Electrolyzed–Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water
Abstract
1. Introduction
1.1. Electrolyzed Oxidizing Water (EOW)
1.2. Electrolyzed Reduced Water (ERW)
ERW and Oxidation Reduction Potential
2. Safety and Concerns of ERW Ingestion
2.1. Safety of High pH Water
2.2. Safety of Electrodes and Metal Degradation
2.3. Hyperkalemia
2.4. Safety of Gut Microbiota
3. Effectiveness of ERW Machines for Making H2 Water
Other Methods of Providing and Administering Molecular Hydrogen
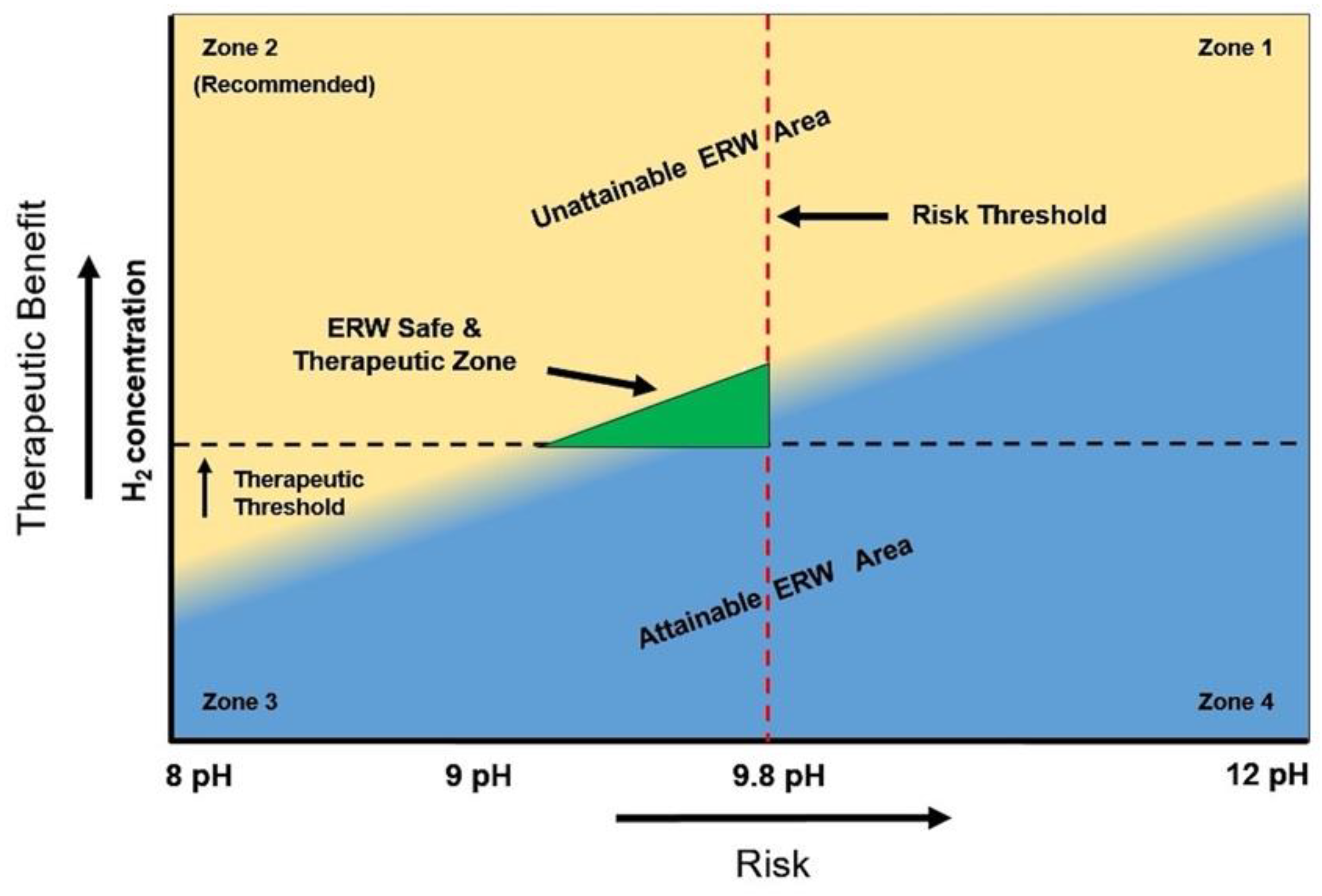
4. Recommendations and Guidelines
- The most effective pH for EOW is between 4.5 and 6.5.
- The HOCl concentration depends on source water chloride concentration, flow rate, applied voltage, electrode configuration, and plate morphology, which may all have wide variations.
- With all other factors being equal, mesh electrodes are more effective at producing HOCl than solid plate electrodes
- If the pH is within the optimal range, the ORP can be used to indicate the sanitizing/disinfecting ability and should generally exceed +700 mV to over +1200 mV
- Using chlorine test strips can also be helpful to ensure the HOCl concentration is high enough.
- Ensure that source water has a mineral concentration (total dissolved solids, TDS) sufficient to permit electrolysis to occur (minimum TDS varies with machine design)
- If source water does have sufficient minerals, be sure to clean the machine as needed.
- ○
- Although some machines have self-cleaning modes that reverse the electrode polarity, this may not be sufficient, and will not prevent scale build-up in other parts of the machine including hoses and solenoid valves.
- ○
- Cleaning the machine with weak acids such as vinegar or citric acid can dissolve the calcium and mineral salts and remove them from the electrodes and other wetted components.
- Frequently measure the H2 concentration of ERW to ensure the level is within the desired range. Be sure to use accurate methods (e.g., gas chromatography, or perhaps the less accurate but useful redox titration reagent (MiZ Japan and H2Blue™, USA) (see also [22]) to measure H2. Specifically, avoid using pH-sensitive methods such as ORP and ORP-based H2 meters (see [22]).
- ○
- A low H2 concentration may indicate insufficient minerals in the source water, a flow rate that is too high, or a need to clean the machine with an acidic solution.
- Ensure that the ERW unit is from a high-quality supplier, the electrodes are coated with high purity-platinum, and the thickness is great enough to withstand normal operation.
- Follow governmental regulations to not ingest ERW that exceeds pH 9.8 to prevent hyperkalemia and other issues associated with ingesting high pH ERW.
- ○
- Remember, even if the setting/button states the pH, this simply alters the applied voltage, thus the actual pH may be higher or lower.
- ○
- If a universal pH indicator is used, the color should not be “dark purple” as this indicates a pH greater than 10.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, B.K.; Wang, C.K. Electrolyzed Water and Its Pharmacological Activities: A Mini-Review. Molecules 2022, 27, 1222. [Google Scholar] [CrossRef]
- Henry, M.; Chambron, J. Physico-Chemical, Biological and Therapeutic Characteristics of Electrolyzed Reduced Alkaline Water (ERAW). Water 2013, 5, 2094–2115. [Google Scholar] [CrossRef]
- Jackson, K.; Dressler, N.; Ben-Shushan, R.S.; Meerson, A.; LeBaron, T.W.; Tamir, S. Effects of alkaline-electrolyzed and hydrogen-rich water, in a high-fat-diet nonalcoholic fatty liver disease mouse model. World J. Gastroenterol. 2018, 24, 5095–5108. [Google Scholar] [CrossRef]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative Stress and Pathways of Molecular Hydrogen Effects in Medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef]
- Zoulias, E.; Varkaraki, E.; Lymberopoulos, N.; Christodoulou, C.N.; Karagiorgis, G.N. A review on water electrolysis. Tcjst 2004, 4, 41–71. [Google Scholar]
- Iram, A.; Wang, X.; Demirci, A. Electrolyzed oxidizing water and its applications as sanitation and cleaning agent. Food Eng. Rev. 2021, 13, 411–427. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, X.; Chen, Y.; Lin, M.; Tang, J.; Lin, Q.; Fang, L.; Li, M.; Hung, Y.C.; Lin, H. Recent trends and applications of electrolyzed oxidizing water in fresh foodstuff preservation and safety control. Food Chem. 2022, 369, 130873. [Google Scholar] [CrossRef]
- Severing, A.L.; Rembe, J.D.; Koester, V.; Stuermer, E.K. Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): Antimicrobial efficacy, cytotoxic impact and physicochemical parameters in vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef]
- Gombas, D.; Luo, Y.; Brennan, J.; Shergill, G.; Petran, R.; Walsh, R.; Hau, H.; Khurana, K.; Zomorodi, B.; Rosen, J.; et al. Guidelines To Validate Control of Cross-Contamination during Washing of Fresh-Cut Leafy Vegetables. J. Food Prot. 2017, 80, 312–330. [Google Scholar] [CrossRef]
- Shen, M.; Bennett, N.; Ding, Y.; Scott, K. A concise model for evaluating water electrolysis. Int. J. Hydrogen Energy 2011, 36, 14335–14341. [Google Scholar] [CrossRef]
- Ye, J.; Li, Y.; Hamasaki, T.; Nakamichi, N.; Komatsu, T.; Kashiwagi, T.; Teruya, K.; Nishikawa, R.; Kawahara, T.; Osada, K.; et al. Inhibitory effect of electrolyzed reduced water on tumor angiogenesis. Biol. Pharm. Bull. 2008, 31, 19–26. [Google Scholar] [CrossRef]
- Tsai, C.F.; Hsu, Y.W.; Chen, W.K.; Chang, W.H.; Yen, C.C.; Ho, Y.C.; Lu, F.J. Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food Chem. Toxicol. 2009, 47, 2031–2036. [Google Scholar] [CrossRef]
- Abe, M.; Sato, S.; Toh, K.; Hamasaki, T.; Nakamichi, N.; Teruya, K.; Katakura, Y.; Morisawa, S.; Shirahata, S. Suppressive Effect of ERW on Lipid Peroxidation and Plasma Triglyceride Level. In Animal Cell Technology: Basic & Applied Aspects, Proceedings of the Fifteenth Annual Meeting of the Japanese Association for Animal Cell Technology (JAACT), Fuchu, Japan, 11–15 November 2002; Springer: Dordrecht, The Netherlands, 2010; Volume 16, pp. 315–321. [Google Scholar]
- Yan, P.; Daliri, E.B.; Oh, D.H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsubo, K.; Morisawa, S.; Katakura, Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun. 1997, 234, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Hsu, S.P.; Yang, C.C.; Ou-Yang, P.; Lee, K.T.; Morisawa, S.; Otsubo, K.; Chien, C.T. Electrolysed-reduced water dialysate improves T-cell damage in end-stage renal disease patients with chronic haemodialysis. Nephrol. Dial. Transpl. 2010, 25, 2730–2737. [Google Scholar] [CrossRef]
- Siswantoro, E.; Purwanto, N.H.; Sutomo. Effectiveness of Alkali Water Consumption to Reduce Blood Sugar Levels in Diabetes Mellitus Type 2. J. Diabetes Mellit. 2017, 07, 249–264. [Google Scholar] [CrossRef]
- McCauley, B. The Miraculous Properties of Ionized Water; Spartan Enterprises, Inc.: Montgomery, TX, USA, 2008. [Google Scholar]
- Parker, P. Ionized Water Protocols; Simple Health Network: New York, NY, USA, 2012. [Google Scholar]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Sharpe, R. ORP should not be used to estimate or compare concentrations of aqueous H2: An in silico analysis and narrative synopsis. Front. Food Sci. Technol. 2022, 2, 1007001. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, D.H.; Yoon, Y.S.; Teng, Y.C.; Chang, B.S.; Lee, K.J. Evaluate of Electrochemical Characteristics in Electrolyzed Reduced Water. Korean J. Microsc. 2008, 38, 321–324. [Google Scholar]
- Saitoh, Y.; Harata, Y.; Mizuhashi, F.; Nakajima, M.; Miwa, N. Biological safety of neutral-pH hydrogen-enriched electrolyzed water upon mutagenicity, genotoxicity and subchronic oral toxicity. Toxicol. Ind. Health 2010, 26, 203–216. [Google Scholar] [CrossRef]
- Lee, K.J.; Jin, D.; Chang, B.S.; Teng, Y.C.; Kim, D.H. The immunological effects of electrolyzed reduced water on the Echinostoma hortense infection in C57BL/6 mice. Biol. Pharm. Bull. 2009, 32, 456–462. [Google Scholar] [CrossRef][Green Version]
- Barancik, M.; Kura, B.; LeBaron, T.W.; Bolli, R.; Buday, J.; Slezak, J. Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems. Antioxidants 2020, 9, 1281. [Google Scholar] [CrossRef]
- Watanabe, T.; Kamata, H.; Fukuda, Y.; Murasugi, E.; Sato, T.; Uwatoko, K.; Pan, I.J. Influences of alkaline ionized water on milk electrolyte concentrations in maternal rats. J. Toxicol. Sci. 2000, 25, 417–422. [Google Scholar] [CrossRef][Green Version]
- Merne, M.E.; Syrjanen, K.J.; Syrjanen, S.M. Systemic and local effects of long-term exposure to alkaline drinking water in rats. Int. J. Exp. Pathol. 2001, 82, 213–219. [Google Scholar] [CrossRef]
- Watanabe, T.; Kishikawa, Y.; Shirai, W. Influence of alkaline ionized water on rat erythrocyte hexokinase activity and myocardium. J. Toxicol. Sci. 1997, 22, 141–152. [Google Scholar] [CrossRef]
- Watanabe, T.; Kishikawa, Y. Degradation of myocardiac myosin and creatine kinase in rats given alkaline ionized water. J. Vet. Med. Sci. 1998, 60, 245–250. [Google Scholar] [CrossRef]
- Watanabe, T.; Pan, I.; Fukuda, Y.; Murasugi, E.; Kamata, H.; Uwatoko, K. Influences of alkaline ionized water on milk yield, body weight of offspring and perinatal dam in rats. J. Toxicol. Sci. 1998, 23, 365–371. [Google Scholar] [CrossRef]
- Watanabe, T.; Shirai, W.; Pan, I.; Fukuda, Y.; Murasugi, E.; Sato, T.; Kamata, H.; Uwatoko, K. Histopathological influence of alkaline ionized water on myocardial muscle of mother rats. J. Toxicol. Sci. 1998, 23, 411–417. [Google Scholar] [CrossRef]
- Stankevič, J.; Audickaitė, A.; Šilovė, S.; Šimčikas, V.; Cesiulis, H.; Skujienė, G.; Bukelskienė, V.; Žalgevičienė, V.; Tutkuvienė, J. Effect of ionised (electrolysed) water on the rat embryo development. J. Environ. Eng. Landsc. Manag. 2020, 28, 28–35. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, H.; Kim, Y.; Park, K. Intratracheal instillation of platinum nanoparticles may induce inflammatory responses in mice. Arch. Pharm. Res. 2010, 33, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Katao, K.; Honma, R.; Kato, S.; Watanabe, S.; Imai, J. Influence of platinum nanoparticles orally administered to rats evaluated by systemic gene expression profiling. Exp. Anim. 2011, 60, 33–45. [Google Scholar] [CrossRef][Green Version]
- Evenepoel, P. Alteration in digestion and absorption of nutrients during profound acid suppression. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Uchinashi, S.; Saihara, Y.; Kikuchi, K.; Okaya, T.; Ogumi, Z. Dissolution of hydrogen and the ratio of the dissolved hydrogen content to the produced hydrogen in electrolyzed water using SPE water electrolyzer. Electrochim. Acta 2003, 48, 4013–4019. [Google Scholar] [CrossRef]
- Sumiyoshi, K. Abstracts from the Functional Water Symposium 96 Held at Fukuoka Prefecture, Japan, November 28 and 29, 1996. Artif. Organs 2008, 21, 1222–1226. [Google Scholar] [CrossRef]
- Sayre, I.M. International standards for drinking water. J. Am. Water Work. Assoc. 1988, 80, 53–60. [Google Scholar] [CrossRef]
- Al-Abri, S.A.; Olson, K.R. Baking soda can settle the stomach but upset the heart: Case files of the Medical Toxicology Fellowship at the University of California, San Francisco. J. Med. Toxicol. 2013, 9, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Parker, P. +100 WAYS TO USE KANGEN WATER. Available online: https://uploads-ssl.webflow.com/5dfe5ea57badfae81f8527c3/5f59047d0104ba54a07b6e92_100%20ways%20to%20use%20Kangen%20Water.pdf (accessed on 15 October 2022).
- Harris, D.C. Quantitative Chemical Analysis; Macmillan: New York, NY, USA, 2010. [Google Scholar]
- Tsujimoto, H.; Hirata, Y.; Ueda, Y.; Kinoshita, N.; Tawa, H.; Tanaka, Y.; Koshiba, R.; Ota, K.; Kojima, Y.; Kakimoto, K.; et al. Effect of a proton-pump inhibitor on intestinal microbiota in patients taking low-dose aspirin. Eur. J. Clin. Pharmacol. 2021, 77, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Gu, J.L.; Cao, J.M. The acute toxic effects of platinum nanoparticles on ion channels, transmembrane potentials of cardiomyocytes in vitro and heart rhythm in vivo in mice. Int. J. Nanomed. 2019, 14, 5595–5609. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Watari, A.; Hayata, Y.; Li, X.; Kondoh, M.; Tsutsumi, Y.; Yagi, K. Hepatotoxicity of sub-nanosized platinum particles in mice. Pharmazie 2013, 68, 178–182. [Google Scholar]
- Yamagishi, Y.; Watari, A.; Hayata, Y.; Li, X.; Kondoh, M.; Yoshioka, Y.; Tsutsumi, Y.; Yagi, K. Acute and chronic nephrotoxicity of platinum nanoparticles in mice. Nanoscale Res. Lett. 2013, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.; Aygun, A.; Gunduz, H.; Sahin, K.; Demir, E.; Akocak, S.; Sen, F. Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids. Surf. B Biointerfaces 2018, 163, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Xinyi, N.; Hande, M.P.; Valiyaveettil, S. DNA damage and p53-mediated growth arrest in human cells treated with platinum nanoparticles. Nanomedicine 2010, 5, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Shinohara, A. Effects of drinking a water product supplemented with the platinum nanocolloid on the oxidative stress and functions of liver and kidney. Biomed. Res. Trace Elem. 2009, 20, 186. [Google Scholar]
- Hiraoka, A.; Inaba, H.; Suzuki, E.; Kasai, K.; Suzuki, H.; Shinohara, A.; Shirao, M.; Kubo, K.; Yoshimura, Y. In Vitro Physicochemical Properties of Neutral Aqueous Solution Systems (Water Products as Drinks) Containing Hydrogen Gas, 2-Carboxyethyl Germanium Sesquioxide, and Platinum Nanocolloid as Additives. J. Health Sci. 2010, 56, 167–174. [Google Scholar] [CrossRef]
- Jan, H.; Gul, R.; Andleeb, A.; Ullah, S.; Shah, M.; Khanum, M.; Ullah, I.; Hano, C.; Abbasi, B.H. A detailed review on biosynthesis of platinum nanoparticles (PtNPs), their potential antimicrobial and biomedical applications. J. Saudi Chem. Soc. 2021, 25, 101297. [Google Scholar] [CrossRef]
- Bodner, M.; Senn, J.; Hacker, V. Degradation Mechanisms and Their Lifetime. Fuel Cells Hydrog. 2018, 139–154. [Google Scholar]
- Enagic, I. Enagic Leveluk Product Owner’s Manual; Enagic: Sapporo, Japan, 2013. [Google Scholar]
- Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y.; Naito, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas Res. 2018, 8, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kiuchi, M.; Higashimura, Y.; Naito, Y.; Koyama, K. The effects of ingestion of hydrogen-dissolved alkaline electrolyzed water on stool consistency and gut microbiota: A double-blind randomized trial. Med. Gas Res. 2021, 11, 138–144. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.M.; Kim, N.; Kim, Y.S.; Nam, R.H.; Park, J.H.; Lee, D.H.; Seok, Y.J.; Kim, Y.R.; Kim, J.H.; Kim, J.M.; et al. Impact of Long-Term Proton Pump Inhibitor Therapy on Gut Microbiota in F344 Rats: Pilot Study. Gut Liver 2016, 10, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jeong, Y.; Park, S.J.; Jin, H.; Lee, J.; Ju, J.H.; Ji, G.E.; Park, S.H. Influence of proton pump inhibitor or rebamipide use on gut microbiota of rheumatoid arthritis patients. Rheumatology 2021, 60, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Okayama, T.; Dohi, O.; Yoshida, N.; et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: An age-sex-matched case-control study. J. Clin. Biochem. Nutr. 2018, 62, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lee, S.Y.; Park, Y.M.; Kim, B.S.; Lee, M.J.; Kim, J.H.; Jeong, S.; Lee, S.H.; Park, M.J.; Rhee, E.S.; et al. Interactions Between IL-17 Variants and Streptococcus in the Gut Contribute to the Development of Atopic Dermatitis in Infancy. Allergy Asthma Immunol. Res. 2021, 13, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Sayols-Baixeras, S.; Dekkers, K.F.; Hammar, U.; Baldanzi, G.; Lin, Y.-T.; Ahmad, S.; Nguyen, D.; Varotsis, G.; Pita, S.; Nielsen, N.; et al. Streptococcus species abundance in the gut is linked to subclinical coronary atherosclerosis in 8973 participants from the SCAPIS cohort. medRxiv 2022. [Google Scholar] [CrossRef]
- Bordoni, L.; Gabbianelli, R.; Fedeli, D.; Fiorini, D.; Bergheim, I.; Jin, C.J.; Marinelli, L.; Di Stefano, A.; Nasuti, C. Positive effect of an electrolyzed reduced water on gut permeability, fecal microbiota and liver in an animal model of Parkinson’s disease. PLoS ONE 2019, 14, e0223238. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.T.; Qin, S.C. Neuroprotective Effects of Molecular Hydrogen: A Critical Review. Neurosci. Bull. 2021, 37, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen–comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The Effects of 24-Week, High-Concentration Hydrogen-Rich Water on Body Composition, Blood Lipid Profiles and Inflammation Biomarkers in Men and Women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 889–896. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kharman, J.; McCullough, M.L. An H2-infused, nitric oxide-producing functional beverage as a neuroprotective agent for TBIs and concussions. J. Integr. Neurosci. 2021, 20, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Alwazeer, D.; Delbeau, C.; Divies, C.; Cachon, R. Use of redox potential modification by gas improves microbial quality, color retention, and ascorbic acid stability of pasteurized orange juice. Int. J. Food Microbiol. 2003, 89, 21–29. [Google Scholar] [CrossRef]
- Hersbach, T.J.P.; Garcia, A.C.; Kroll, T.; Sokaras, D.; Koper, M.T.M.; Garcia-Esparza, A.T. Base-Accelerated Degradation of Nanosized Platinum Electrocatalysts. ACS Catal. 2021, 11, 9904–9915. [Google Scholar] [CrossRef]
- Kumar, S.; Alam, M.T.; Connell, Z.; Haque, M.A. Electromigration stress induced deformation mechanisms in free-standing platinum thin films. Scr. Mater. 2011, 65, 277–280. [Google Scholar] [CrossRef]
- van der Sloot, H.A.; van Zomeren, A.; Meeussen, J.C.L.; Seignette, P.; Bleijerveld, R. Test method selection, validation against field data, and predictive modelling for impact evaluation of stabilised waste disposal. J. Hazard. Mater. 2007, 141, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
| Potentially Harmful Effects | Comment | References |
|---|---|---|
| Growth retardation | Only animal studies and drinking very high pH constantly | [28,33] |
| Tissue necrosis and damage | Only animal studies. Could be due to electrode degradation | [29,30,31,32] |
| Electrode toxicity platinum nanoparticles (PtNPs) and other heavy metals | Depends on electrode purity, voltage, duration, etc. | [32,34,35] |
| Accumulation of harmful substances in drinking water | If harmful substances were not removed, some substances may accumulate in the alkaline drinking water | [33] |
| Impaired mineral, vitamin, and nutrient absorption | Unlikely for a healthy individual, but easy to induce with ingestion of high-volume high pH water | [36] |
| harmful bacterial overgrowth | Bacteria do not survive for long under pH 4. Neutralization of stomach acid could be problematic | [36] |
| Hyperkalemia | Observed in animal and human studies if pH exceeds 10. Cause remains unknown | [32,37,38] |
| Excessive thirst | Commonly reported when pH exceeds 10. Animals also drink more water. May be due to irritation of the mucosal lining | [3,28] |
| Exacerbation of gastric reflux | Commonly reported in some individuals, whereas in others it seems to help. | Anecdotally reported. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed–Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water. Int. J. Mol. Sci. 2022, 23, 14508. https://doi.org/10.3390/ijms232314508
LeBaron TW, Sharpe R, Ohno K. Electrolyzed–Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water. International Journal of Molecular Sciences. 2022; 23(23):14508. https://doi.org/10.3390/ijms232314508
Chicago/Turabian StyleLeBaron, Tyler W., Randy Sharpe, and Kinji Ohno. 2022. "Electrolyzed–Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water" International Journal of Molecular Sciences 23, no. 23: 14508. https://doi.org/10.3390/ijms232314508
APA StyleLeBaron, T. W., Sharpe, R., & Ohno, K. (2022). Electrolyzed–Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water. International Journal of Molecular Sciences, 23(23), 14508. https://doi.org/10.3390/ijms232314508










