Recent Advances in Light-Controlled Activation of Pt(IV) Prodrugs
Abstract
:1. Introduction
2. Light-Controlled Activation of Pt(IV) Prodrugs
2.1. Photoinduced Catalytical Reduction of Mixtures of Pt(IV) Prodrugs
2.2. Photoinduced Reduction of Pt(IV) Prodrugs with Photoabsorbers as Axial Moieties
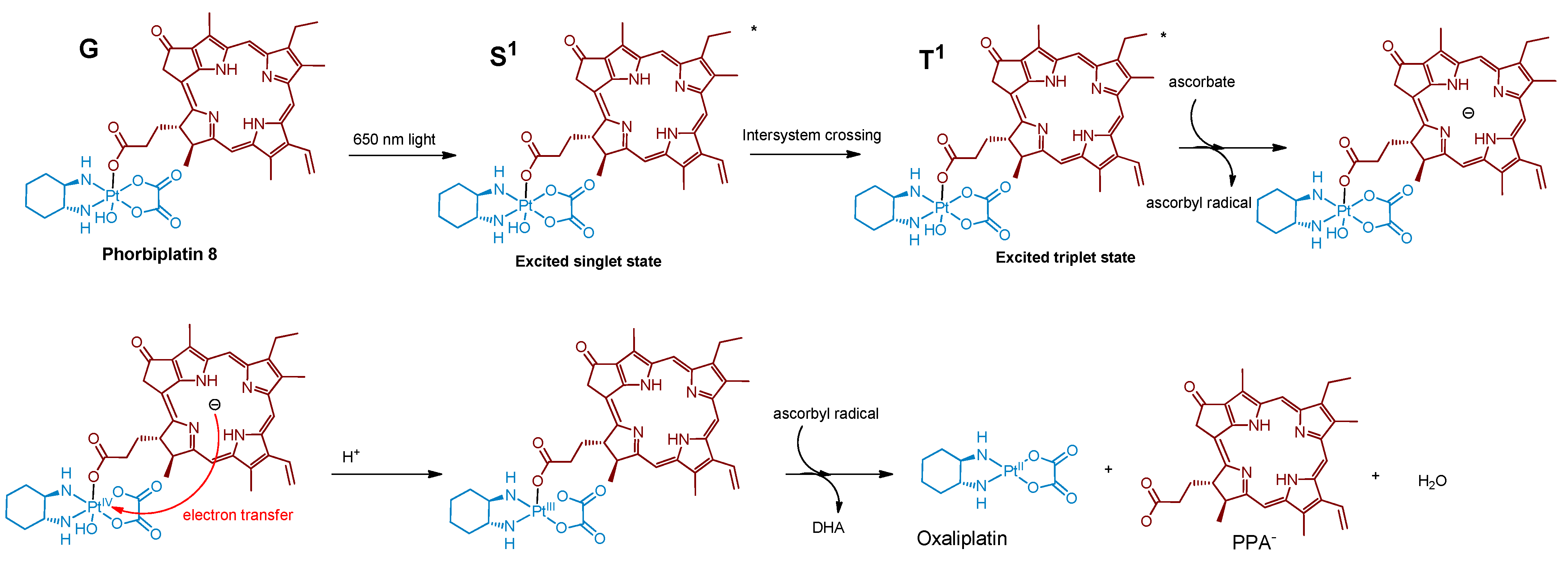
2.2.1. Pt(IV) Prodrug with Red-Absorbing Ligand Pyropheophorbide in the Axial Position
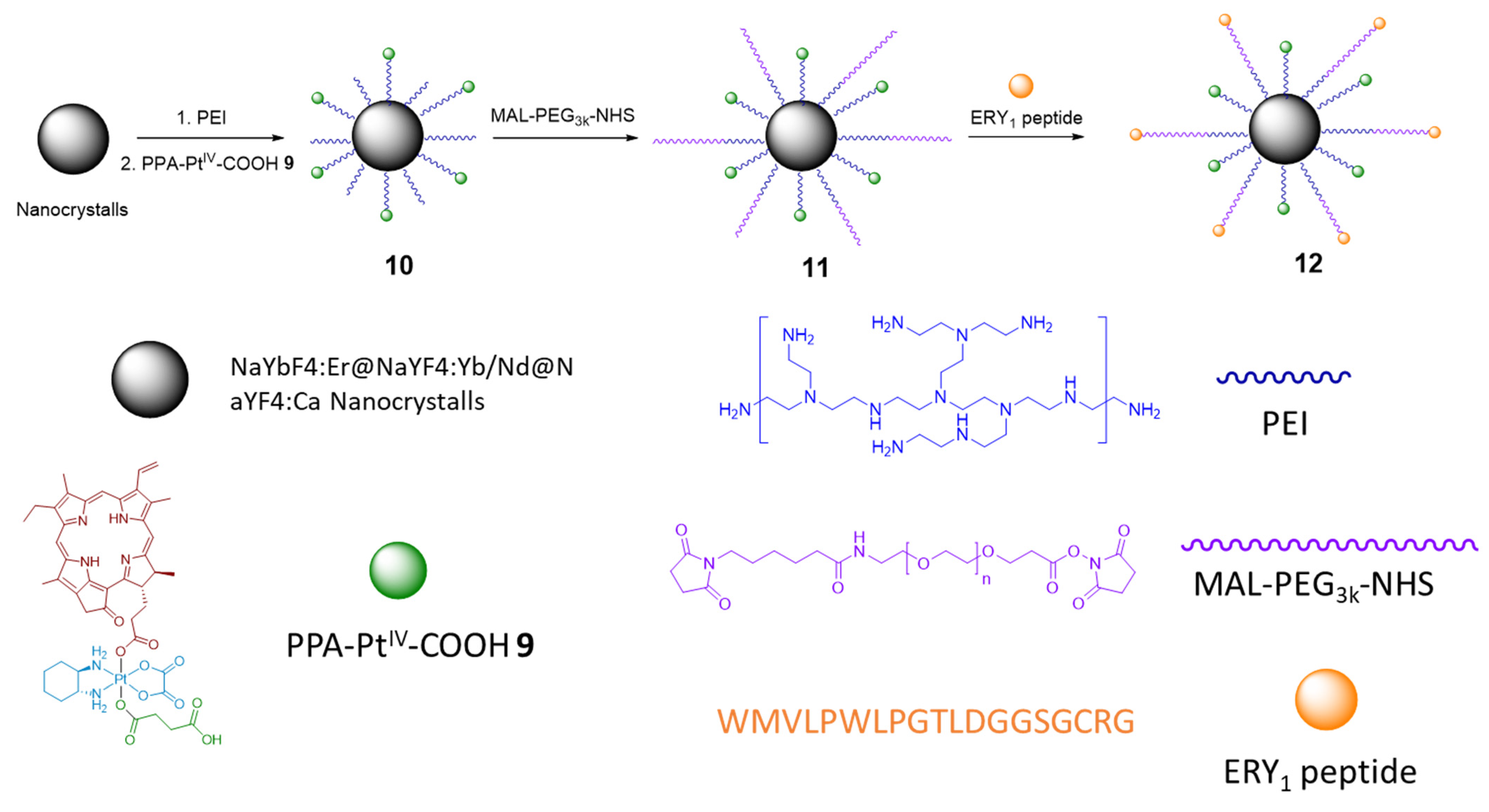
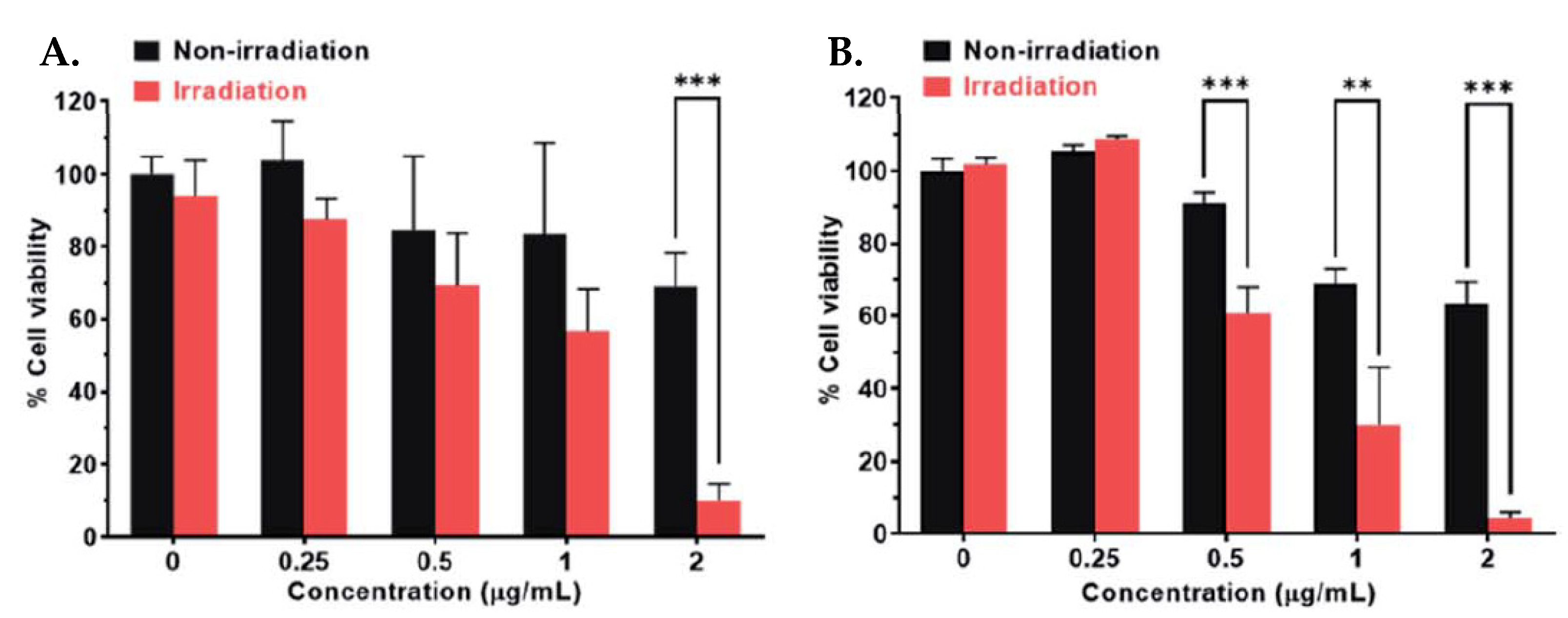
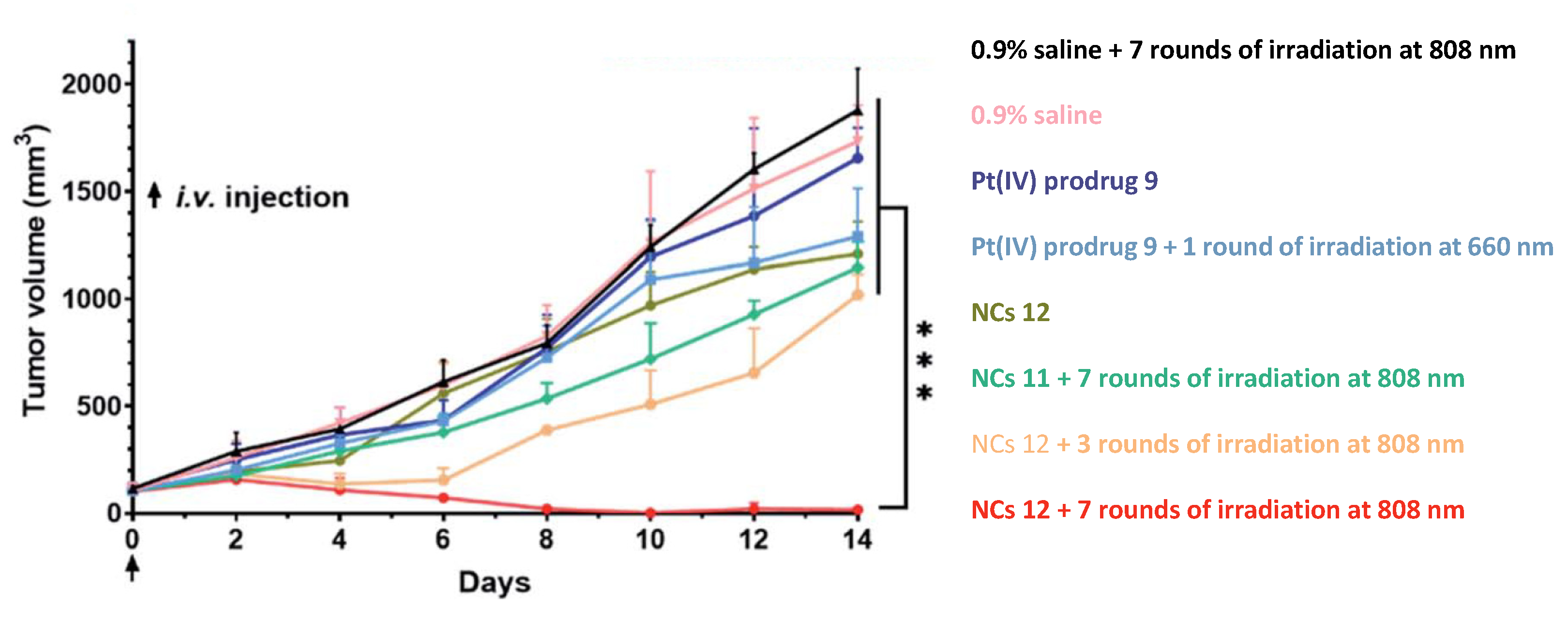
2.2.2. Phorbiplatin-Containing Nanoprodrug with NIR Absorption
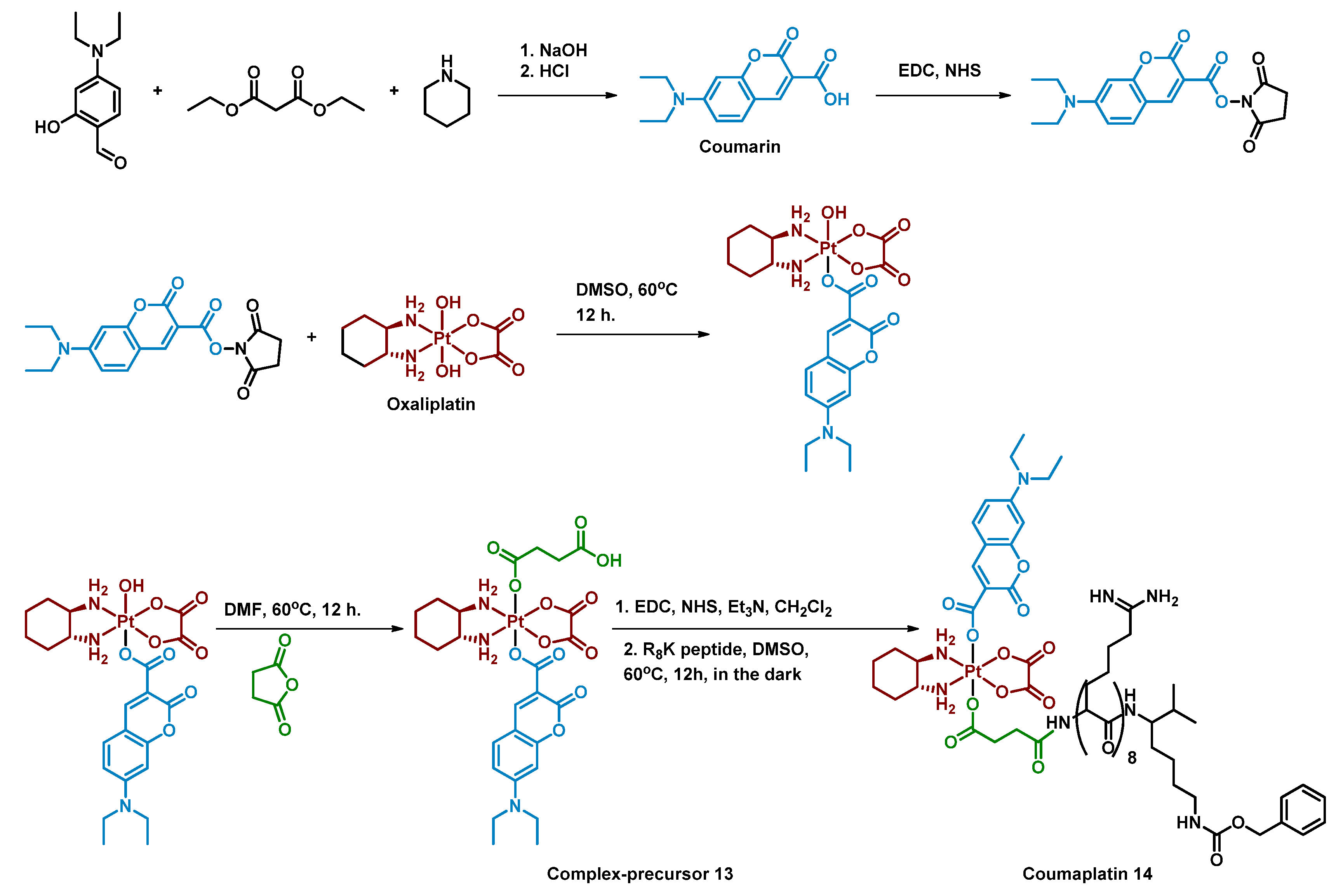
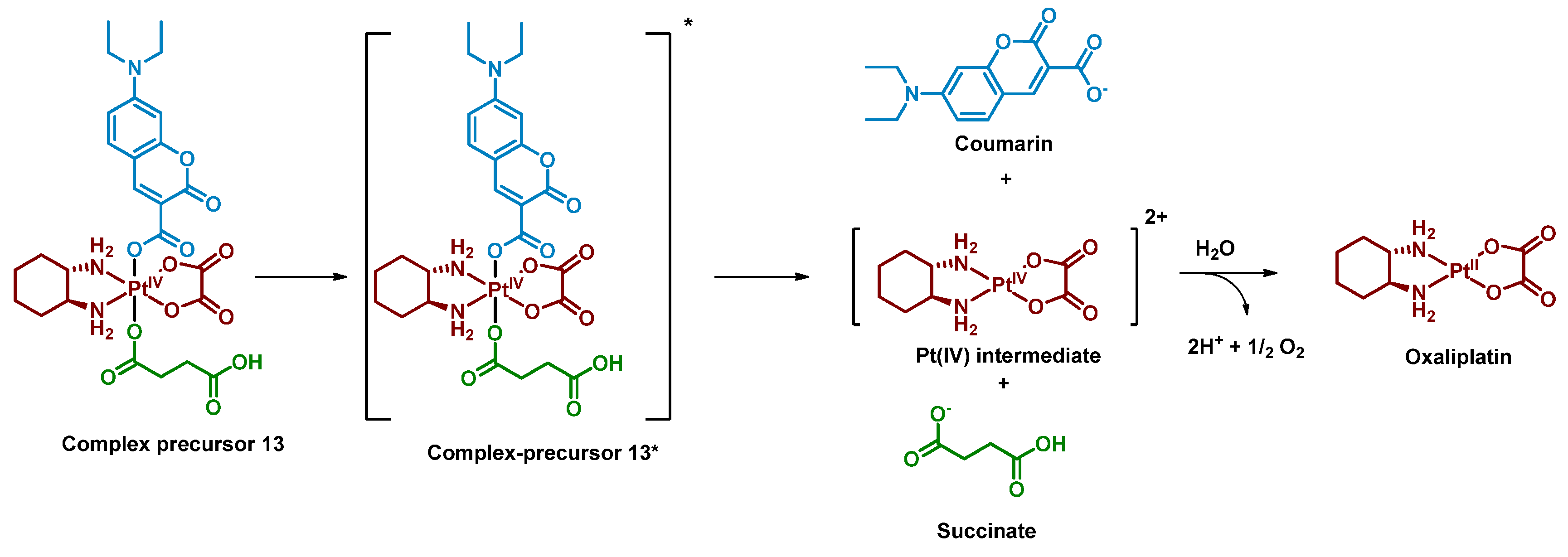
2.2.3. Pt(IV) Prodrug with Coumarin and Cell-Penetrating Peptide in the Axial Positions
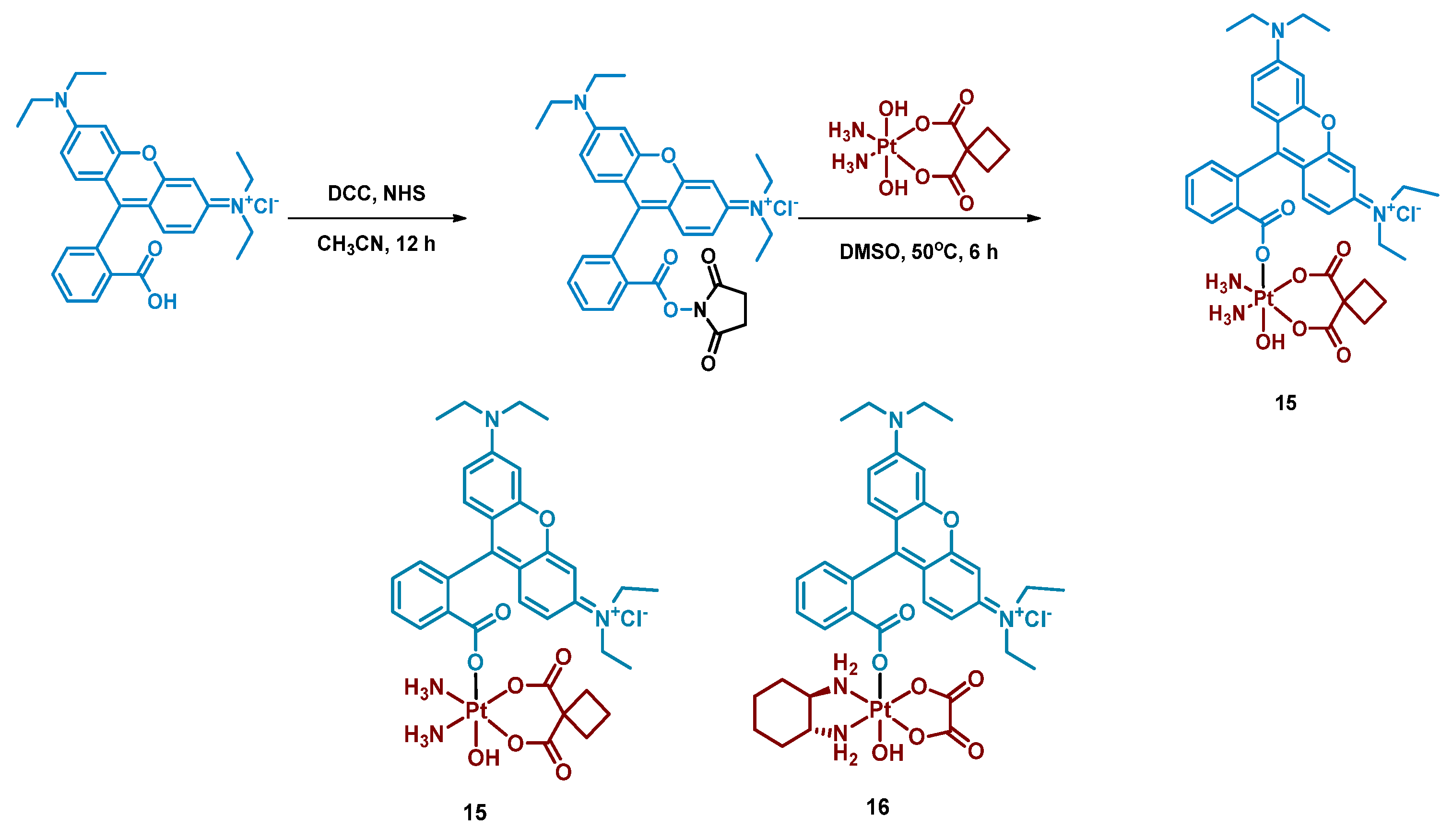
2.2.4. Pt(IV) Prodrugs with Rhodamine B in the Axial Position
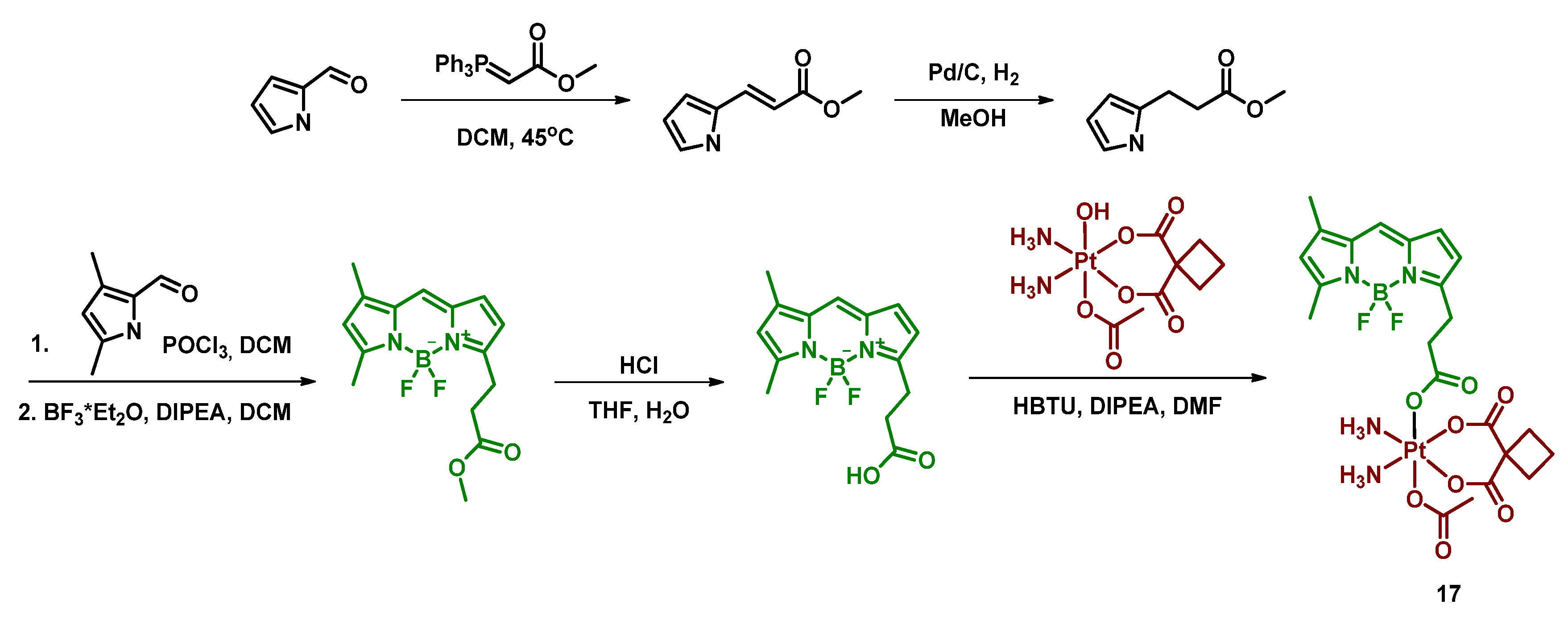
2.2.5. Carboplatin-Based Pt(IV) Prodrug with Bodipy Derivative in the Axial Position
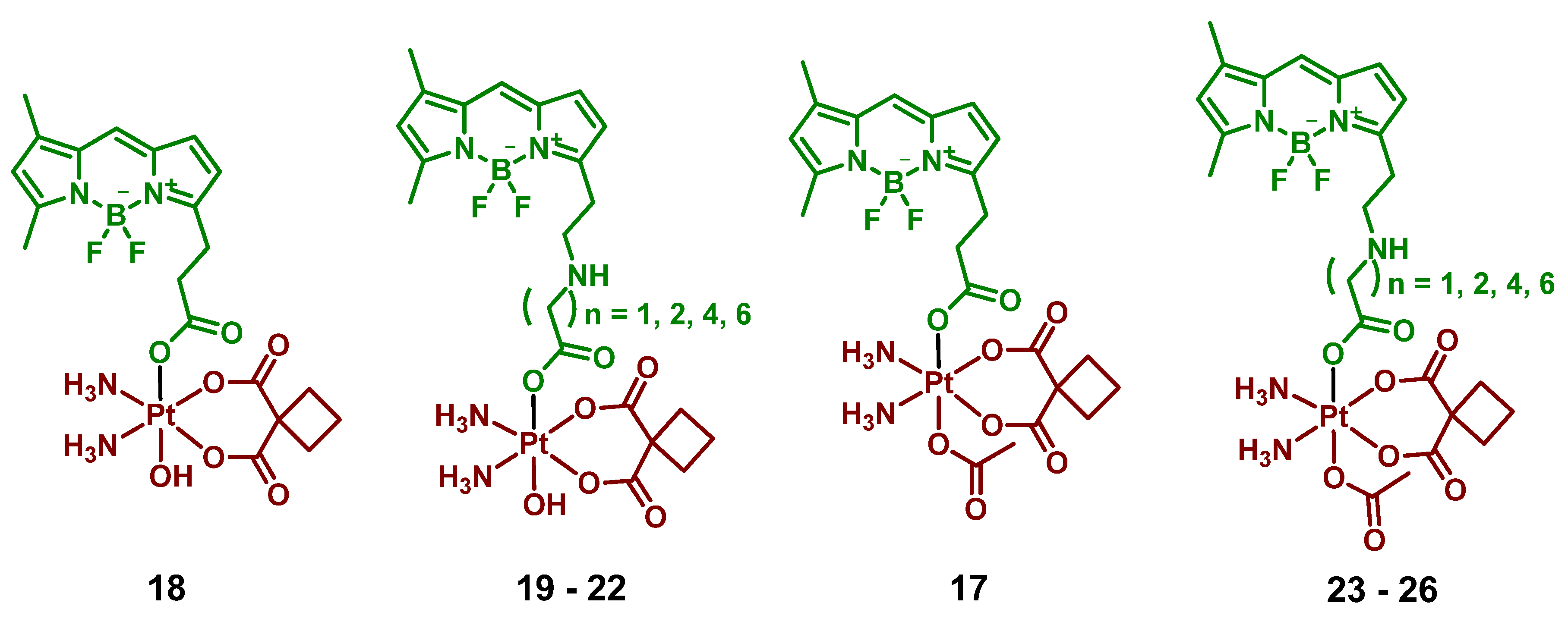
2.2.6. Pt(IV) Prodrugs with Linkers of Various Lengths between Pt(IV) Center and Bodipy Ligand
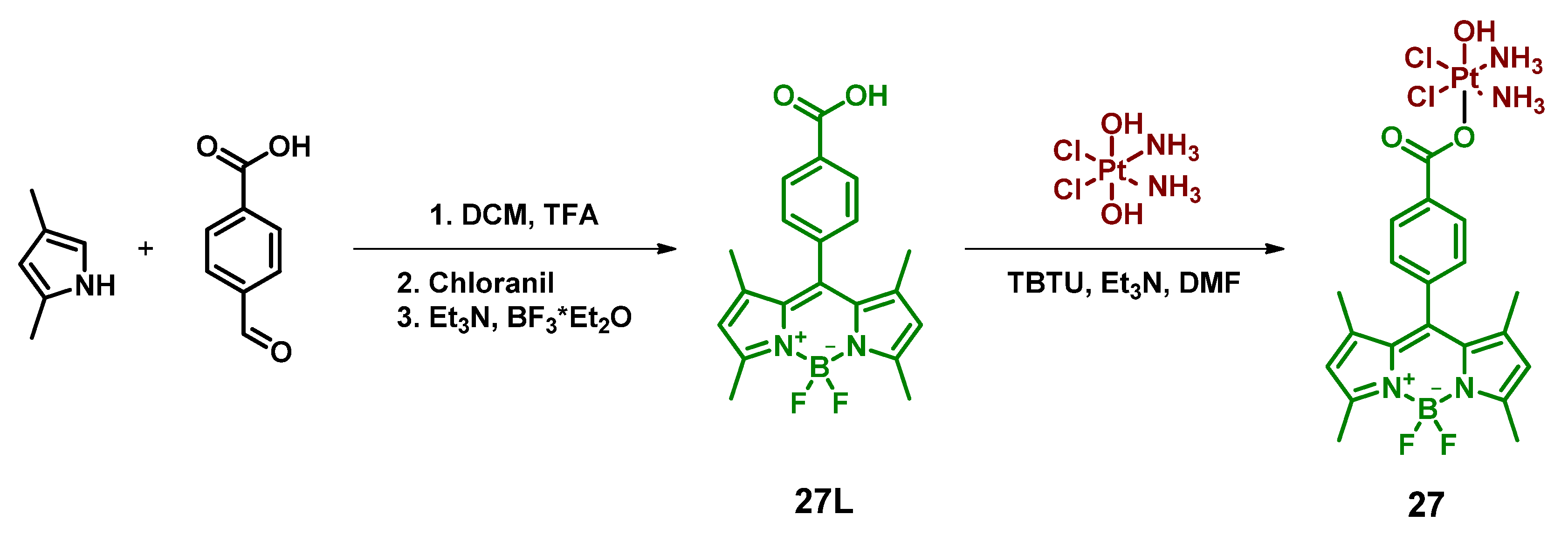
2.2.7. Pt(IV) Prodrug Conjugated with Bodipy Derivative via Benzoic Acid Moiety in Meso-Position
2.2.8. Pt(IV) Prodrug with Red-Light Absorbing Bodipy Derivative in Axial Position
2.2.9. Pt(IV) Prodrug with Two Cyanine-Based Ligands in Axial Position
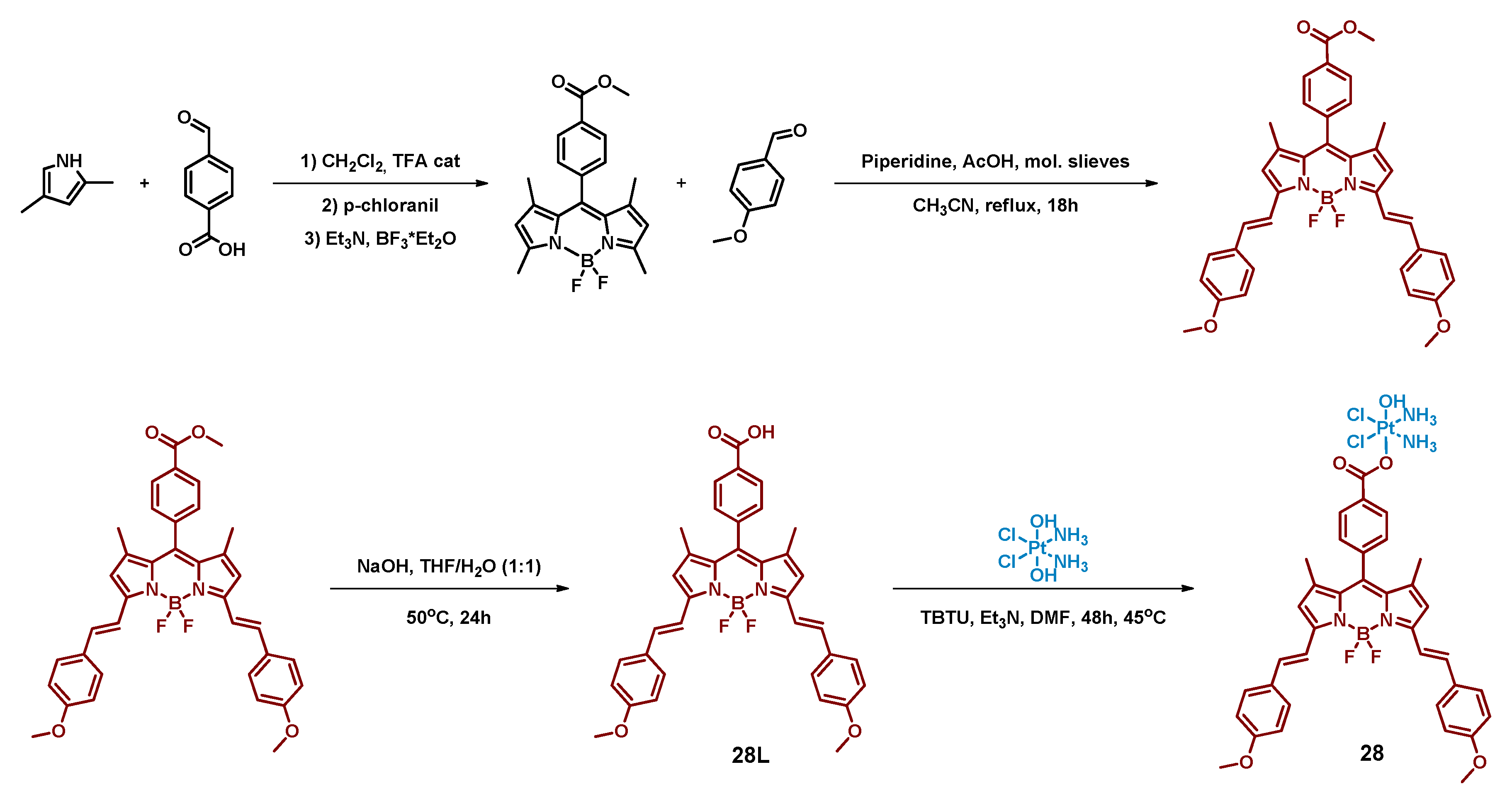
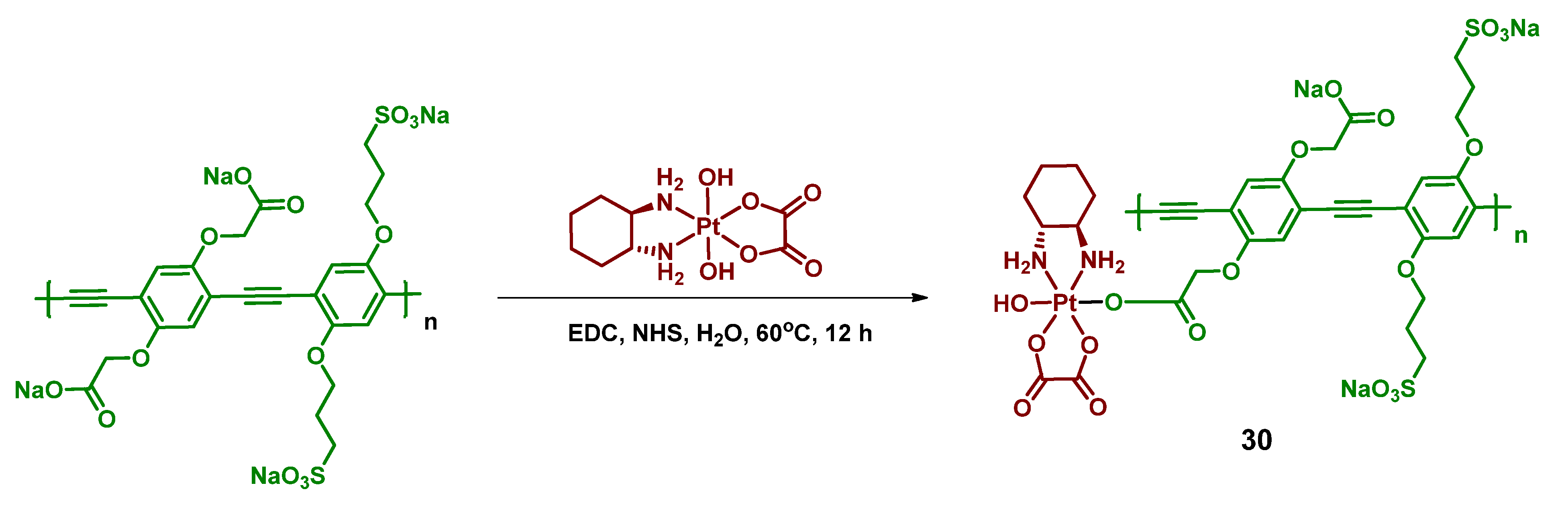
2.2.10. Pt(IV) Prodrug-Functionalized Photoactive Polymer
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Glossary
References
- Stordal, B.; Pavlakis, N.; Davey, R. Oxaliplatin for the Treatment of Cisplatin-Resistant Cancer: A Systematic Review. Cancer Treat. Rev. 2007, 33, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Cosaert, J.; Quoix, E. Platinum Drugs in the Treatment of Non-Small-Cell Lung Cancer. Br. J. Cancer 2002, 87, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The Status of Platinum Anticancer Drugs in the Clinic and in Clinical Trials. Dalton Trans. 2010, 39, 8113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Lippard, S.J. Cellular Processing of Platinum Anticancer Drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, J.T.; Lipp, H.P. Toxicity of Platinum Compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Tixier, F.; Ranchon, F.; Iltis, A.; Vantard, N.; Schwiertz, V.; Bachy, E.; Bouafia-Sauvy, F.; Sarkozy, C.; Tournamille, J.F.; Gyan, E.; et al. Comparative Toxicities of 3 Platinum-Containing Chemotherapy Regimens in Relapsed/Refractory Lymphoma Patients. Hematol. Oncol. 2017, 35, 584–590. [Google Scholar] [CrossRef]
- Langer, T.; Am Zehnhoff-Dinnesen, A.; Radtke, S.; Meitert, J.; Zolk, O. Understanding Platinum-Induced Ototoxicity. Trends Pharmacol. Sci. 2013, 34, 458–469. [Google Scholar] [CrossRef]
- Park, S.B.; Lin, C.S.Y.; Krishnan, A.V.; Goldstein, D.; Friedlander, M.L.; Kiernan, M.C. Oxaliplatin-Induced Neurotoxicity: Changes in Axonal Excitability Precede Development of Neuropathy. Brain 2009, 132, 2712–2723. [Google Scholar] [CrossRef]
- Ohmichi, M.; Hayakawa, J.; Tasaka, K.; Kurachi, H.; Murata, Y. Mechanisms of Platinum Drug Resistance. Trends Pharmacol. Sci. 2005, 26, 113–116. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for Design and Development of Platinum(IV) Anticancer Complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef]
- Dolman, R.C.; Deacon, G.B.; Hambley, T.W. Studies of the Binding of a Series of Platinum (IV) Complexes to Plasma Proteins. J. Inorg. Biochem. 2002, 88, 260–267. [Google Scholar] [CrossRef]
- Wexselblatt, E.; Gibson, D. What Do We Know about the Reduction of Pt(IV) pro-Drugs? J. Inorg. Biochem. 2012, 117, 220–229. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Gou, S. A Cisplatin-Based Platinum(IV) Prodrug Containing a Glutathione s-Transferase Inhibitor to Reverse Cisplatin-Resistance in Non-Small Cell Lung Cancer. J. Inorg. Biochem. 2019, 193, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Petruzzella, E.; Sirota, R.; Solazzo, I.; Gandin, V.; Gibson, D. Triple Action Pt(Iv) Derivatives of Cisplatin: A New Class of Potent Anticancer Agents That Overcome Resistance. Chem. Sci. 2018, 9, 4299–4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spector, D.V.; Pavlov, K.G.; Akasov, R.A.; Vaneev, A.N.; Erofeev, A.S.; Gorelkin, P.V.; Nikitina, V.N.; Lopatukhina, E.V.; Semkina, A.S.; Vlasova, K.Y.; et al. Pt(IV) Prodrugs with Non-Steroidal Anti-Inflammatory Drugs in the Axial Position. J. Med. Chem. 2022, 65, 8227–8244. [Google Scholar] [CrossRef]
- Pathak, R.K.; Marrache, S.; Choi, J.H.; Berding, T.B.; Dhar, S. The Prodrug Platin-A: Simultaneous Release of Cisplatin and Aspirin. Angew. Chem. Int. Ed. 2014, 53, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Massaguer, A.; González-Cantó, A.; Escribano, E.; Barrabés, S.; Artigas, G.; Moreno, V.; Marchán, V. Integrin-Targeted Delivery into Cancer Cells of a Pt(IV) pro-Drug through Conjugation to RGD-Containing Peptides. Dalton Trans. 2015, 44, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.K.J.; Zhang, J.Z.; Aitken, J.B.; Hambley, T.W. Influence of Equatorial and Axial Carboxylato Ligands on the Kinetic Inertness of Platinum(IV) Complexes in the Presence of Ascorbate and Cysteine and within Dld-1 Cancer Cells. J. Med. Chem. 2013, 56, 8757–8764. [Google Scholar] [CrossRef]
- Kastner, A.; Poetsch, I.; Mayr, J.; Burda, J.V.; Roller, A.; Heffeter, P.; Keppler, B.K.; Kowol, C.R. A Dogma in Doubt: Hydrolysis of Equatorial Ligands of PtIV Complexes under Physiological Conditions. Angew. Chem. Int. Ed. 2019, 58, 7464–7469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spector, D.; Krasnovskaya, O.; Pavlov, K.; Erofeev, A.; Gorelkin, P.; Beloglazkina, E.; Majouga, A. Pt(IV) Prodrugs with Nsaids as Axial Ligands. Int. J. Mol. Sci. 2021, 22, 3817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Yang, X.; Pu, K. Photoactivatable Protherapeutic Nanomedicine for Cancer. Adv. Mater. 2020, 32, 1–19. [Google Scholar] [CrossRef]
- Hu, J.J.; Lei, Q.; Zhang, X.Z. Recent Advances in Photonanomedicines for Enhanced Cancer Photodynamic Therapy. Prog. Mater. Sci. 2020, 114, 100685. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.A.; Sholina, N.V.; Khochenkov, D.A.; Alova, A.V.; Gorelkin, P.V.; Erofeev, A.S.; Generalova, A.N.; Khaydukov, E.V. Photodynamic Therapy of Melanoma by Blue-Light Photoactivation of Flavin Mononucleotide. Sci. Rep. 2019, 9, 9679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Wu, J.B.; Pan, D. Review on Near-Infrared Heptamethine Cyanine Dyes as Theranostic Agents for Tumor Imaging, Targeting, and Photodynamic Therapy. J. Biomed. Opt. 2016, 21, 050901. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Yuan, Y.; Wan, Y.; Li, J.; Song, Y.; Chen, W.; Zhao, D.; Chi, Y.; Li, M.; Lee, C.; et al. Near-Infrared Thermally Activated Delayed Fluorescence Nanoparticle: A Metal-Free Photosensitizer for Two-Photon-Activated Photodynamic Therapy at the Cell and Small Animal Levels. Small 2022, 18, 2106215. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Guo, W.; Liu, Z. Light Responsive Nucleic Acid for Biomedical Application. Exploration 2022, 2, 20210099. [Google Scholar] [CrossRef]
- Imran, M.; Ayub, W.; Butler, I.S. Zia-ur-Rehman Photoactivated Platinum-Based Anticancer Drugs. Coord. Chem. Rev. 2018, 376, 405–429. [Google Scholar] [CrossRef]
- Kratochwil, N.A.; Zabel, M.; Range, K.J.; Bednarski, P.J. Synthesis and X-ray Crystal Structure of trans,cis-[Pt(OAc)2I2(En)]: A Novel Type of Cisplatin Analog That Can Be Photolyzed by Visible Light to DNA-Binding and Cytotoxic Species In Vitro. J. Med. Chem. 1996, 39, 2499–2507. [Google Scholar] [CrossRef]
- Kratochwil, N.A.; Parkinson, J.A.; Bednarski, P.J.; Sadler, P.J. Nucleotide Platination Induced by Visible Light. Angew. Chem.-Int. Ed. 1999, 38, 1460–1463. [Google Scholar] [CrossRef]
- Kratochwil, N.A.; Bednarski, P.J. Relationships between Reduction Properties and Cancer Cell Growth Inhibitory Activities of cis-Dichloro- and cis-Diiodo-Pt(IV)-ethylenediamines. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 279–285. [Google Scholar] [CrossRef]
- Bednarski, P.J.; Grünert, R.; Zielzki, M.; Wellner, A.; Mackay, F.S.; Sadler, P.J. Light-Activated Destruction of Cancer Cell Nuclei by Platinum Diazide Complexes. Chem. Biol. 2006, 13, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissleder, R. A Clearer Vision for In Vivo Imaging: Progress Continues in the Development of Smaller, More Penetrable Probes for Biological Imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting Hypoxia in Cancer Therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Deng, Z.; Zhu, G. Recent Advances in the Synthesis, Stability, and Activation of Platinum(IV) Anticancer Prodrugs. Coord. Chem. Rev. 2021, 442, 213991. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, Z. Photoactivatable Platinum-Based Anticancer Drugs: Mode of Photoactivation and Mechanism of Action. Molecules 2020, 25, 5167. [Google Scholar] [CrossRef]
- Ravera, M.; Gabano, E.; McGlinchey, M.J.; Osella, D. A View on Multi-Action Pt(IV) Antitumor Prodrugs. Inorg. Chim. Acta 2019, 492, 32–47. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. 100 Years of Metal Coordination Chemistry: From Alfred Werner to Anticancer Metallodrugs. Pure Appl. Chem. 2014, 86, 1897–1910. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Imberti, C.; Sadler, P.J. Diazido Platinum(IV) Complexes for PhotoactIVated Anticancer Chemotherapy. Inorg. Chem. Front. 2019, 6, 1623–1638. [Google Scholar] [CrossRef] [Green Version]
- Massey, V. The Chemical and Biological Versatility of Riboflavin. Biochem. Soc. Trans. 2000, 28, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V. Synthetic applications of flavin photocatalysis: A review. RSC Adv. 2021, 11, 14251–14259. [Google Scholar] [CrossRef]
- Alonso-De Castro, S.; Ruggiero, E.; Ruiz-De-Angulo, A.; Rezabal, E.; Mareque-Rivas, J.C.; Lopez, X.; López-Gallego, F.; Salassa, L. Riboflavin as a Bioorthogonal Photocatalyst for the Activation of a PtIV Prodrug. Chem. Sci. 2017, 8, 4619–4625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-de Castro, S.; Cortajarena, A.L.; López-Gallego, F.; Salassa, L. Bioorthogonal Catalytic Activation of Platinum and Ruthenium Anticancer Complexes by FAD and Flavoproteins. Angew. Chem. —Int. Ed. 2018, 57, 3143–3147. [Google Scholar] [CrossRef] [Green Version]
- Alonso-de Castro, S.; Terenzi, A.; Hager, S.; Englinger, B.; Faraone, A.; Martínez, J.C.; Galanski, M.; Keppler, B.K.; Berger, W.; Salassa, L. Biological Activity of PtIV Prodrugs Triggered by Riboflavin-Mediated Bioorthogonal Photocatalysis. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzei, L.F.; Martínez, Á.; Trevisan, L.; Rosa-Gastaldo, D.; Cortajarena, A.L.; Mancin, F.; Salassa, L. Toward Supramolecular Nanozymes for the Photocatalytic Activation of Pt(IV) Anticancer Prodrugs. Chem. Commun. 2020, 56, 10461–10464. [Google Scholar] [CrossRef]
- Gurruchaga-Pereda, J.; Martínez-Martínez, V.; Rezabal, E.; Lopez, X.; Garino, C.; Mancin, F.; Cortajarena, A.L.; Salassa, L. Flavin Bioorthogonal Photocatalysis toward Platinum Substrates. ACS Catal. 2020, 10, 187–196. [Google Scholar] [CrossRef]
- Gurruchaga-Pereda, J.; Martínez-Martínez, V.; Formoso, E.; Azpitarte, O.; Rezabal, E.; Lopez, X.; Cortajarena, A.L.; Salassa, L. Enhancing the Photocatalytic Conversion of Pt(IV) Substrates by Flavoprotein Engineering. J. Phys. Chem. Lett. 2021, 12, 4504–4508. [Google Scholar] [CrossRef]
- Berera, R.; Van Grondelle, R.; Kennis, J.T.M. Ultrafast Transient Absorption Spectroscopy: Principles and Application to Photosynthetic Systems. Photosynth. Res. 2009, 101, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.; Li, C.; Chen, S.; Zhou, Q.; Xu, Z.; Wang, Z.; Yao, H.; Hirao, H.; Zhu, G. An Intramolecular Photoswitch Can Significantly Promote Photoactivation of Pt(IV) Prodrugs. Chem. Sci. 2021, 12, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. Platinum (IV) Anticancer Prodrugs–Hypotheses and Facts. Dalton Trans. 2016, 45, 12983–12991. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Cheng, S.C.; Xu, K.; Deng, Z.; Chen, S.; Xu, Z.; Xie, K.; Tse, M.K.; Shi, P.; et al. Phorbiplatin, a Highly Potent Pt(IV) Antitumor Prodrug That Can Be Controllably Activated by Red Light. Chem 2019, 5, 3151–3165. [Google Scholar] [CrossRef]
- Wang, N.; Deng, Z.; Zhu, Q.; Zhao, J.; Xie, K.; Shi, P.; Wang, Z.; Chen, X.; Wang, F.; Shi, J.; et al. An Erythrocyte-Delivered Photoactivatable Oxaliplatin Nanoprodrug for Enhanced Antitumor Efficacy and Immune Response. Chem. Sci. 2021, 12, 14353–14362. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, N.; Liu, Y.; Xu, Z.; Wang, Z.; Lau, T.C.; Zhu, G. A photocaged, water-oxidizing, and nucleolus-targeted Pt (IV) complex with a distinct anticancer mechanism. J. Am. Chem. Soc. 2020, 142, 7803–7812. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, S.; Deng, Z.; Tse, M.K.; Matsuda, Y.; Zhu, G. BODI-Pt, a Green-Light-Activatable and Carboplatin-Based Platinum(IV) Anticancer Prodrug with Enhanced Activation and Cytotoxicity. Inorg. Chem. 2020, 59, 11823–11833. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Gunawan, Y.F.; Liu, G.; Tse, M.K.; Zhu, G. Optimization of Axial Ligands to Promote the Photoactivation of BODIPY-Conjugated Platinum(IV) Anticancer Prodrugs. Dalton Trans. 2021, 50, 13737–13747. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Gautam, S.; Raza, M.K.; Kondaiah, P.; Chakravarty, A.R. Oxoplatin-B, a Cisplatin-Based Platinum(IV) Complex with Photoactive BODIPY for Mitochondria Specific “Chemo-PDT” Activity. J. Inorg. Biochem. 2021, 223, 111526. [Google Scholar] [CrossRef]
- Bera, A.; Gautam, S.; Sahoo, S.; Pal, A.K.; Kondaiah, P.; Chakravarty, A.R. Red Light Active Pt(IV)–BODIPY Prodrug as a Mitochondria and Endoplasmic Reticulum Targeted Chemo-PDT Agent. RSC Med. Chem. 2022. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Qi, Y.; Tang, Z.; Li, X.; Huang, Y. A Red-Light Activatable and Mitochondrion-Targeting PtIV Complex to Overcome Drug Resistance. Chem. Commun. 2022, 58, 8404–8407. [Google Scholar] [CrossRef]
- Sun, H.; Yee, S.S.; Gobeze, H.B.; He, R.; Martinez, D.; Risinger, A.L.; Schanze, K.S. One- and Two-Photon Activated Release of Oxaliplatin from a Pt(IV)-Functionalized Poly(Phenylene Ethynylene). ACS Appl. Mater. Interfaces 2022, 14, 15996–16005. [Google Scholar] [CrossRef]
- Stamati, I.; Kuimova, M.K.; Lion, M.; Yahioglu, G.; Phillips, D.; Deonarain, M.P. Novel Photosensitisers Derived from Pyropheophorbide-a: Uptake by Cells and Photodynamic Efficiency In Vitro. Photochem. Photobiol. Sci. 2010, 9, 1033–1041. [Google Scholar] [CrossRef]
- Richoux, M.C.; Neta, P.; Harriman, A.; Baral, S.; Hambright, P. One- and Two-Electron Reduction of Metalloporphyrins. Radlation Chemical, Photochemical, and Electrochernlcal Studies. Kinetics of the Decay of π-Radical Anions. J. Phys. Chem. 1986, 90, 2462–2468. [Google Scholar] [CrossRef]
- Suboch, V.P.; Losev, A.P.; Gurinovitch, G.P. Photoreduction of Protochlorophyll and Its Derivatives. Photochem. Photobiol. 1974, 20, 183–190. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Chen, X.; Hung, T.F.; Wang, B.; Zhu, G.; Yu, S.F.; Wang, F. Upconverting Near-Infrared Light through Energy Management in Core-Shell-Shell Nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 13419–13423. [Google Scholar] [CrossRef]
- Kontos, S.; Kourtis, I.C.; Dane, K.Y.; Hubbell, J.A. Engineering Antigens for in Situ Erythrocyte Binding Induces T-Cell Deletion. Proc. Natl. Acad. Sci. USA 2013, 110, E60–E68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, J.; Barton, J.K. Targeting DNA Mismatches with Rhodium Intercalators Functionalized with a Cell-Penetrating Peptide. Biochemistry 2006, 45, 12295–12302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piella, J.; Merkoçi, F.; Genç, A.; Arbiol, J.; Bastús, N.G.; Puntes, V. Probing the Surface Reactivity of Nanocrystals by the Catalytic Degradation of Organic Dyes: The Effect of Size, Surface Chemistry and Composition. J. Mater. Chem. A 2017, 5, 11917–11929. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; MacK, J.; Yang, Y.; Shen, Z. Structural Modification Strategies for the Rational Design of Red/NIR Region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, Oncosis, and Necrosis: An Overview of Cell Death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar]
- Guarr, T.; McGuire, M.; Strauch, S.; McLendon, G. Collisionless Photoinduced Electron Transfer from Ruthenium Tris(Bipyridine)2+* Homologues to Methyl Viologen (MV2+) in Rigid Glycerol Solution. J. Am. Chem. Soc. 1983, 105, 616–618. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheung, Y.K.; Ma, C.; Zhao, S.; Gao, D.; Lo, P.C.; Fong, W.P.; Wong, K.S.; Ng, D.K.P. Endoplasmic Reticulum-Localized Two-Photon-Absorbing Boron Dipyrromethenes as Advanced Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2018, 61, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of Cyanine Derived Dyes in Photodynamic Therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Obah Kosso, A.R.; Sellet, N.; Baralle, A.; Cormier, M.; Goddard, J.P. Cyanine-Based near Infra-Red Organic Photoredox Catalysis. Chem. Sci. 2021, 12, 6964–6968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lv, F.; Liu, L.; Wang, S. Water-Soluble Conjugated Organic Molecules as Optical and Electrochemical Materials for Interdisciplinary Biological Applications. Acc. Chem. Res. 2019, 52, 3211–3222. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Liu, X.; Phan, S.; Lv, F.; Belfield, K.D.; Wang, S.; Schanze, K.S. Two-Photon Absorption of Cationic Conjugated Polyelectrolytes: Effects of Aggregation and Application to 2-Photon-Sensitized Fluorescence from Green Fluorescent Protein. Chem. Mater. 2017, 29, 3295–3303. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Ahn, H.Y.; Belfield, K.D.; Schanze, K.S. Two-Photon Excited Fluorescence of a Conjugated Polyelectrolyte and Its Application in Cell Imaging. ACS Appl. Mater. Interfaces 2010, 2, 2744–2748. [Google Scholar] [CrossRef] [PubMed]
- Funston, A.M.; Silverman, E.E.; Miller, J.R.; Schanze, K.S. Charge Transfer through Terthiophene End-Capped Poly(arylene ethynylene)s. J. Phys. Chem. B 2004, 108, 1544–1555. [Google Scholar] [CrossRef]
- Jagadesan, P.; Valandro, S.R.; Schanze, K.S. Ultrafast Photoinduced Electron Transfer in Conjugated Polyelectrolyte-Acceptor Ion Pair Complexes. Mater. Chem. Front. 2020, 4, 3649–3659. [Google Scholar] [CrossRef]
- Kenny, R.G.; Chuah, S.W.; Crawford, A.; Marmion, C.J. Platinum(IV) Prodrugs—A Step Closer to Ehrlich’s Vision? Eur. J. Inorg. Chem. 2017, 2017, 1596–1612. [Google Scholar] [CrossRef]
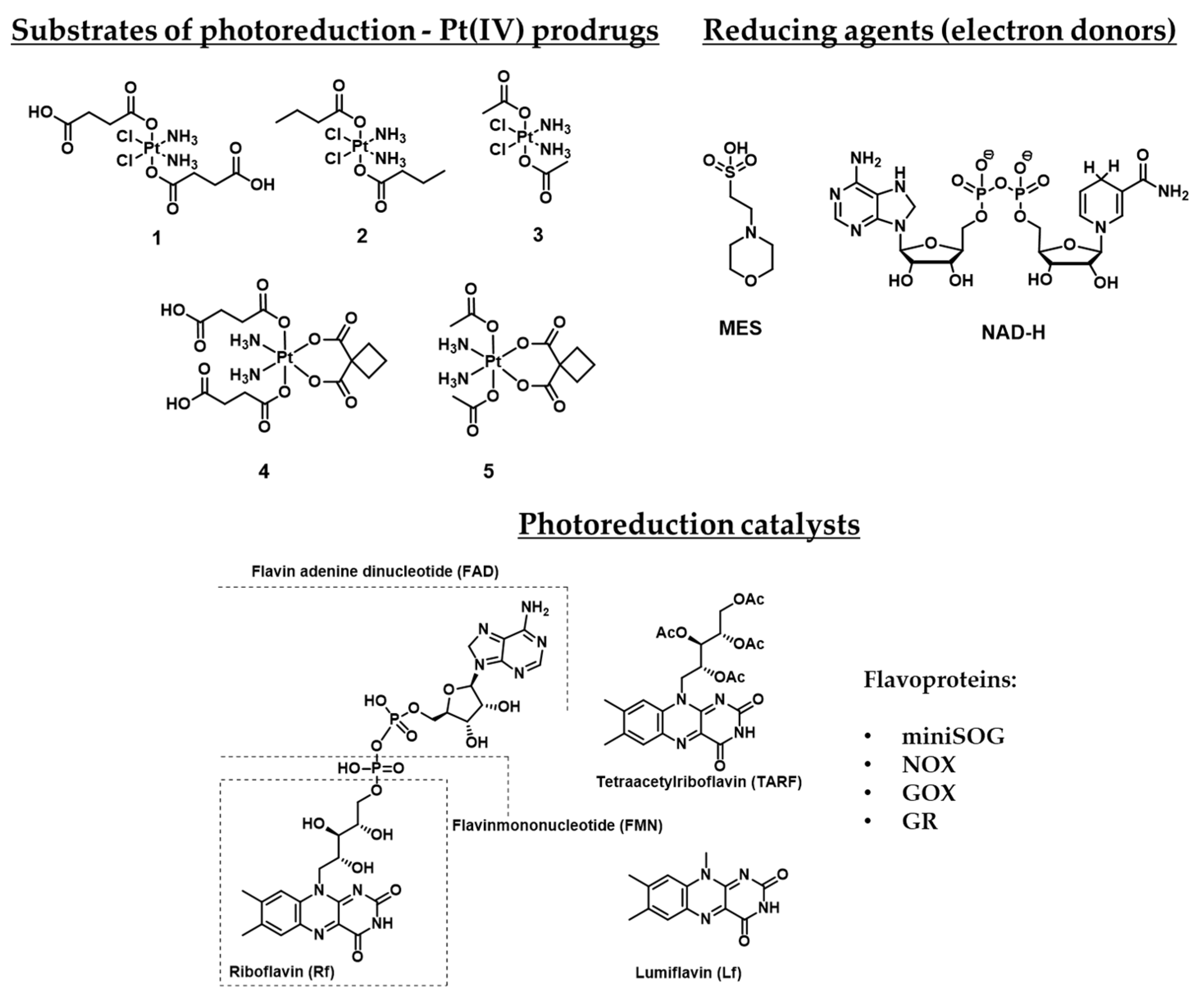


| Pt(IV) Prodrugs Used in Paper | Flavines | Electron-Donor | Results | Reference |
|---|---|---|---|---|
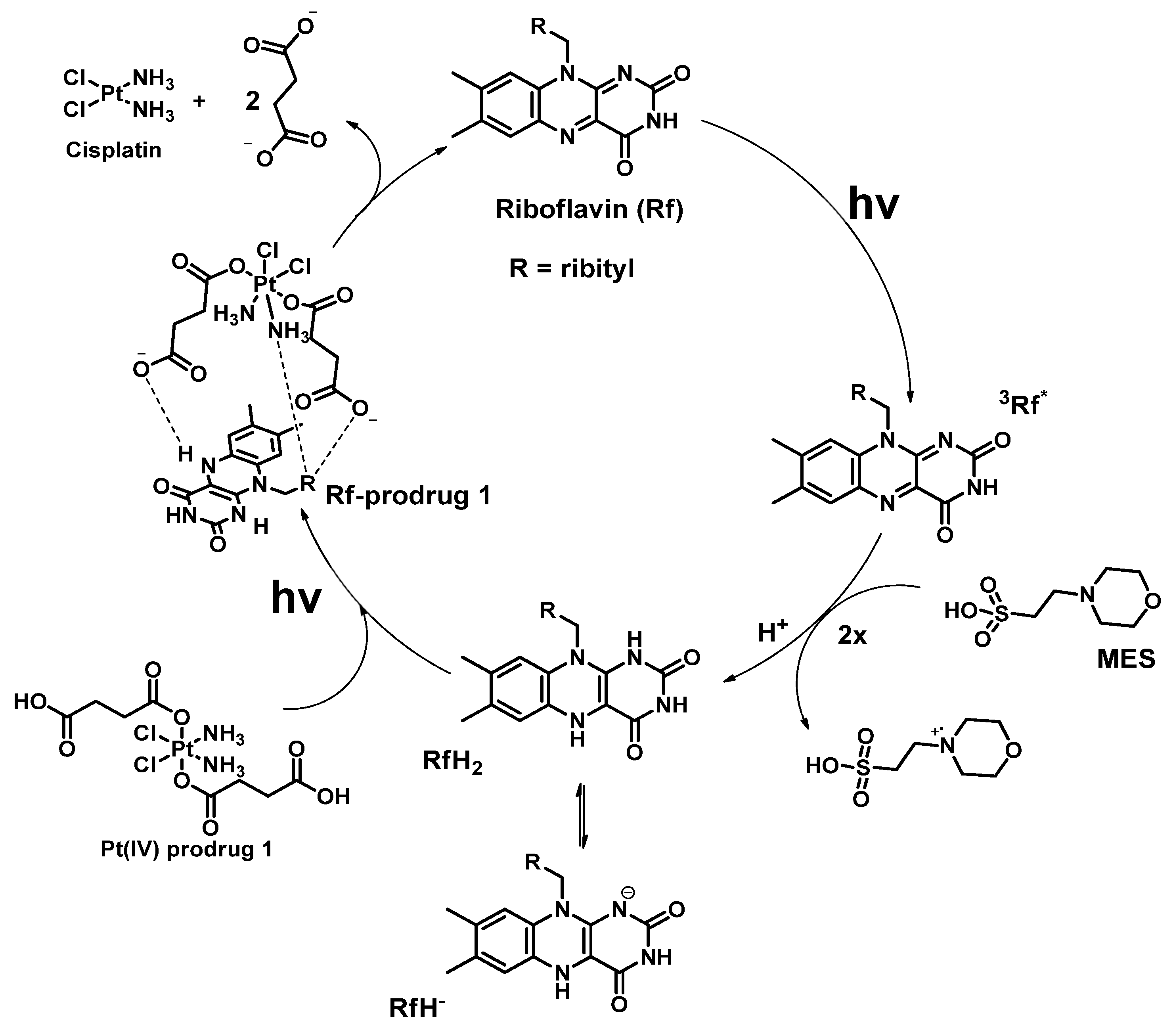
| 1 | Riboflavin (Rf) | 2-(N-morpholino)ethanesulfonic acid (MES) | Proven that riboflavin might reduce Pt(IV) prodrug 1 in presence of a reducing agent. | [51] |
| 1, 2, 4 | Flavin adenine dinucleotide (FAD), mini Singlet Oxygen Generator (miniSOG), NAD-H oxidase (NOX), Glutathione reductase (GR) | MES, NAD-H | Demonstrated that activation of Pt(IV) prodrugs 1, 2, and 4 may be induced by flavoproteins. | [52] |
| 1, 4 | Riboflavin | MES | Demonstrated that mixtures Rf-1 and Rf-4 are active toward the PDT-resistant Capan-1 cell line. | [53] |
| 1 | FMN incorporated in TACN-modified Au nanoparticles | TACN-modified Au nanoparticles | A system that contains TACN-modified Au nanoparticles and incorporated FMN was developed, which may cause photocatalytic reduction of Pt(IV) prodrug 1 without other reducing agents. | [54] |
| 1, 3, 4, 5 | Rf, FMN, Tetraacetylriboflavin (TARF), Lumiflavin (Lf), miniSOG | NAD-H | Defined photocatalytic properties of other riboflavin derivatives and photocatalytic mechanism proved by flash-photolysis. | [55] |
| 1, 3 | miniSOG and its mutants Q103V, Q50E, Q50W | NAD-H | The photocatalytic ability of several mutant miniSOG proteins was defined. | [56] |
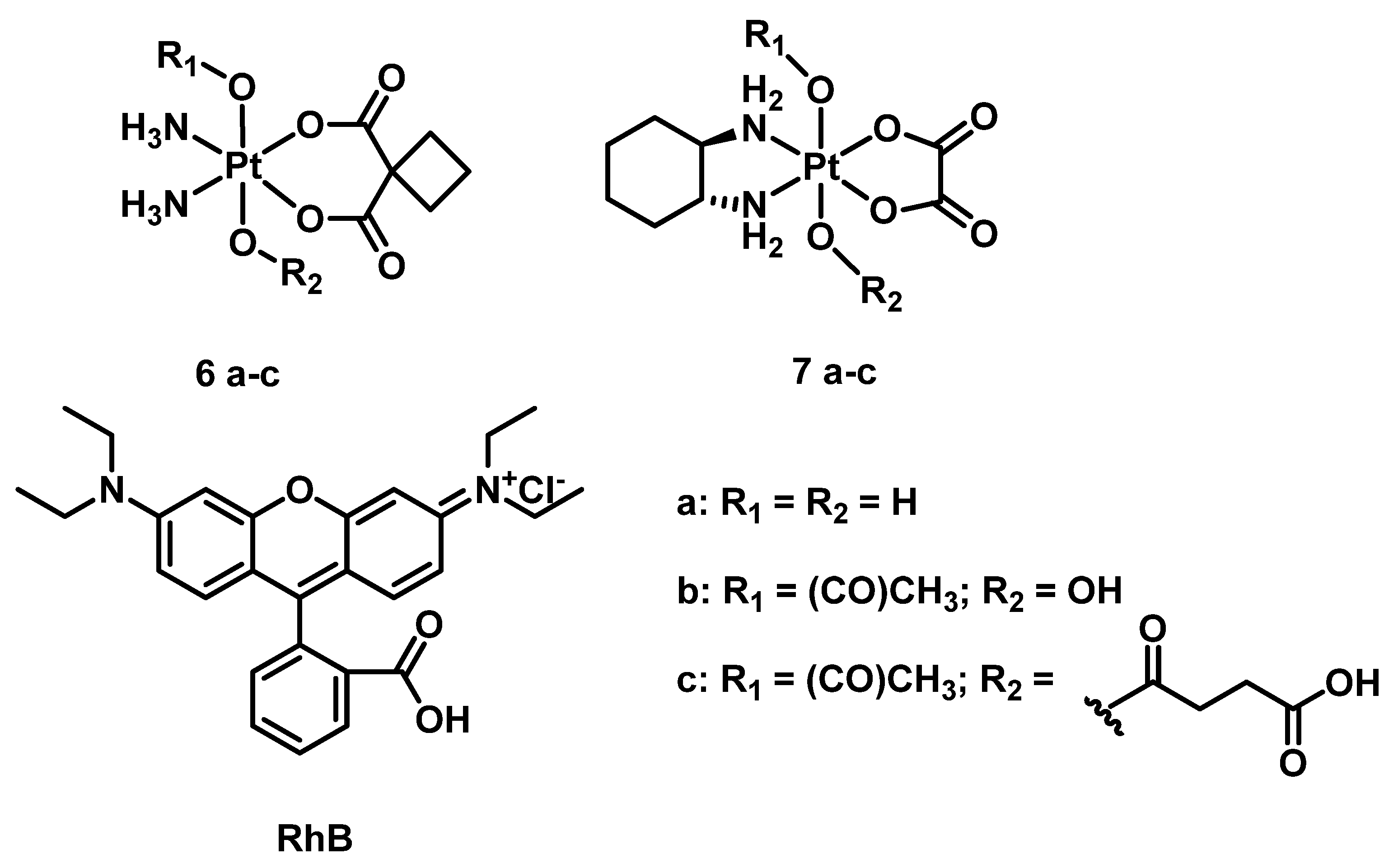
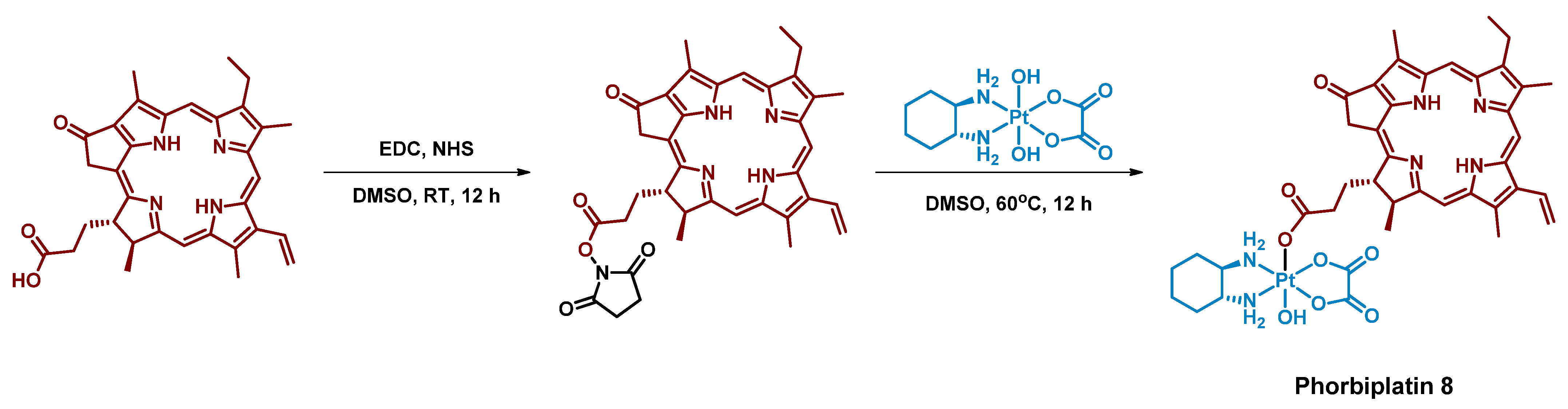
| №/Name | Prodrug Pt(IV) Structure | Nature of Axial Moiety | Fold Increase: IC50 of Pt(II) Drug Under Irradiation/IC50 of Pt(IV) Prodrug Under Irradiation | Irradiation, Wavelength λ (nm), and Dosage (Irradiation Power and Period) | Reference |
|---|---|---|---|---|---|
| 8 Phorbiplatin | 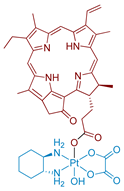 | Porphyrin | 974 (A2780cisR) | 650 nm, 6.3 J/cm2 (7 mW/cm2, 15 min) | [60] |
| 9 Phorbiplatin-COOH | 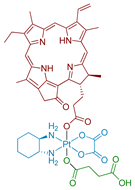 | Porphyrin | - | - | [61] |
| 10 |  | Nanocrystals, modified with 9 | ≈16 (A2780cisR) | 808 nm, 150 J/cm2 (500 mW/cm2, 5 min) | [61] |
| 11 |  | Nanocrystals, modified with 9 and PEG | - | - | [61] |
| 12 |  | Nanocrystals, modified with 9 and ERY1 peptide | - | In Vivo: 808 nm, 900 J/cm2 (500 mW/cm2, 30 min) per round of irradiation | [61] |
| 13 |  | Coumarin | >2 (A2780cisR) | 450 nm, 28.8 J/cm2 (8 mW/cm2) | [62] |
| 14 Coumaplatin | 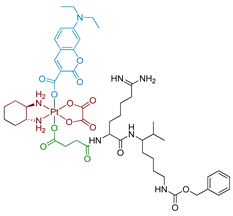 | Coumarin | 26 (A2780) | 450 nm, 28.8 J/cm2 (8 mW/cm2) | [62] |
| 15 Rhodaplatin |  | Rhodamine B | 9.8 (A2780cisR) | 400–760 nm, 7.2 J/cm2 (4 mW/cm2, 30 min) | [58] |
| 16 Rhodaplatin | 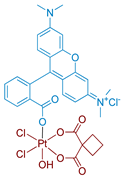 | Rhodamine B | 9.8 (A2780cisR) | 400–760 nm, 7.2 J/cm2 (4 mW/cm2, 30 min) | [58] |
| 17 Bodi-Pt | 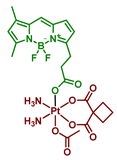 | Bodipy | 7.2 (A2780) | ≈490 nm, 23.4 J/cm2 (13 mW/cm2, 30 min) | [63] |
| 18 |  | Bodipy | >>2 (A2780) | 400–760 nm, 3.6 J/cm2 (2 mW/cm2, 30 min) | [64] |
| 19–22 | 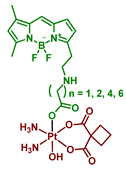 | Bodipy | >>2 (A2780) | 400–760 nm, 3.6 J/cm2 (2 mW/cm2, 30 min) | [64] |
| 23–26 | 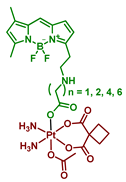 | Bodipy | >>2 (A2780) | 400–760 nm, 3.6 J/cm2 (2 mW/cm2, 30 min) | [64] |
| 27 Oxoplatin B | 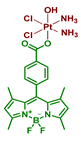 | Bodipy | 33 (HeLa) | 400–700 nm, 10 J/cm2 (13 mW/cm2, 30 min) | [65] |
| 28 | 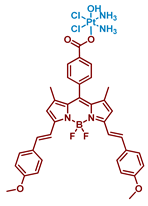 | Bodipy | 117 (HeLa) | 600–720 nm, 30 J/cm2 | [66] |
| 29 | 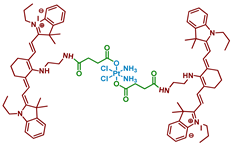 | Heptamethine cyanine | >4 (A2780cisR) | 650 nm, 18 J/cm2 (10 mW/cm2, 30 min) | [67] |
| 30 | 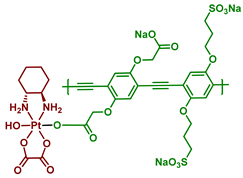 | Poly(phenylene ethynylene) | 460, 8.4 J/cm2 (7 mW/cm2, 20 min) | [68] |
| Cell line | IC50 [µM] | FI | |||||
|---|---|---|---|---|---|---|---|
| Oxaliplatin | Pyropheophorbide a (PPA) | Phorbiplatin 8 | |||||
| Light | Dark | Light | Dark | Light | Dark | ||
| A2780 | 68 ± 9 | 76 ± 4 | 0.34 ± 0.05 | >10 | 0.13 ± 0.01 | >10 | 523 |
| A2780cisR | 185 ± 8 | 162 ± 9 | 0.23 ± 0.01 | >10 | 0.19 ± 0.01 | >10 | 974 |
| MCF-7 | 78.6 ± 8.7 | 110 ± 4.3 | 0.20 ± 0.02 | >10 | 0.044 ± 0.004 | >10 | 1786 |
| 4T1 | 7.6 ± 1.3 | 8.7 ± 0.9 | 0.16 ± 0.02 | >10 | 0.13 ± 0.004 | >10 | 58 |
| MRC-5 | 122 ± 5.2 | >10 | >10 | ||||
| Cell Line | IC50 (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Oxaliplatin | Pt(IV) Prodrug 13 | Coumaplatin 14 | PI | FI | ||||
| Light | Dark | Light | Dark | Light | Dark | |||
| HCT116 p53+/+ | 29.3 ± 3.2 | 31.6 ± 5.2 | 52.8 ± 6.6 | >100 | 0.9 ± 0.2 | 47.1 ± 5.9 | 52 | 32 |
| HCT116 p53−/− | 124.9 ± 9.7 | 127.2 ± 11.4 | 89.3 ± 5.6 | >100 | 1.3 ± 0.3 | 80.6 ± 7.1 | 62 | 96 |
| HT29 | 37.8 ± 3.1 | 41.2 ± 2.7 | 81.5 ± 5.5 | >100 | 2.7 ± 0.4 | 51.6 ± 4.2 | 19 | 14 |
| HeLa | 34.1 ± 3.3 | 38.2 ± 2.4 | 61.8 ± 4.8 | >100 | 5.6 ± 1.1 | 38.8 ± 4.2 | 7 | 6 |
| MCF-7 | 44.1 ± 7.7 | 46.5 ± 8.1 | 86.2 ± 93 | >100 | 5.5 ± 2.1 | 52.1 ± 6.9 | 10 | 8 |
| MDA-MB-231 | 40.5 ± 5.61 | 42.3 ± 7.5 | 79.4 ± 6.6 | >100 | 8.1 ± 3.0 | 69.3 ± 5.2 | 9 | 5 |
| A2780 | 25.2 ± 4.7 | 26.6 ± 4.1 | 56.2 ± 5.6 | >100 | 3.9 ± 0.6 | 42.1 ± 4.7 | 11 | 6 |
| A2780cisR | 127.4 ± 8.7 | 132.8 ± 9.2 | >100 | >100 | 4.9 ± 0.8 | 72.6 ± 4.8 | 15 | 26 |
| A549 | 61.9 ± 7.0 | 64.4 ± 6.1 | 81.3 ± 6.7 | >100 | 6.9 ± 1.1 | 49.2 ± 4.2 | 7 | 9 |
| A549cisR | 172 ± 6.9 | 175.7 ± 10.2 | >100 | >100 | 4 ± 0.5 | 114.8 ± 7.7 | 29 | 43 |
| MRC-5 | 59.7 ± 7.1 | >100 | 93.9 ± 6.8 | – | – | |||
| Cell Line | IC50 [µM] | IC50 [µM] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboplatin | Rhodaplatin 15 | PI | FI | Oxaliplatin | Rhodaplatin 16 | PI | FI | |||||
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | |||||
| A2780 | 322 ± 33 | 301 ± 28 | 44 ± 5 | 220 ± 17 | 5 | 7.3 | 64 ± 6 | 68 ± 6 | 25 ± 2 | 108 ± 9 | 4.4 | 2.6 |
| A2780cisR (RF) | >400 (—) | >400 | 41 ± 5 (0.9) | 250 ± 18 | 6.1 | >9.8 | 199 ± 21 | 187 ± 19 | 20 ± 7 (0.8) | 136 ± 13 | 6.7 | 9.8 |
| MCF-7 | >400 | >400 | 77 ± 9 | 245 ± 17 | 3.2 | >5.2 | 103 ± 12 | 113 ± 13 | 43 ± 3 | 133 ± 11 | 3.1 | 2.4 |
| A549 | >400 | >400 | 57 ± 5 | 251 ± 15 | 4.4 | >7.0 | 87 ± 6 | 95 ± 12 | 29 ± 5 | 104 ± 15 | 3.7 | 3.1 |
| A549cisR (RF) | >400 (—) | >400 | 61 ± 6 (1.1) | 289 ± 17 | 4.7 | >6.5 | 218 ± 10 (2.5) | 212 ± 9 | 33 ± 5 (1.1) | 142 ± 10 | 4 | 5 |
| HCT116 | >400 | >400 | 47 ± 5 | 248 ± 18 | 5.3 | >10.7 | 60 ± 4 | 58 ± 4 | 18 ± 1 | 112 ± 6 | 6.3 | 3.4 |
| MRC-5 | >400 | >300 | — | — | 82 ± 7 | 116 ± 7 | — | — | ||||
| Cell Line | IC50 [µM] | ||||||
|---|---|---|---|---|---|---|---|
| BODI-Pt 17 | Carboplatin | PI | FI | ||||
| Light | Dark | PI | Light | Dark | |||
| MCF-7 | 15.7 ± 1.0 | 173.4 ± 8.8 | 11 | 642.6 ± 51.4 | 608.1 ± 61.0 | 0.9 | 38.7 |
| MDA-MB-231 | 19.1 ± 4.7 | 162 ± 8.4 | 8.5 | 527.7 ± 54.6 | 498.3 ± 28.9 | 0.9 | 26.1 |
| SKOV3 | 22.4 ± 0.8 | >200 | >8.9 | 991.7 ± 62.3 | 962.5 ± 110.7 | 1 | 43 |
| A2780 | 31.0 ± 7.1 | 68.5 ± 5.0 | 2.2 | 254.0 ± 31.0 | 222.6 ± 11.8 | 0.9 | 7.2 |
| HeLa | 37 ± 1.5 | >200 | >5.4 | 600.1 ± 38.1 | 799.8 ± 33.0 | 1.3 | 21.6 |
| A549 | 60.5 ± 14 | 148.9 ± 17.3 | 2.5 | 418.6 ± 75.0 | 395.9 ± 69.2 | 0.9 | 6.5 |
| WI-38 | >200 | 432.7 ± 35.7 | |||||
| Compounds | IC50 [µM] | |
|---|---|---|
| A2780 | ||
| Light | Dark | |
| 18 | >100 | >100 |
| 19 | 43.9 ± 5.2 | 69.8 ± 4.9 |
| 20 | 48.3 ± 2.0 | 74.8 ± 8.2 |
| 21 | 76.8 ± 9.9 | >100 |
| 22 | >100 | >100 |
| 17 | 66.6 ± 7.0 | >100 |
| 23 | 43.8 ± 3.9 | >100 |
| 24 | 88.6 ± 15.7 | >100 |
| 25 | >100 | >100 |
| 26 | >100 | >100 |
| IC50 [µM] | |||||||
|---|---|---|---|---|---|---|---|
| Cell Line | Pt(IV) Prodrug 27 | 27L | Cisplatin | ||||
| Light | Dark | Light | Dark | Light | Dark | FI | |
| MCF-7 | 3.8 ± 0.4 | >50 | 33.4 ± 1.1 | >50 | - | 28 ± 3 | 7.4 |
| HeLa | 2.1 ± 0.1 | >50 | 31 ± 1 | >50 | - | 71 ± 3 | 33.8 |
| A549 | 1.1 ± 0.3 | >50 | 33 ± 1 | >50 | - | - | |
| HPL1D | 46 ± 1 | >50 | >50 | >50 | - | - | |
| Cell Line | IC50 [µM] | |||||||
|---|---|---|---|---|---|---|---|---|
| Pt(IV) Prodrug 28 | 28L | Cisplatin | ||||||
| Light | Dark | PI | Light | Dark | PI | Light | Dark | |
| HeLa | 0.58 ± 0.02 | >100 | >172 | 6.3 ± 0.1 | >100 | >15 | 68.7 ± 3.4 | 71.3 ± 2.9 |
| MCF-7 | 0.76 ± 0.05 | >100 | >131 | 4.4 ± 0.4 | >100 | >23 | — | 69.7 ± 1.2 |
| HPL1D | 9.4 ± 0.6 | >100 | >11 | 37.6 ± 2.2 | >100 | >2 | ||
| IC50 [µM] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | 29L | Cisplatin | 29L + Cisplatin | Pt(IV) Prodrug 29 | ||||
| Dark | Light | Dark | Light | Dark | Light | Dark | Light | |
| MCF-7 | >50 | 29.3 | 35.3 | 32.6 | 25 | 17.1 | 35.3 | 7.7 |
| MCF-7cisR | >50 | 44.3 | >50 | >50 | >50 | 31 | 44.9 | 8.9 |
| A2780 | >50 | 27.4 | 29.9 | 25.2 | 16.4 | 9.7 | 38 | 12 |
| A2780cisR | >50 | 33.2 | >50 | >50 | >50 | 29.6 | 44.6 | 12.5 |
| A549 | >50 | 27.7 | 49.7 | 43.3 | 35.5 | 19.7 | 38.6 | 11.1 |
| A549cisR | >50 | 33.8 | >50 | >50 | >50 | 29.1 | >50 | 13.8 |
| τ1, ps (α1) | τ2, ps (α2) | τ3, ps (α3) | Φf | |
|---|---|---|---|---|
| PPE | 160 (0.89) | 380 (0.11) | 0.19 | |
| Pt(IV) prodrug 30 | 17 (0.96) | 220 (0.04) | 920 (0.01) | 0.013 |
| τ1, ps (α1) | τ2, ps (α2) | τ3, ps (α3) | |
|---|---|---|---|
| PPE | 1.7 (0.43) | 30 (0.29) | 245 (0.28) |
| Pt(IV) prodrug 30 | 0.63 (0.54) | 9.6 (0.31) | 350 (0.15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spector, D.; Pavlov, K.; Beloglazkina, E.; Krasnovskaya, O. Recent Advances in Light-Controlled Activation of Pt(IV) Prodrugs. Int. J. Mol. Sci. 2022, 23, 14511. https://doi.org/10.3390/ijms232314511
Spector D, Pavlov K, Beloglazkina E, Krasnovskaya O. Recent Advances in Light-Controlled Activation of Pt(IV) Prodrugs. International Journal of Molecular Sciences. 2022; 23(23):14511. https://doi.org/10.3390/ijms232314511
Chicago/Turabian StyleSpector, Daniil, Kirill Pavlov, Elena Beloglazkina, and Olga Krasnovskaya. 2022. "Recent Advances in Light-Controlled Activation of Pt(IV) Prodrugs" International Journal of Molecular Sciences 23, no. 23: 14511. https://doi.org/10.3390/ijms232314511
APA StyleSpector, D., Pavlov, K., Beloglazkina, E., & Krasnovskaya, O. (2022). Recent Advances in Light-Controlled Activation of Pt(IV) Prodrugs. International Journal of Molecular Sciences, 23(23), 14511. https://doi.org/10.3390/ijms232314511







