Nitrogen-Doped Bismuth Nanosheet as an Efficient Electrocatalyst to CO2 Reduction for Production of Formate
Abstract
1. Introduction
2. Results and Discussion
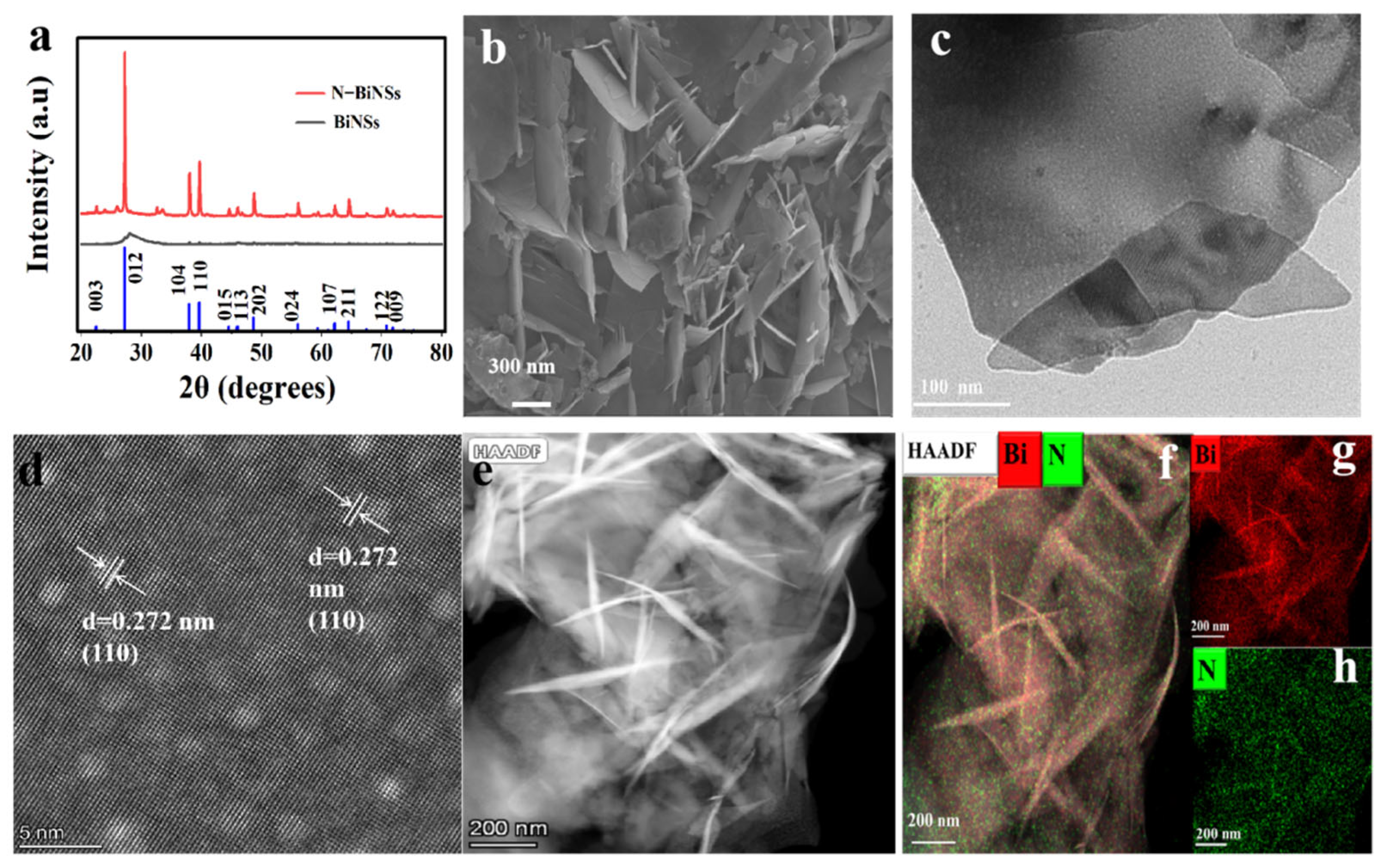
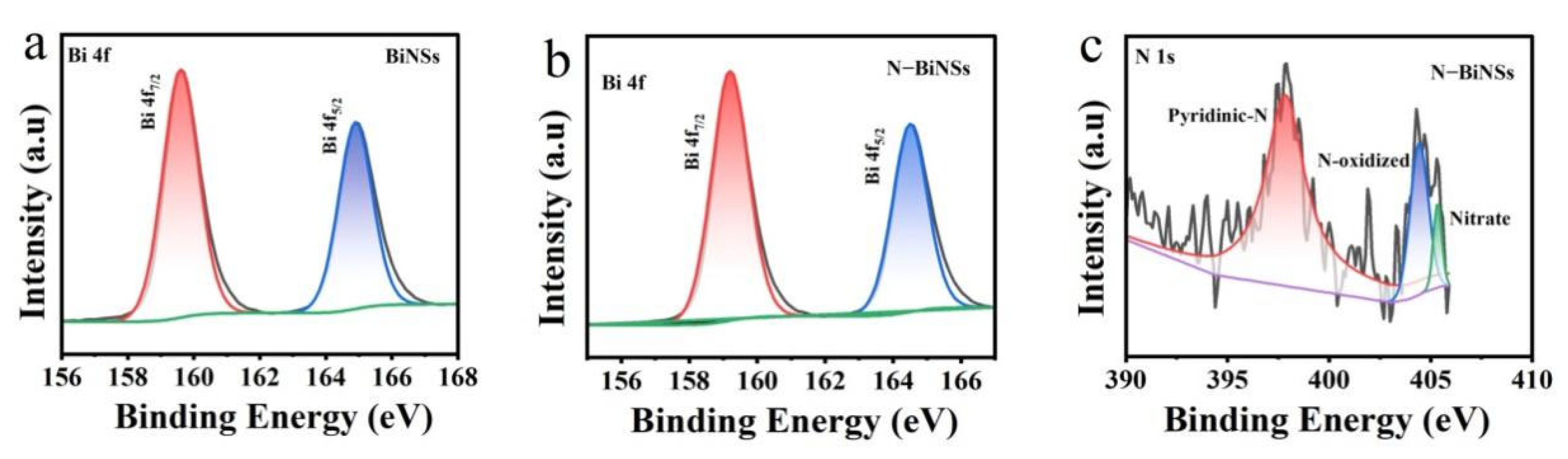
2.1. Morphology and Structure Analysis
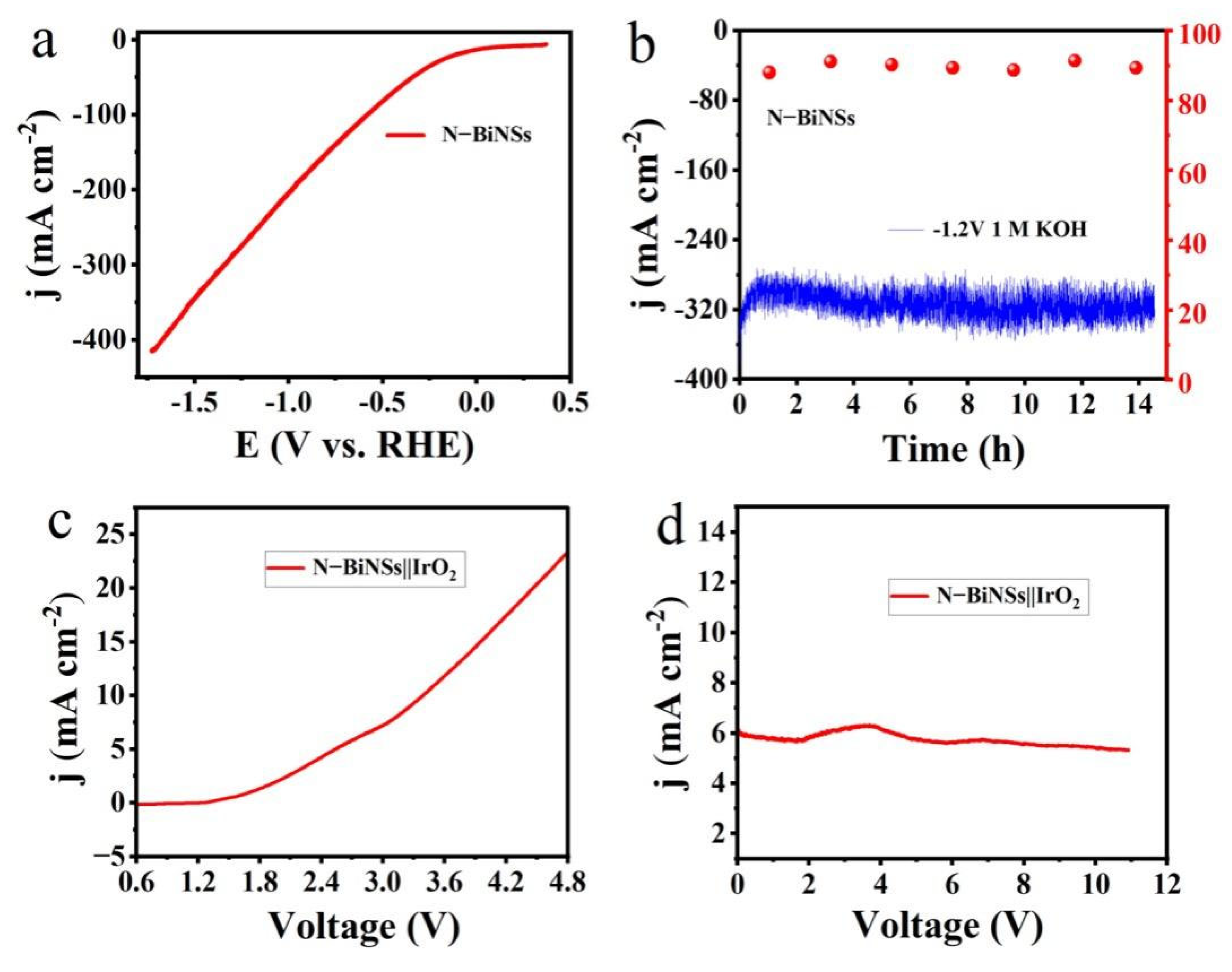
2.2. Electrocatalytic Perfomance
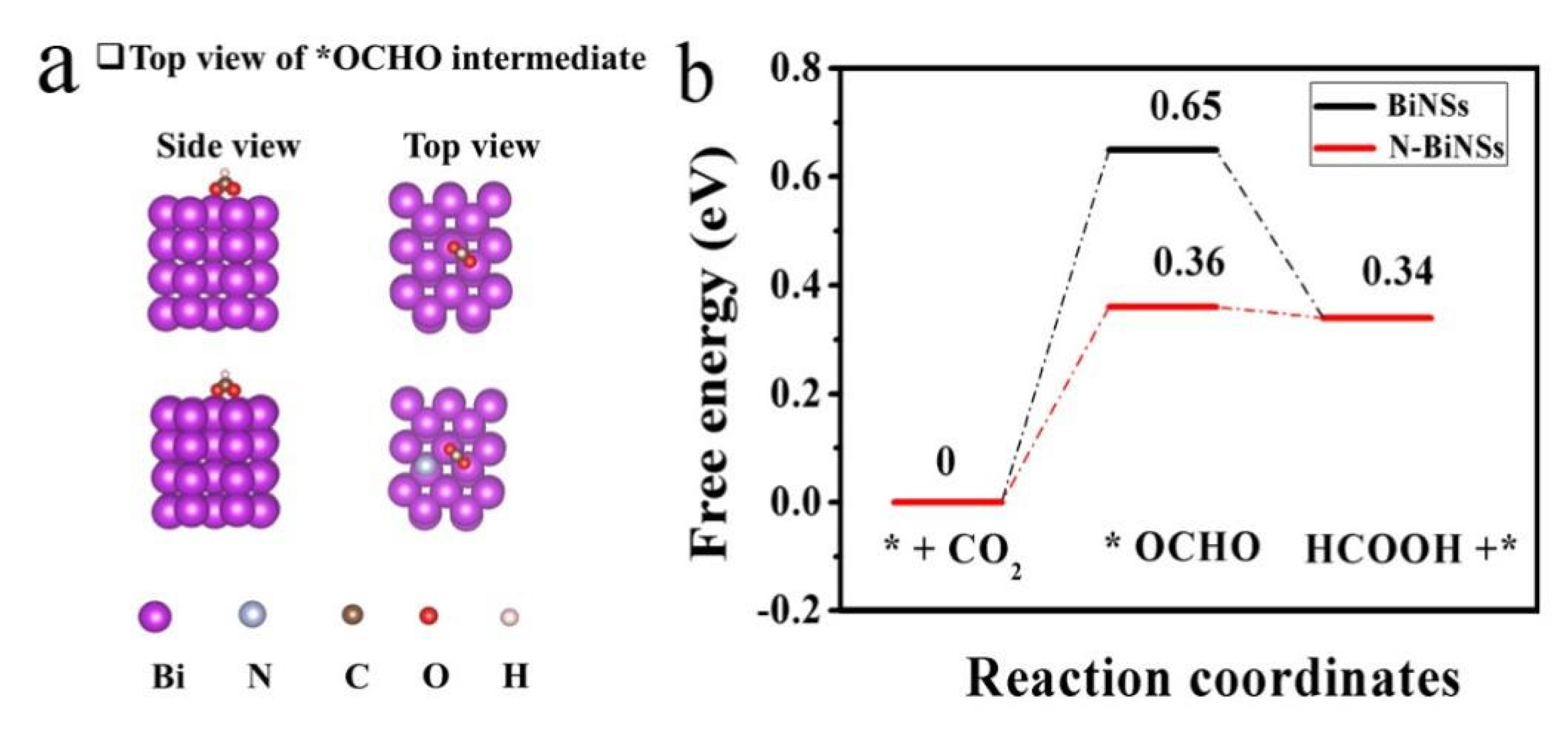
2.3. Catalytic Mechanisms Revealed by DFT Computations
3. Materials and Methods
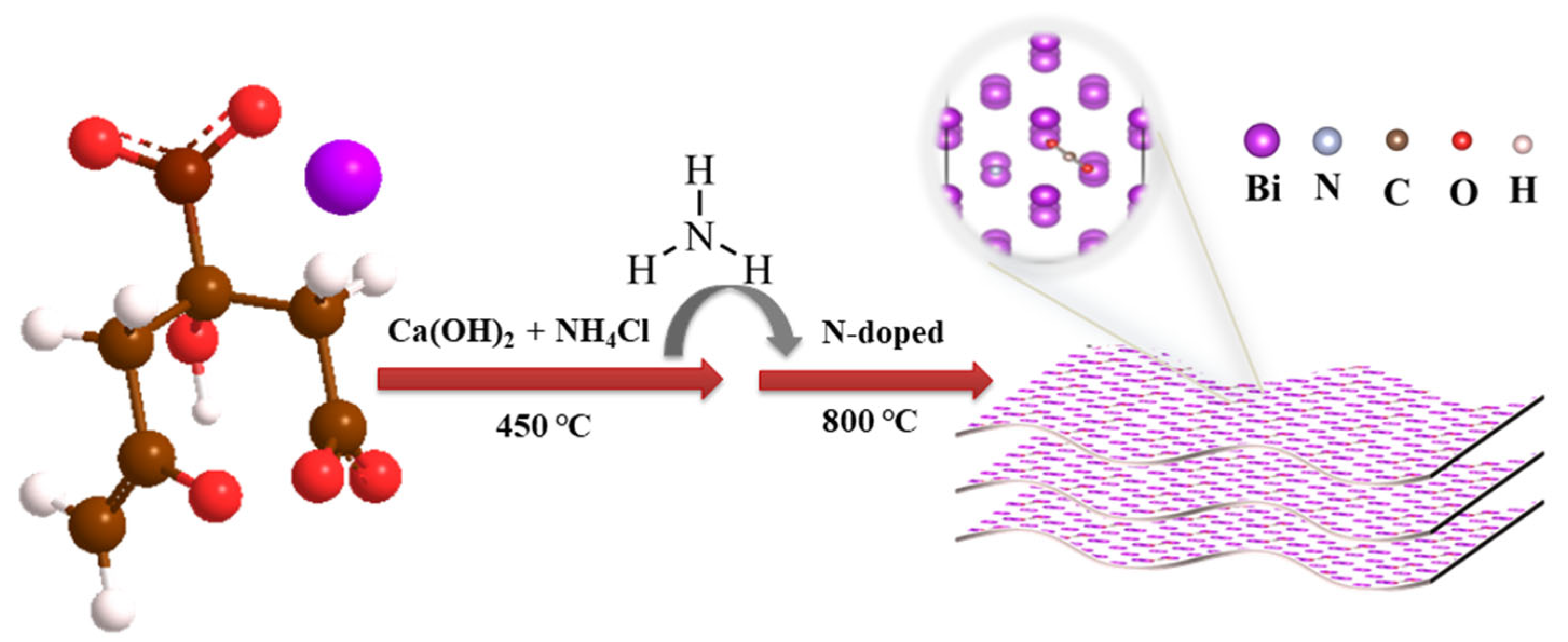
3.1. Synthesis of Nitrogen-Doped Bismuth Nanosheets (N-BiNSs)
3.2. Preparation of Working Electrode
3.3. Electrochemical Measurements
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B.A.; Haasch, R.; Abiade, J.; Yarin, A.L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 2819. [Google Scholar] [CrossRef]
- Feng, X.; Zou, H.; Zheng, R.; Wei, W.; Wang, R.; Zou, W.; Lim, G.; Hong, J.; Duan, L.; Chen, H. Bi2O3/BiO2 Nanoheterojunction for Highly Efficient Electrocatalytic CO2 Reduction to Formate. Nano Lett. 2022, 22, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Boutin, E.; Wang, M.; Lin, J.C.; Mesnage, M.; Mendoza, D.; Lassalle-Kaiser, B.; Hahn, C.; Jaramillo, T.F.; Robert, M. Aqueous Electrochemical Reduction of Carbon Dioxide and Carbon Monoxide into Methanol with Cobalt Phthalocyanine. Angew. Chem. Int. Ed. 2019, 58, 16172–16176. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, Y.; Shi, Y.; Lan, X.; Wang, Y.; Yu, Y.; Zhang, B. Electrocatalytic Reduction of CO2 to Ethanol at Close to Theoretical Potential via Engineering Abundant Electron-Donating Cu(delta+) Species. Angew. Chem. Int. Ed. 2022, 134, e202205909. [Google Scholar] [CrossRef]
- Liu, B.; Yao, X.; Zhang, Z.; Li, C.; Zhang, J.; Wang, P.; Zhao, J.; Guo, Y.; Sun, J.; Zhao, C. Synthesis of Cu2O Nanostructures with Tunable Crystal Facets for Electrochemical CO2 Reduction to Alcohols. ACS Appl. Mater. Interfaces 2021, 13, 39165–39177. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Q.; Liang, X.; Wang, Z.; Zheng, Z.; Wang, P.; Liu, Y.; Dai, Y.; Whangbo, H.M.; Huang, B. Cu2O Nanoparticles with Both {100} and {111} Facets for Enhancing the Selectivity and Activity of CO2 Electroreduction to Ethylene. Adv. Sci. 2020, 7, 1902820. [Google Scholar] [CrossRef]
- Jung, H.; Lee, S.Y.; Lee, C.W.; Cho, M.K.; Won, D.H.; Kim, C.; Oh, H.S.; Min, B.K.; Hwang, Y.J. Electrochemical Fragmentation of Cu2O Nanoparticles Enhancing Selective C-C Coupling from CO2 Reduction Reaction. J. Am. Chem. Soc. 2019, 141, 4624–4633. [Google Scholar] [CrossRef]
- Tan, X.; Yu, C.; Zhao, C.; Huang, H.; Yao, X.; Han, X.; Guo, W.; Cui, S.; Huang, H.; Qiu, J. Restructuring of Cu2O to Cu2O@Cu-Metal-Organic Frameworks for Selective Electrochemical Reduction of CO2. ACS Appl. Mater. Interfaces 2019, 11, 9904–9910. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Jiang, W.-J.; Yao, W.; Liu, Z.-L.; Liu, Z.; Yang, Y.; Gao, L.-Z. Advances in electrochemical reduction of carbon dioxide to formate over bismuth-based catalysts. Rare Met. 2021, 40, 2327–2353. [Google Scholar] [CrossRef]
- Pander, J.E.; Lum, J.W.J.; Yeo, B.S. The importance of morphology on the activity of lead cathodes for the reduction of carbon dioxide to formate. J. Mater. Chem. A 2019, 7, 4093–4101. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, J.J.; Tang, T.; Niu, S.; Zhang, L.B.; Zhang, J.N.; Hu, J.S. Regulating surface In–O in In@ InOx core-shell nanoparticles for boosting electrocatalytic CO2 reduction to formate. Chin. J. Catal. 2022, 43, 1674–1679. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, T.; Pei, J.; Shang, H.; Zhou, D.; Li, H.; Dong, J.; Wang, Y.; Cao, R.; Zhuang, Z.; et al. Discovery of main group single Sb–N4 active sites for CO2 electroreduction to formate with high efficiency. Energy Environ. Sci. 2020, 13, 2856–2863. [Google Scholar] [CrossRef]
- Wen, G.; Lee, D.U.; Ren, B.; Hassan, F.M.; Jiang, G.; Cano, Z.P.; Gostick, J.; Croiset, E.; Bai, Z.; Yang, L.; et al. Orbital Interactions in Bi-Sn Bimetallic Electrocatalysts for Highly Selective Electrochemical CO2 Reduction toward Formate Production. Adv. Energy Mater. 2018, 8, 1802427. [Google Scholar] [CrossRef]
- Cheng, Y.; Hou, J.; Kang, P. Integrated Capture and Electroreduction of Flue Gas CO2 to Formate Using Amine Functionalized SnOx Nanoparticles. ACS Energy Lett. 2021, 6, 3352–3358. [Google Scholar] [CrossRef]
- Gao, S.; Jiao, X.C.; Sun, Z.T.; Zhang, W.H.; Sun, Y.F.; Wang, C.M.; Hu, Q.T.; Zu, X.L.; Yang, F.; Yang, S.Y.; et al. Ultrathin Co3O4 layers realizing optimized CO2 electroreduction to formate. Angew. Chem. Int. Ed. 2016, 55, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Y.; Wang, Y.; Xu, N.; Lou, W.; Liu, P.; Cai, D.; Huang, H.; Qiao, J. Separated growth of Bi-Cu bimetallic electrocatalysts on defective copper foam for highly converting CO2 to formate with alkaline anion-exchange membrane beyond KHCO3 electrolyte. Appl. Catal. B Environ. 2021, 288, 120003. [Google Scholar] [CrossRef]
- Tao, Z.X.; Wu, Z.S.; Yuan, X.L.; Wu, Y.S.; Wang, H.L. Copper–gold interactions enhancing formate production from electrochemical CO2 reduction. ACS Catal. 2019, 9, 10894–10898. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, X.; Mao, X.; Xu, J.; Han, N.; Yang, H.; Pan, B.; Li, Y.; Wang, L.; Li, Y. Large-Area Vertically Aligned Bismuthene Nanosheet Arrays from Galvanic Replacement Reaction for Efficient Electrochemical CO2 Conversion. Adv. Mater. 2021, 33, e2100910. [Google Scholar] [CrossRef]
- Xie, L.; Liu, X.; Huang, F.; Liang, J.; Liu, J.; Wang, T.; Yang, L.; Cao, R.; Li, Q. Regulating Pd-catalysis for electrocatalytic CO2 reduction to formate via intermetallic PdBi nanosheets. Chin. J. Catal. 2022, 43, 1680–1686. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Yang, H.; Deng, J.; Wu, J.; Li, Y.; Li, Y. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nat. Commun. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Wu, J.; Yu, X.; He, H.; Yang, C.; Xia, D.; Wang, L.; Huang, J.; Zhao, N.; Tang, F.; Deng, L.; et al. Bismuth-Nanosheet-Based Catalysts with a Reconstituted Bi0 Atom for Promoting the Electrocatalytic Reduction of CO2 to Formate. Ind. Eng. Chem. Res. 2022, 61, 12383–12391. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, H.; Cai, W.; Wen, Z.; Jia, B.; Wang, L.; Jin, W.; Ma, T. Engineering Bismuth-Tin Interface in Bimetallic Aerogel with a 3D Porous Structure for Highly Selective Electrocatalytic CO2 Reduction to HCOOH. Angew. Chem. Int. Ed. 2021, 60, 12554–12559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Zhao, P.; Xue, X.; Chen, R.; Yang, S.; Ma, J.; Liu, J.; et al. Liquid-phase exfoliated ultrathin Bi nanosheets: Uncovering the origins of enhanced electrocatalytic CO2 reduction on two-dimensional metal nanostructure. Nano Energy 2018, 53, 808–816. [Google Scholar] [CrossRef]
- Yi, L.; Chen, J.; Shao, P.; Huang, J.; Peng, X.; Li, J.; Wang, G.; Zhang, C.; Wen, Z. Molten-Salt-Assisted Synthesis of Bismuth Nanosheets for Long-term Continuous Electrocatalytic Conversion of CO2 to Formate. Angew. Chem. Int. Ed. 2020, 59, 20112–20119. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Hu, X.; Feng, X. Tuning the microenvironment in gas-diffusion electrodes enables high-rate CO2 electrolysis to formate. ACS Energy Lett. 2021, 6, 1694–1702. [Google Scholar] [CrossRef]
- Fan, K.; Jia, Y.; Jia, Y.F.; Ji, Y.F.; Kuang, Y.P.; Zhu, B.C.; Liu, X.Y.; Yu, J.G. Curved surface boosts electrochemical CO2 reduction to formate via bismuth nanotubes in a wide potential window. ACS Catal. 2019, 10, 358–364. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Yang, J.; Wang, L. Electrodeposited Bi dendrites/2D black phosphorus nanosheets composite used for boosting formic acid production from CO2 electroreduction. J. CO2 Util. 2020, 38, 32–38. [Google Scholar] [CrossRef]
- Li, W.; Bandosz, T.J. Analyzing the effect of nitrogen/sulfur groups’ density ratio in porous carbons on the efficiency of CO2 electrochemical reduction. Appl. Surf. Sci. 2021, 569, 151066. [Google Scholar] [CrossRef]
- Zhao, M.; Gu, Y.; Gao, W.; Cui, P.; Tang, H.; Wei, X.; Zhu, H.; Li, G.; Yan, S.; Zhang, X.; et al. Atom vacancies induced electron-rich surface of ultrathin Bi nanosheet for efficient electrochemical CO2 reduction. Appl. Catal. B: Environ. 2020, 266, 118625. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, S.; Zhang, J.; Zhou, T.; Zhang, N.; Zheng, X.S.; Chu, W.; Hu, Z.; Wu, C.; Xie, Y. Surface Nitrogen-Injection Engineering for High Formation Rate of CO2 Reduction to Formate. Nano Lett. 2020, 20, 6097–6103. [Google Scholar] [CrossRef]
- Chen, Z.; Mou, K.; Wang, X.; Liu, L. Nitrogen-Doped Graphene Quantum Dots Enhance the Activity of Bi2O3 Nanosheets for Electrochemical Reduction of CO2 in a Wide Negative Potential Region. Angew. Chem. Int. Ed. 2018, 57, 12790–12794. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.; Zhang, M.; Hou, P.; Kang, P. Nitrogen doped tin oxide nanostructured catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Energy Chem. 2017, 26, 825–829. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, N.; Li, S.; Min, S.; Peng, J.; Liu, W.; Zhan, D.; Bai, H. A Mn single atom catalyst with Mn–N2O2 sites integrated into carbon nanosheets for efficient electrocatalytic CO2 reduction. J. Mater. Chem. A 2022, 10, 10892–10901. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, H.J.; Zhou, X.; Liu, K.L.; Zhang, C.; Zhou, Z.Y.; Wang, C.; Lin, W.B. Electrocatalytic reduction of CO2 to CO with 100% faradaic efficiency by using pyrolyzed zeolitic imidazolate frameworks supported on carbon nanotube networks. J. Mater. Chem. A 2017, 5, 24867–24873. [Google Scholar] [CrossRef]
- Varela, A.S.; Sahraie, N.R.; Steinberg, J.; Ju, W.; Oh, H.S.; Strasser, P. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons. Angew. Chem. Int. Ed. 2015, 54, 10758–10762. [Google Scholar] [CrossRef]
- Kwon, I.S.; Debela, T.T.; Kwak, I.H.; Seo, H.W.; Park, K.; Kim, D.; Yoo, S.J.; Kim, J.-G.; Park, J.; Kang, H.S. Selective electrochemical reduction of carbon dioxide to formic acid using indium–zinc bimetallic nanocrystals. J. Mater. Chem. A 2019, 7, 22879–22883. [Google Scholar] [CrossRef]
- Tian, W.L.; Zhang, J.; Feng, H.; Wen, H.; Sun, X.; Guan, X.; Zheng, D.C.; Liao, J.; Yan, M.L.; Yao, Y.D. Fuels, A hierarchical CoP@ NiCo-LDH nanoarray as an efficient and flexible catalyst electrode for the alkaline oxygen evolution reaction. Sustain. Energy Fuels 2021, 5, 391–395. [Google Scholar] [CrossRef]
- Yoo, J.S.; Christensen, R.; Vegge, T.; Norskov, J.K.; Studt, F. Theoretical Insight into the Trends that Guide the Electrochemical Reduction of Carbon Dioxide to Formic Acid. ChemSusChem 2016, 9, 358–363. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Lei, Z. One-step preparation of ultrathin nitrogen-doped carbon nanosheets with ultrahigh pore volume for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 17297–17301. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens Matter. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B Condens Matter. 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens Matter. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W. Comment on “Generalized Gradient Approximation Made Simple”. Phys. Rev Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerh of functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Xing, Y.; Kong, X.; Guo, X.; Liu, Y.; Li, Q.; Zhang, Y.; Sheng, Y.; Yang, X.; Geng, Z.; Zeng, J. Bi@Sn Core-Shell Structure with Compressive Strain Boosts the Electroreduction of CO2 into Formic Acid. Adv Sci. 2020, 7, 1902989. [Google Scholar] [CrossRef]
- Li, F.; Xue, M.; Li, J.; Ma, X.; Chen, L.; Zhang, X.; MacFarlane, D.R.; Zhang, J. Unlocking the electrocatalytic activity of antimony for CO2 reduction by two-dimensional engineering of the bulk material. Angew. Chem. Int. Ed. 2017, 129, 14910–14914. [Google Scholar] [CrossRef]
- Watanabe, M.; Shibata, M.; Kato, A.; Azuma, M.; Sakata, T. Design of alloy electrocatalysts for CO2 reduction: III. The selective and reversible reduction of on Cu alloy electrodes. J. Electrochem. Soc. 1991, 138, 3382. [Google Scholar] [CrossRef]
- Sreekanth, N.; Nazrulla, M.A.; Vineesh, T.V.; Sailaja, K.; Phani, K.L. Metal-free boron-doped graphene for selective electroreduction of carbon dioxide to formic acid/formate. Chem. Commun. 2015, 51, 16061–16064. [Google Scholar] [CrossRef] [PubMed]
- Won, D.H.; Choi, C.H.; Chung, J.; Chung, M.W.; Kim, E.H.; Woo, S.I. Rational design of a hierarchical tin dendrite electrode for efficient electrochemical reduction of CO2. ChemSusChem 2015, 8, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; De Luna, P.; García de Arquer, F.P.; Zhang, B.; Becknell, N.; Ross, M.B.; Li, Y.; Banis, M.N.; Li, Y.; Liu, M.; et al. Sulfur-modulated tin sites enable highly selective electrochemical reduction of CO2 to formate. Joule 2017, 1, 794–805. [Google Scholar] [CrossRef]
- He, S.; Ni, F.; Ji, Y.; Wang, L.; Wen, Y.; Bai, H.; Liu, G.; Zhang, Y.; Li, Y.; Zhang, B.; et al. The p-Orbital delocalization of main-group metals to boost CO2 electroreduction. Angew. Chem. Int. Ed. 2018, 130, 16346–16351. [Google Scholar] [CrossRef]
- Kumar, B.; Atla, V.; Brian, J.P.; Kumari, S.; Nguyen, T.Q.; Sunkara, M.; Spurgeon, J.M. Reduced SnO2 porous nanowires with a high density of grain boundaries as catalysts for efficient electrochemical CO2-into-HCOOH conversion. Angew. Chem. Int. Ed. 2017, 56, 3645–3649. [Google Scholar] [CrossRef]
- Liang, C.; Kim, B.; Yang, S.; Yang Liu, Y.L.; Francisco Woellner, C.; Li, Z.; Vajtai, R.; Yang, W.; Wu, J.; Kenis, P.J.A.; et al. High efficiency electrochemical reduction of CO2 beyond the two-electron transfer pathway on grain boundary rich ultra-small SnO2 nanoparticles. J. Mater. Chem. A 2018, 6, 10313–10319. [Google Scholar] [CrossRef]
- Lee, C.W.; Hong, J.S.; Yang, K.D.; Jin, K.; Lee, J.H.; Ahn, H.-Y.; Seo, H.; Sung, N.-E.; Nam, K.T. Selective Electrochemical Production of Formate from Carbon Dioxide with Bismuth-Based Catalysts in an Aqueous Electrolyte. ACS Catal. 2018, 8, 931–937. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Zhang, X.; Williams, T.; Easton, C.D.; Bond, A.M.; Zhang, J. Electrochemical reduction of CO2 on defect-rich Bi derived from Bi2S3 with enhanced formate selectivity. J. Mater. Chem. A 2018, 6, 4714–4720. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, J.; Wang, C.; Ma, J.; Wallace, G.G. Tunable and efficient tin modified nitrogen-doped carbon nanofibers for electrochemical reduction of aqueous carbon dioxide. Adv. Energy Mater. 2018, 8, 1702524. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Kang, Y.; Mo, C.; Peng, Y.; Ma, H.; Peng, J. Nitrogen-Doped Bismuth Nanosheet as an Efficient Electrocatalyst to CO2 Reduction for Production of Formate. Int. J. Mol. Sci. 2022, 23, 14485. https://doi.org/10.3390/ijms232214485
Li S, Kang Y, Mo C, Peng Y, Ma H, Peng J. Nitrogen-Doped Bismuth Nanosheet as an Efficient Electrocatalyst to CO2 Reduction for Production of Formate. International Journal of Molecular Sciences. 2022; 23(22):14485. https://doi.org/10.3390/ijms232214485
Chicago/Turabian StyleLi, Sanxiu, Yufei Kang, Chenyang Mo, Yage Peng, Haijun Ma, and Juan Peng. 2022. "Nitrogen-Doped Bismuth Nanosheet as an Efficient Electrocatalyst to CO2 Reduction for Production of Formate" International Journal of Molecular Sciences 23, no. 22: 14485. https://doi.org/10.3390/ijms232214485
APA StyleLi, S., Kang, Y., Mo, C., Peng, Y., Ma, H., & Peng, J. (2022). Nitrogen-Doped Bismuth Nanosheet as an Efficient Electrocatalyst to CO2 Reduction for Production of Formate. International Journal of Molecular Sciences, 23(22), 14485. https://doi.org/10.3390/ijms232214485





