [Google Scholar] [CrossRef]
- Chien, C.-i.; Kirollos, K.S.; Linderman, R.J.; Dauterman, W.C. α,β-Unsaturated carbonyl compounds: Inhibition of rat liver glutathione S-transferase isozymes and chemical reaction with reduced glutathione. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1994, 1204, 175–180. [Google Scholar] [CrossRef]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Sansone, C.; Braca, A.; Ercolesi, E.; Romano, G.; Palumbo, A.; Casotti, R.; Francone, M.; Ianora, A. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS ONE 2014, 9, e101220. [Google Scholar] [CrossRef] [Green Version]
- Graveland, A. Enzymatic oxidations of linoleic acid and glycerol-1-monolinoleate in doughs and flour-water suspensions. J. Am. Oil Chem. Soc. 1970, 47, 352–361. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Polyesters in higher plants. Adv. Biochem. Eng. Biotechnol. 2001, 71, 1–49. [Google Scholar] [CrossRef]
- Ustünes, L.; Claeys, M.; Laekeman, G.M.; Herman, A.G.; Vlietinck, A.J.; Ozer, A. Isolation and identification of two isomeric trihydroxy octadecenoic acids with prostaglandin E-like activity from onion bulbs (Allium cepa). Prostaglandins 1985, 29, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G.; Avetissian, G.M.; Mnatsakanian, V.A.; Batrakov, S.G.; Vartanian, S.A.; Gabrielian, E.S.; Amroyan, E.A. Unsaturated polyhydroxy acids having prostaglandin-like activity from Bryonia alba II. Major components. Planta Med. 1983, 47, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Fenet, B.; Michaud, S.; Tomczyk, N.; Véricel, E.; Lagarde, M.; Guichardant, M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009, 583, 3478–3484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Chen, P.; Véricel, E.; Lelli, M.; Béguin, L.; Lagarde, M.; Guichardant, M. Characterization and biological effects of di-hydroxylated compounds deriving from the lipoxygenation of ALA. J. Lipid Res. 2013, 54, 2083–2094. [Google Scholar] [CrossRef] [Green Version]
- Dofuor, A.K.; Ayertey, F.; Bolah, P.; Djameh, G.I.; Kyeremeh, K.; Ohashi, M.; Okine, L.K.; Gwira, T.M. Isolation and Antitrypanosomal Characterization of Furoquinoline and Oxylipin from Zanthoxylum zanthoxyloides. Biomolecules 2020, 10, 1670. [Google Scholar] [CrossRef] [PubMed]
- Hayshi, Y.; Nishikawa, Y.; Mori, H.; Tamura, H.; Matsushita, Y.-I.; Matsui, T. Antitumor activity of (10E,12Z)-9-hydroxy-10,12-octadecadienoic acid from rice bran. J. Ferment. Bioeng. 1998, 86, 149–153. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nishikawa, Y.; Mori, H.; Matsushita, Y.; Sugamoto, K.; Matsui, T. Evaluation of the Cytotoxic Activity of Chiral (E)-13-Hydroxy-10-oxo-11-octadecenoic Acid and Its Lactone. Biosci. Biotechnol. Biochem. 1998, 62, 1771–1773. [Google Scholar] [CrossRef] [Green Version]
- D’Abrosca, B.; Ciaramella, V.; Graziani, V.; Papaccio, F.; Della Corte, C.M.; Potenza, N.; Fiorentino, A.; Ciardiello, F.; Morgillo, F. Urtica dioica L. inhibits proliferation and enhances cisplatin cytotoxicity in NSCLC cells via Endoplasmic Reticulum-stress mediated apoptosis. Sci. Rep. 2019, 9, 4986. [Google Scholar] [CrossRef] [Green Version]
- Samarakoon, K.W.; Ko, J.-Y.; Lee, J.-H.; Kwon, O.N.; Kim, S.-W.; Jeon, Y.-J. Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom, Phaeodactylum tricornutum. J. Funct. Foods 2014, 6, 231–240. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Talero, E.; de Los Reyes, C.; Zubía, E.; Motilva, V.; García-Mauriño, S. Cytotoxic Activity of Microalgal-derived Oxylipins against Human Cancer Cell lines and their Impact on ATP Levels. Nat. Prod. Commun. 2016, 11, 1871–1875. [Google Scholar] [CrossRef] [Green Version]
- Ávila-Román, J.; Talero, E.; de Los Reyes, C.; García-Mauriño, S.; Motilva, V. Microalgae-derived oxylipins decrease inflammatory mediators by regulating the subcellular location of NFκB and PPAR-γ. Pharmacol. Res. 2018, 128, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Nishizawa, T.; Egawa, K.; Kawada, T.; Nishikawa, Y.; Uenakai, K.; Ohigashi, H. New class of linoleic acid metabolites biosynthesized by corn and rice lipoxygenases: Suppression of proinflammatory mediator expression via attenuation of MAPK- and Akt-, but not PPARγ-, dependent pathways in stimulated macrophages. Biochem. Pharm. 2005, 70, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W. Lipid Enzymes: Lipases, Lipoxygenases, and “Hydroperoxidases”. In Autoxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Springer: Boston, MA, USA, 1980; pp. 447–504. [Google Scholar] [CrossRef]
- Pace-Asciak, C.R.; Keiko, M.; Shozo, Y. The enzymatic conversion of arachidonic acid into 8,11,12-trihydroxyeicosatrienoic acid Resolution of rat lung enzyme into two active fractions. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1982, 712, 142–145. [Google Scholar] [CrossRef]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.-T.; Rosahl, S.; et al. Evaluation of the Antimicrobial Activities of Plant Oxylipins Supports Their Involvement in Defense against Pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef] [Green Version]
- Nugteren, D.H. Arachidonate lipoxygenase in blood platelets. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1975, 380, 299–307. [Google Scholar] [CrossRef]
- Falardeau, P.; Hamberg, M.; Samuelsson, B. Metabolism of 8,11,14-eicosatrienoic acid in human platelets. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1976, 441, 193–200. [Google Scholar] [CrossRef]
- Hamberg, M. Isolation and structures of two divinyl ether fatty acids from Clematis vitalba. Lipids 2004, 39, 565–569. [Google Scholar] [CrossRef]
- Galano, J.M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25years of research in chemistry and biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef]
- Ahmed, O.S.; Galano, J.M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: Where are we now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Karg, K.; Dirsch, V.M.; Vollmar, A.M.; Cracowski, J.L.; Laporte, F.; Mueller, M.J. Biologically active oxidized lipids (phytoprostanes) in the plant diet and parenteral lipid nutrition. Free Radic. Res. 2007, 41, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L.; Salvi, R.; Lavinia Salvatori, M.; Ajmone-Cat, M.A.; De Nuccio, C.; Visentin, S.; Bultel-Poncé, V.; Oger, C.; Guy, A.; Galano, J.M.; et al. Nonenzymatic oxygenated metabolites of α-linolenic acid B1- and L1-phytoprostanes protect immature neurons from oxidant injury and promote differentiation of oligodendrocyte progenitors through PPAR-γ activation. Free Radic. Biol. Med. 2014, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.-M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Pino Ramos, L.L.; Jiménez-Aspee, F.; Theoduloz, C.; Burgos-Edwards, A.; Domínguez-Perles, R.; Oger, C.; Durand, T.; Gil-Izquierdo, Á.; Bustamante, L.; Mardones, C.; et al. Phenolic, oxylipin and fatty acid profiles of the Chilean hazelnut (Gevuina avellana): Antioxidant activity and inhibition of pro-inflammatory and metabolic syndrome-associated enzymes. Food Chem. 2019, 298, 125026. [Google Scholar] [CrossRef]
- Martínez Sánchez, S.; Domínguez-Perles, R.; Montoro-García, S.; Gabaldón, J.A.; Guy, A.; Durand, T.; Oger, C.; Ferreres, F.; Gil-Izquierdo, A. Bioavailable phytoprostanes and phytofurans from Gracilaria longissima have anti-inflammatory effects in endothelial cells. Food Funct. 2020, 11, 5166–5178. [Google Scholar] [CrossRef]
- Traidl-Hoffmann, C.; Mariani, V.; Hochrein, H.; Karg, K.; Wagner, H.; Ring, J.; Mueller, M.J.; Jakob, T.; Behrendt, H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 2005, 201, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Gutermuth, J.; Bewersdorff, M.; Traidl-Hoffmann, C.; Ring, J.; Mueller, M.J.; Behrendt, H.; Jakob, T. Immunomodulatory effects of aqueous birch pollen extracts and phytoprostanes on primary immune responses in vivo. J. Allergy Clin. Immunol. 2007, 120, 293–299. [Google Scholar] [CrossRef]
- Mariani, V.; Gilles, S.; Jakob, T.; Thiel, M.; Mueller, M.J.; Ring, J.; Behrendt, H.; Traidl-Hoffmann, C. Immunomodulatory mediators from pollen enhance the migratory capacity of dendritic cells and license them for Th2 attraction. J. Immunol. 2007, 178, 7623–7631. [Google Scholar] [CrossRef] [Green Version]
- Galano, J.M.; Lee, J.C.; Gladine, C.; Comte, B.; Le Guennec, J.Y.; Oger, C.; Durand, T. Non-enzymatic cyclic oxygenated metabolites of adrenic, docosahexaenoic, eicosapentaenoic and alpha-linolenic acids; bioactivities and potential use as biomarkers. Biochim. Et Biophys. Acta 2015, 1851, 446–455. [Google Scholar] [CrossRef]
- Bernardo, A.; De Simone, R.; De Nuccio, C.; Visentin, S.; Minghetti, L. The nuclear receptor peroxisome proliferator-activated receptor-γ promotes oligodendrocyte differentiation through mechanisms involving mitochondria and oscillatory Ca2+ waves. Biol. Chem. 2013, 394, 1607–1614. [Google Scholar] [CrossRef]
- Gilles, S.; Mariani, V.; Bryce, M.; Mueller, M.J.; Ring, J.; Jakob, T.; Pastore, S.; Behrendt, H.; Traidl-Hoffmann, C. Pollen-Derived E1-Phytoprostanes Signal via PPAR-γ and NF-κB-Dependent Mechanisms. J. Immunol. 2009, 182, 6653–6658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campillo, M.; Medina, S.; Fanti, F.; Gallego-Gómez, J.I.; Simonelli-Muñoz, A.; Bultel-Poncé, V.; Durand, T.; Galano, J.M.; Tomás-Barberán, F.A.; Gil-Izquierdo, Á.; et al. Phytoprostanes and phytofurans modulate COX-2-linked inflammation markers in LPS-stimulated THP-1 monocytes by lipidomics workflow. Free Radic. Biol. Med. 2021, 167, 335–347. [Google Scholar] [CrossRef]
- Gutierrez-Pajares, J.L.; Ben Hassen, C.; Oger, C.; Galano, J.M.; Durand, T.; Frank, P.G. Oxidized Products of α-Linolenic Acid Negatively Regulate Cellular Survival and Motility of Breast Cancer Cells. Biomolecules 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Raychaudhuri, B.; Banerjee, S.; Das, B.; Mukhopadhaya, S.; Datta, S.C. Momordicatin purified from fruits of Momordica charantia is effective to act as a potent antileishmania agent. Parasitol. Int. 2010, 59, 192–197. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Talero, E.; Rodríguez-Luna, A.; García-Mauriño, S.; Motilva, V. Anti-inflammatory effects of an oxylipin-containing lyophilised biomass from a microalga in a murine recurrent colitis model. Br. J. Nutr. 2016, 116, 2044–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruocco, N.; Albarano, L.; Esposito, R.; Zupo, V.; Costantini, M.; Ianora, A. Multiple Roles of Diatom-Derived Oxylipins within Marine Environments and Their Potential Biotechnological Applications. Mar. Drugs 2020, 18, 342. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, A.; Gupta, P.; Chand, K.; Samant, M.; Maurya, R.; Dube, A. Evaluation of antileishmanial potential of Tinospora sinensis against experimental visceral leishmaniasis. Parasitol. Res. 2008, 102, 561–565. [Google Scholar] [CrossRef]
- Czyzewska, M.M.; Chrobok, L.; Kania, A.; Jatczak, M.; Pollastro, F.; Appendino, G.; Mozrzymas, J.W. Dietary acetylenic oxylipin falcarinol differentially modulates gabaa receptors. J. Nat. Prod. 2014, 77, 2671–2677. [Google Scholar] [CrossRef]





| Oxylipin | Concentration | The Studied System | Effect | Refs. |
|---|---|---|---|---|
| Derivatives of acetylenic fatty acids | ||||
| Falcarinol (31) | 0.5–20 μM | Tissue culture, mouse model | Stimulates neuritoge-nesis, restores memory mechanisms, neuropro-tective properties | [134,135] |
| 0.016–2 μg/mL | Human gastric adenocarcinoma, leukemia like, mouse fibroblast-derived tumor, mouse melanoma | Anti-cancer properties | [80] | |
| 0.1–10 μM | Transfected cells of human embryonic kidney | reversible agonist of cannabinoid receptors | [261] | |
| Falcarinol (31) and didehydrofalcarinol (32) | 20–200 g/mL | Fungal spores | Antifungal properties | [82,118] |
| Falcarinol (31), falcarindiol (33) and falcarindiol-3-acetate (34) | 0.5–20 μM | Mice macrophages cell lines | Anti-inflammatory, NO production inhibition, no cytotoxicity | [131] |
| 100 μg/m1 | Rabbit blood | Antiplatelet effect | [113] | |
| Falcarindiol (33), falcarinol (31) | 10 μg/mL MIC 16.4 μM, | In vitro activity | Antimicrobe and antimycobacterial | [119,120,121,122] |
| Falcarindiol-8-methyl ether (35); panaxydiol (40) | IC50 3.5 μM/l | Human cancer cell lines | Cytotoxic effect | [80,124] |
| Furanocoumarin ethers of falcarindiol (38) | ED50, 3.2–8.5 μg/mL | Tissue culture | Antiproliferative activity | [125] |
| 11(S),16(R)-Dihydroxy-octadeca-9Z,17-dien-12,14-diyn-1-yl acetate (39); falcarindiol (33) | IC50 24 μM; 73 μM, | In vitro activity | Inhibition of 5-, 12-, and 15-lipoxygenases, cyclooxygenase (COX-1) | [83,128,129] |
| 11(S),16(R)-Dihydroxy-octadeca-9Z,17-dien-12,14-diyn-1-yl acetate (39); (3R, 8S)-falcarindiol (33) | IC50 118 μM | In vitro radiolabeled ligand binding | Serotonin receptor binding, serotonergic, antidepressant potential | [136] |
| 1,2-Dihydro-11-acetoxy-falcarindiol (36); 11-Acetoxy-falcarindiol (37) | 0.001–100 μM; IC50 0.3–29 μM | Human cancer cell lines | Inhibition of cell proliferation | [127] |
| Jasmonates | ||||
| JA, 3-oxo-2-(2-pentenyl) cyclopentaneacetic acid (1); MeJA (3) | 0.5–3 µM | Human T lymphoblastic leukemia, breast carcinoma, melanoma, androgen-responsive prostate adenocarcinoma cells; mouse T lymphoma cells | Cell death and inhibition of cell proliferation in cancer cells, no damage to normal lymphocytes | [157] |
| MeJA (3) | 1–5 mM | Human carcinoma cell lines | Cell death and growth inhibition | [161] |
| 20 µM | Microglial cell line BV-2 | Protects against β-amyloid-induced oxidative stress and inflammation | [177] | |
| MeJA analogs | 12.5–100 µM | RAW264.7 murine macrophage cells | Inhibition of biosynthesis of pro-inflammatory mediators | [170,181] |
| Jasmonate derivative, (3-hydroxy-2-pentylcyclopentyl)-acetic acid (4) | 10 µM | Epidermal primary keratinocytes and reconstituted skin epidermis | Induce expression of major skin proteoglycans, skin healing, accelerated epithelial repair in vivo | [195] |
| 12-oxo-phytodienoic acid (2) | 7.5–30 μM | Mouse microglial cells | Suppression of LPS-induced expression of the inflammatory cytokines and NO production | [180] |
| Hydroperoxide lyase branch oxylipins | ||||
| Traumatic acid (6) | 1–10 µM | Fibroblast cell line | Antioxidant and stimulatory effects on collagen biosynthesis | [203] |
| 0.5–1000 µM | ZR-75-1 cell line, treated with mesotrione to enhance growth | Anticancer activity | [205] | |
| 0.5–1000 µM | Breast cancer cell lines and normal breast cell lines | Reduction of pesticide-induced cancer cell division | [207] | |
| (E)-2-hexenal (7); (2E,6Z)-2,6-nonadienal (8); (E)-2-nonenal (9) | 4–314 mg/g | Mites Acarus siro L., Tyrophagus putrescentiae (Schrank), Aleuroglyphus ovatus | Growth inhibition (4–35 mg/g), death (36–314) mg/g | [215] |
| (2E,4E)-2,4-decadienal (10); (2E,4E)-2,4-octadienal (11); (2E,4E)-2,4-heptadienal (12) | 2.5–10 µM | Lung and colon cancer cell lines; normal lung/brunch epithelial cell line | Cytotoxic effect against cancerous but not normal cells | [220] |
| Oxy-, hydroxy-, and epoxy-derivatives of fatty acids | ||||
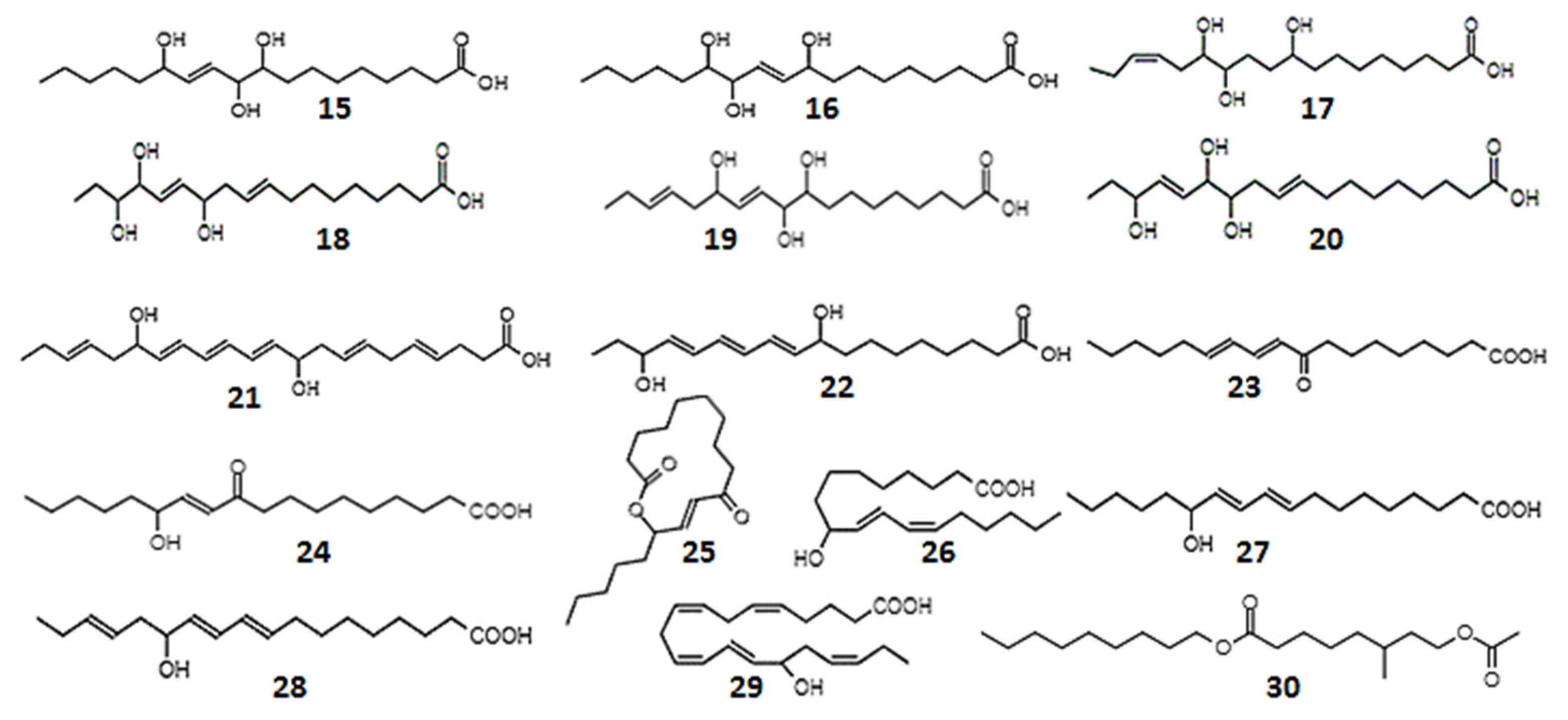
| 9,10,13-trihydroxy-11-octadecenoic (15); 9,12,13-trihydroxy-l0-octadecenoic acid (16) | - | Rabbit coeliac and mesenteric arteries; rat fundus strip; cascade superfusion system | Prostaglandin-like activity–smooth muscle relaxation | [223] |
| 9,12,13-trihydroxy-15-octadecadienoic (17); 12,15,16-trihydroxy-9,13-octadecadienoic (18); 9,10,13-trihydroxy-11,15-octadecadienoic (19); 12,13,16-trihydroxy-9,14-octadecadienoic acid (20) | - | Rat colon, suspended strip | Prostaglandin-like activity–smooth muscle relaxation | [224] |
| 10,17-dihydroxy-docosahexa-4,7,11,13,15,19-enoic acid (21) | 0.3-10 µM | Platelet suspension | Inhibited collagen-induced platelet aggregation in a dose-dependent manner. | [225] |
| 9,16-dihydroxy-10,12,14-octadecatrienoic acid, isomers (22) | 1 µM | Platelet suspensions, leukocyte suspensions, recombinant COX protein | Anti-inflammatory, antithrombotic effects, inhibition COX-1 | [226] |
| 9-oxo-10, 12-octadecadienoic acid (23) | EC50 1.2 µM | Blood stream T. brucei form, mouse macrophages | T. brucei growth inhibition | [227] |
| 13-hydroxy-10-oxo-11-octadecenoic acid (24); 10-oxo-11-octadecen-13-olide, enantiomers (25) | - | Mouse leukemia cells | Cytotoxicity | [229] |
| 9-hydroxy-10,12-octadecadienoic acid (26) 13-hydroxy-9,11-octadecadienoic acid (27) | - | Mouse fibroblast cells, simian virus 40-transformed cells | Cytotoxicity | [229] |
| 9-hydroxy-10,12-octadecadienoic acid; 13-hydroxy-10-oxo-11-octadecenoic acid; 10-oxo-11-octadecen-13-olide | 0.8–100 µM | Murine macrophages, monkey kidney cells | Anti-inflammatory | [234] |
| 13-hydroxyoctadeca-9,11,15-trienoic acid (28); 15-hydroxyeicosa-5,8,11,13,17-pentaenoic acid (29) | 10 mM | Human colonic adenocarcinoma and melanoma cell lines | Cytotoxicity | [232] |
| Nonyl 8-acetoxy-6-methyloctanoate (30) | 25, 50 mg/mL | Human leukemia cells; lung carcinoma; mouse melanoma | Anticancer effects | [231] |
| 13-hydroxy-9,11,15-octadecantrienoic acid (28) | - | Non-small cell lung cancer | Anti-proliferative activity | [230] |
| Phytoprostanes, phytofurans | ||||
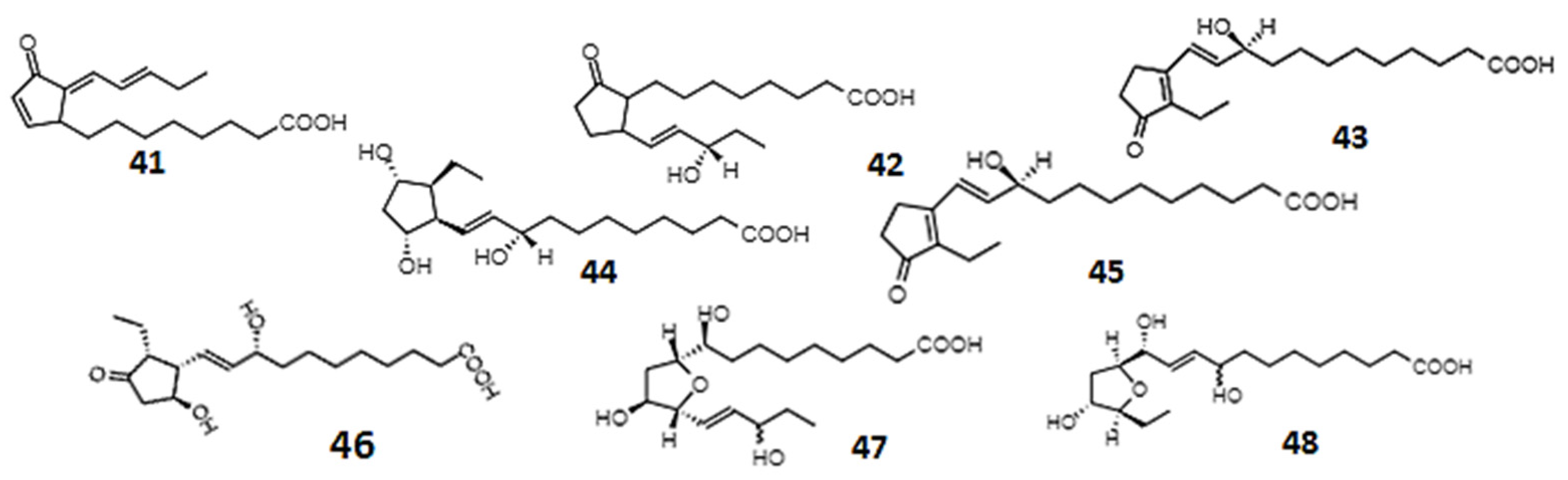
| B1-Phytoprostanes | 0.1–25 µM | Undifferentiated neuroblastoma cells | Neuroprotective activity | [245,252] |
| E1-Phytoprostanes | - | Culture of monocyte-derived dendritic cells | Anti-inflammatory activity | [249,254] |
| Phytoprostanes A1, E1, and deoxy-J1 (41) | 10–80 µM | Healthy males ages 18–35 years, human embryonic kidney cells, macrophage-like cells | Anti-inflammatory, apoptosis-inducing activity | [244] |
| Phytoprostanes from olive oil | - | In vitro inhibition of α-glucosidase and α-amylase | Antidiabetic activity | [246] |
| 16-B-1 (42)- and 9-L1-phytoprostanes (43) | - | Human neuroblastoma cells | Antioxidant activity | [245] |
| Phytoprostanes: 9-F1t, 9-epi-9-F1t (44), ent-16-F1t, ent-16-epi-16-F1t (45), 9-D1t, 9-epi-9-D1t (46), 16-B1 (42), and 9-L1 (43); Phytofurans: ent-16(RS)-9-epi-STΔ14-10, ent-9(RS)-12-epi-ST-Δ10-13 (48), and ent-16(RS)-13-epi-ST-9-Δ14-9 | - | Human colorectal adenocarcinoma and human endothelial cell lines | Anti-inflammatory | [248] |
| Betulla alba pollen phytoprostane | Monocyte-derived dendritic cells, T cells | Modulation of human dendritic cells function | [249] | |
| Phytoprostanes: 16-F1t, 16-epi-16-F1t (45), 16-B1, Ent-16-B1 (42), 9-L1, Ent-9-L1 (43), 9-E1; Phytofurans: Ent-9-(RS)-12-epi-ST-9-∆10-13 (48) | 0.1–100 µM | Human breast cancer cell lines | Anticancer effects | [256] |
| Phytoprostanes: 9-F1t, 9-epi-9-F1t (44), ent-16-F1t, ent-16-epi-16-F1t (45), 9-D1t, 9-epi-9-D1t (46), 16-B1 (42), 9-L1 (43); Phytofurans: ent-16(RS)-9-epi-STΔ14-10, ent-9(RS)-12-epi-ST-Δ10-13 (48), ent-16(RS)-13-epi-ST-Δ14-9 (47) | 0.002–100 µM | The monocytic human (THP-1) cell line | Anti-inflammatory activity | [255] |
| Unusual complex oxylipins | ||||
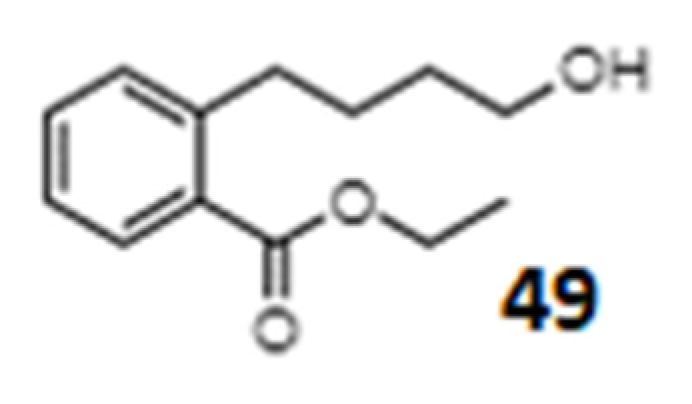
| Momordicatin (49) 4-(o-carboethoxyphenyl) butanol | - | L. donovani strain | Antileishmania agent | [257] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savchenko, T.; Degtyaryov, E.; Radzyukevich, Y.; Buryak, V. Therapeutic Potential of Plant Oxylipins. Int. J. Mol. Sci. 2022, 23, 14627. https://doi.org/10.3390/ijms232314627
Savchenko T, Degtyaryov E, Radzyukevich Y, Buryak V. Therapeutic Potential of Plant Oxylipins. International Journal of Molecular Sciences. 2022; 23(23):14627. https://doi.org/10.3390/ijms232314627
Chicago/Turabian StyleSavchenko, Tatyana, Evgeny Degtyaryov, Yaroslav Radzyukevich, and Vlada Buryak. 2022. "Therapeutic Potential of Plant Oxylipins" International Journal of Molecular Sciences 23, no. 23: 14627. https://doi.org/10.3390/ijms232314627
APA StyleSavchenko, T., Degtyaryov, E., Radzyukevich, Y., & Buryak, V. (2022). Therapeutic Potential of Plant Oxylipins. International Journal of Molecular Sciences, 23(23), 14627. https://doi.org/10.3390/ijms232314627






