Molecular Mechanisms of Cartilage Repair and Their Possible Clinical Uses: A Review of Recent Developments
Abstract
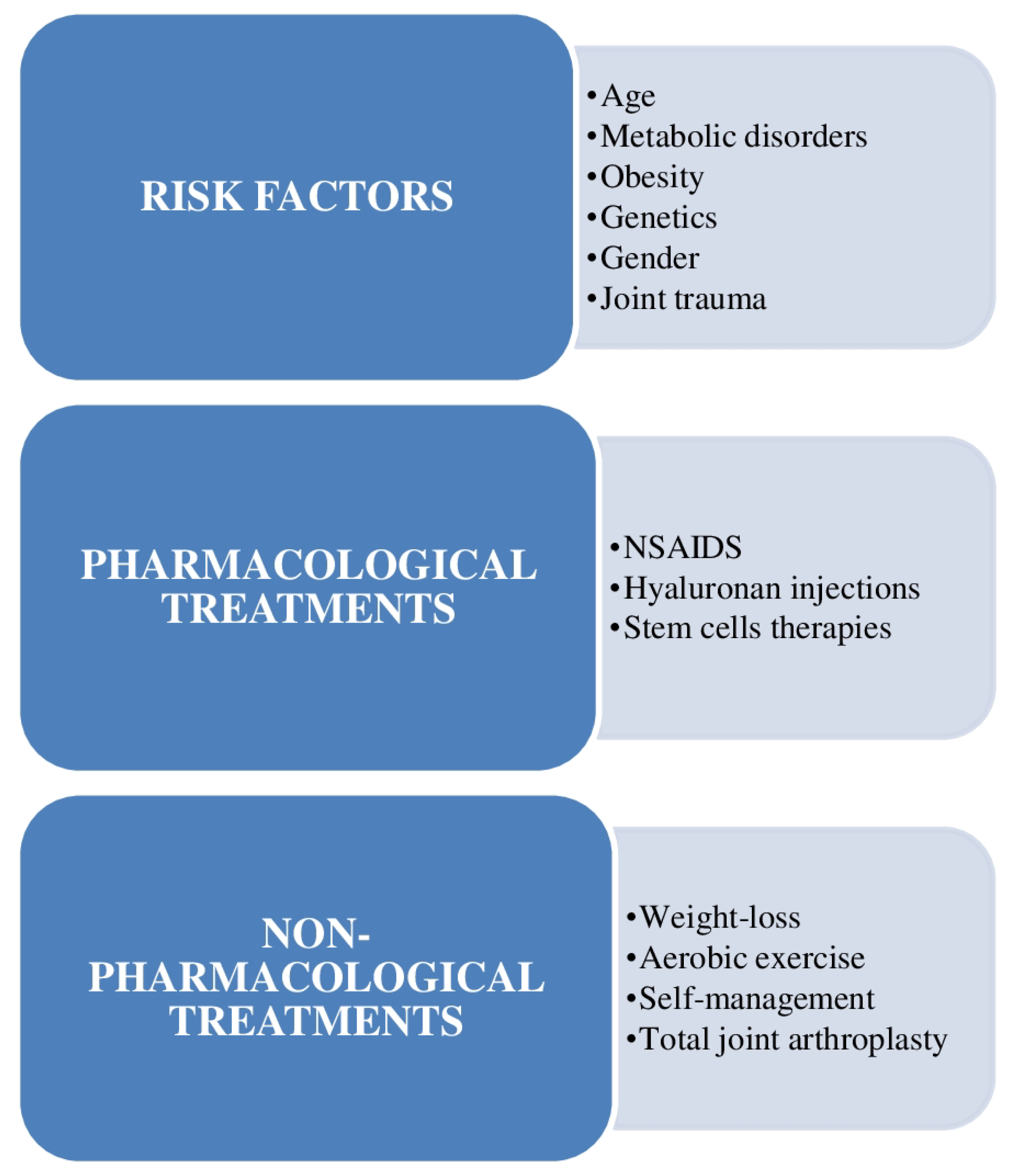
1. Introduction
2. Basic Concepts
3. Recent Developments
3.1. Hyaluronic Acid
3.2. Mesenchymal Stem Cells (MSCs)
3.3. Mechanisms of Diffusion in Articular Cartilage (AC)
3.4. Mechanisms of miRNA Control of the Transcriptome of Tissue-Engineered Cartilage in Reaction to IL-1β and TNF-α
3.5. SIRT1 has an Essential Coordination Function in BMP2-Induced Chondrogenic Differentiation of Stem Cells and Cartilage Preservation under Oxidative Stress
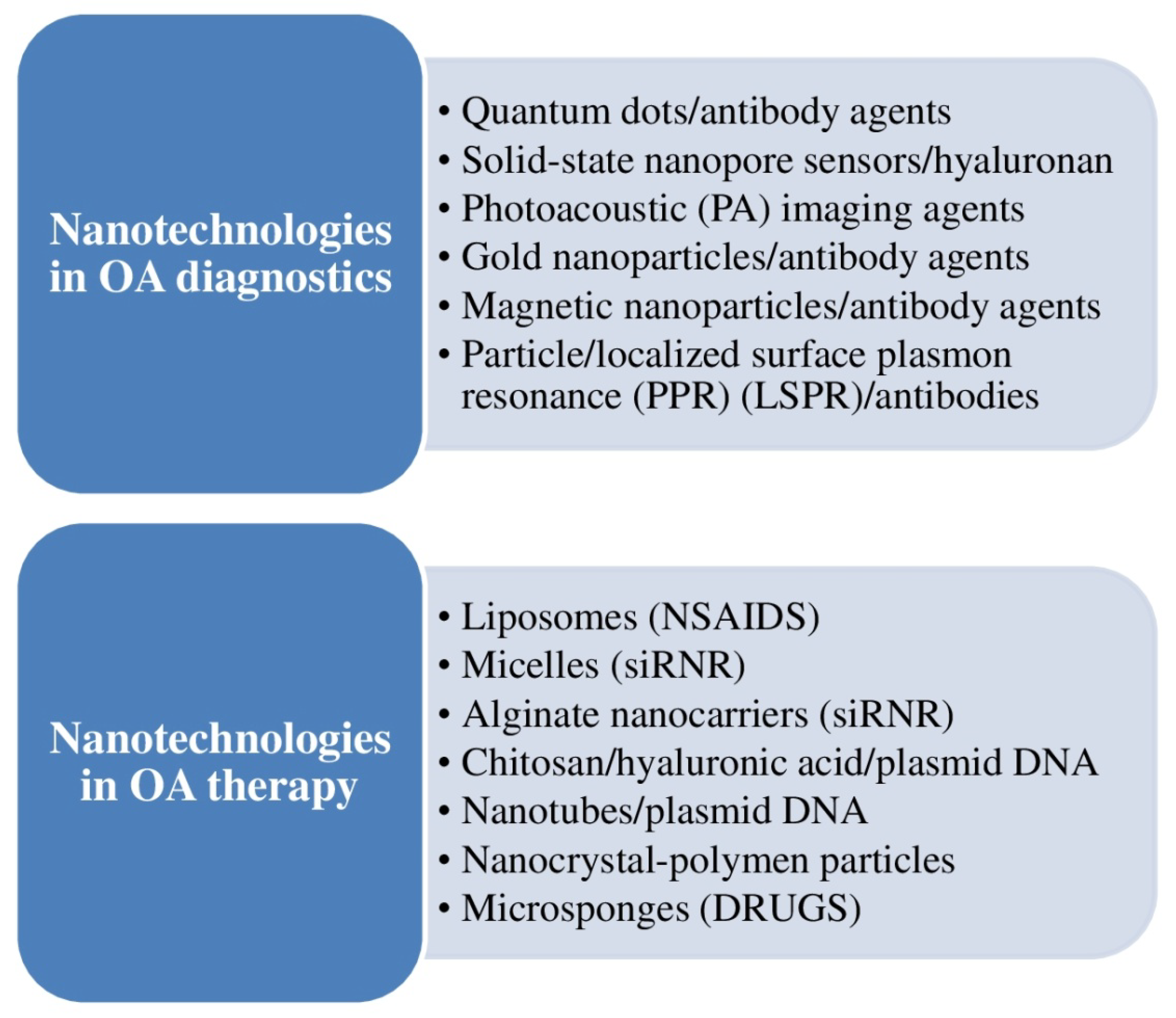
3.6. Nanotechnology for OA Clinical Management
3.7. Ezh2 Ameliorates Osteoarthritis (OA) by Triggering TNFSF13B
3.8. Hesperetin (HPT) Averts Cartilage Degradation
3.9. Intrinsic Restoration Reaction in Cartilage, Mediated by Aggrecan-Dependent Sodium Flux
3.10. Clock Knockdown Attenuated Reactive Oxygen Species-Mediated Senescence of Chondrocytes via Re-Establishing Autophagic Flux
3.11. HMGB2 Facilitates Chondrocyte Proliferation under Negative Pressure via the Phosphorylation of AKT
3.12. Regenerative Potential of OA Human Infrapatellar Fat Pad (IPFP)-Derived Progenitors Osteochondral Defects
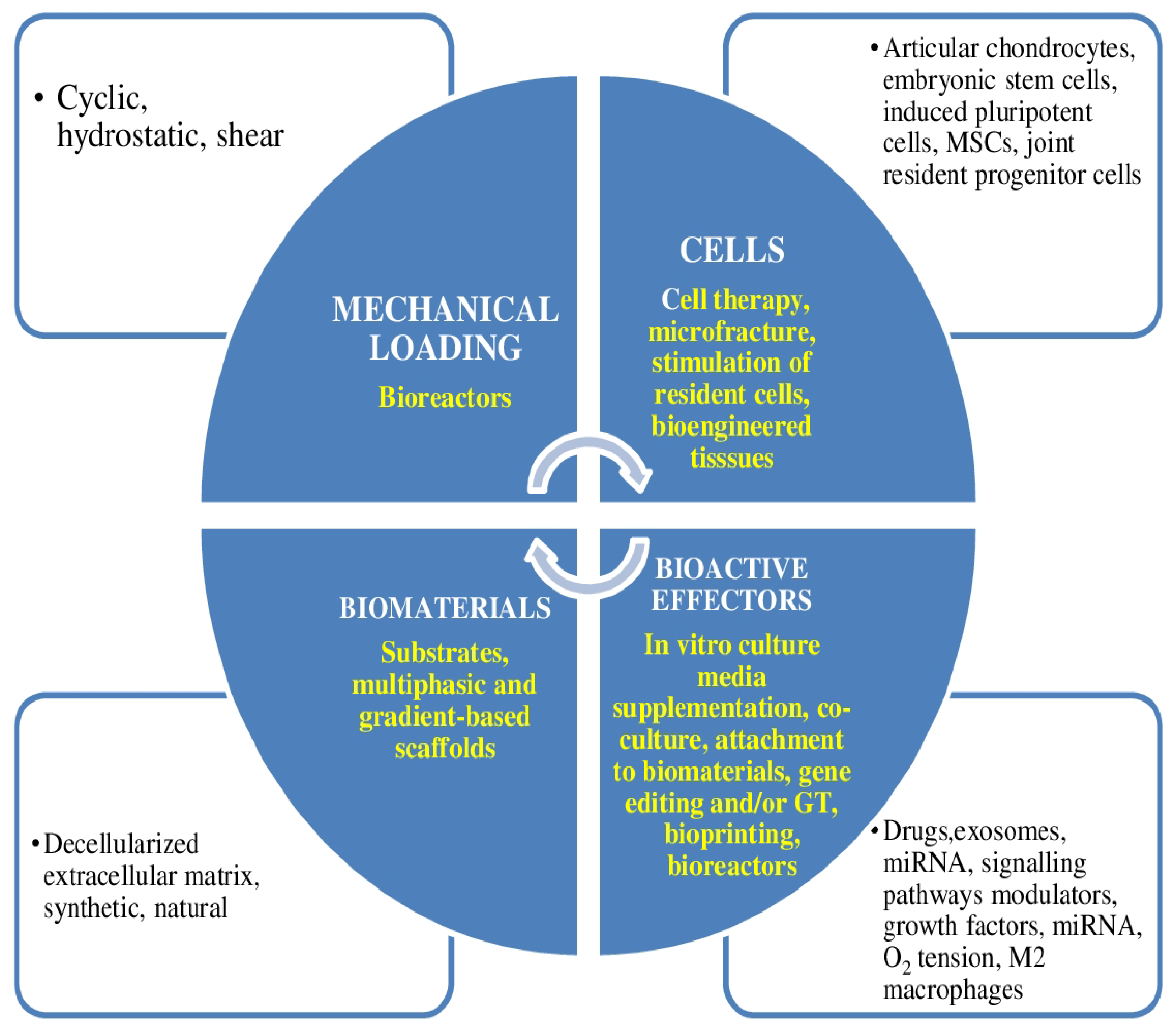
3.13. Function and Mechanism of Arginine–Glycine–Aspartic Acid (Arg–Gly–Asp, RGD) Peptide Family in Cartilage Tissue Engineering
3.14. 3D Bioprinting Gradient-Structured Constructs for Anisotropic Cartilage Regeneration
3.15. LncRNA-CRNDE Regulates BMSC Chondrogenic Differentiation to Promote Cartilage Repair in Osteoarthritis through SIRT1/SOX9
3.16. Manipulation of Histone Signals to Control Chondrocyte Roles or Manage Injuries of the Cartilaginous Tissue
3.17. The Function of Histone Deacetylase 4 (HDAC4)-Controlled Chondrocyte Hypertrophy in the Start and Appearance of Age-Related OA
3.18. The Impact of Osmolarity and FK506 (Tacrolimus, Fujimycin) on Calcineurin Action, Cell Expansion, Extracellular Matrix Quality, and BMP- and TGF-β (Transforming Growth Factor Beta) Signaling
3.19. Mechanism by which OA Is Started and Advances in the Cartilage Extracellular Matrix (ECM)
3.20. Proteoglycan 4 (Prg4)/Lubricin Functions in a Protective Manner against OA
3.21. Chondroinductive/Chondroconductive Peptides and Their Functionalized Biomaterials for Cartilage Tissue Engineering
3.22. Reversal of Tissue Degradation Detected with Joint Distraction
3.23. α2-Macroglobulin (α2M) Is Important to Chondral Protection in Post-traumatic OA
3.24. The Potential Role of Nano-Antioxidants
3.25. The Possible Role of Mitochondria Targeting in Tissue Repairing
3.26. The Use of Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells in Articular Cartilage Regeneration
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keppie, S.J.; Mansfield, J.C.; Tang, X.; Philp, C.J.; Graham, H.K.; Önnerfjord, P.; Wall, A.; McLean, C.; Winlove, C.P.; Sherratt, M.J.; et al. Matrix-bound growth factors are released upon cartilage compression by an aggrecan-dependent sodium flux that is lost in osteoarthritis. Function 2021, 2, zqab037. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, B.; Chen, Y.; Song, Y.; Ye, J.; Pan, Y.; Zhou, B.; Wang, Y.; Mao, F.; Dong, Y.; et al. Anti-friction MSCs delivery system improves the therapy for severe osteoarthritis. Adv. Mater. 2021, 33, e2104758. [Google Scholar] [CrossRef] [PubMed]
- Travascio, F.; Valladares-Prieto, S.; Jackson, A.R. Effects of solute size and tissue composition on molecular and macromolecular diffusivity in human knee cartilage. Osteoarthr. Cartil. Open 2020, 2, 100087. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, F.; Zhou, P.; Xie, M.; Wang, H.; Yang, H.; Huo, W.; Zhang, M.; Yu, S.; Wang, M. HMGB2 promotes chondrocyte proliferation under negative pressure through the phosphorylation of AKT. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119115. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, L.; Wang, L.; He, S.; Ren, H.; Zhou, N.; Hu, Z. The role of SIRT1 in BMP2-induced chondrogenic differentiation and cartilage maintenance under oxidative stress. Aging 2020, 12, 9000–9013. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, B.; Wu, B.; Shang, J.; Jiang, N.; Cui, A.; Chen, Y.; Xu, X.; Lu, H. Clock knockdown attenuated reactive oxygen species-mediated senescence of chondrocytes through restoring autophagic flux. Life Sci. 2021, 269, 119036. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, W.; Wang, J. lncRNA-CRNDE regulates BMSC chondrogenic differentiation and promotes cartilage repair in osteoarthritis through SIRT1/SOX9. Mol. Cell. Biochem. 2021, 476, 1881–1890. [Google Scholar] [CrossRef]
- Yan, B.; Lv, S.; Tong, P.; Yan, L.; Chen, Z.; Zhou, L.; Yuan, Q.; Guo, L.; Shan, L. Intra-articular injection of adipose-derived stem cells ameliorates pain and cartilage anabolism/catabolism in osteoarthritis: Preclinical and clinical evidences. Front. Pharmacol. 2022, 13, 854025. [Google Scholar] [CrossRef]
- Jaabar, I.L.; Cornette, P.; Miche, A.; Wanherdrick, K.; Dupres, V.; Ehkirch, F.P.; Cambon Binder, A.; Berenbaum, F.; Houard, X.; Landoulsi, J. Deciphering pathological remodelling of the human cartilage extracellular matrix in osteoarthritis at the supramolecular level. Nanoscale 2022, 14, 8691–8708. [Google Scholar] [CrossRef]
- Takahata, Y.; Hagino, H.; Kimura, A.; Urushizaki, M.; Yamamoto, S.; Wakamori, K.; Murakami, T.; Hata, K.; Nishimura, R. Regulatory mechanisms of Prg4 and Gdf5 expression in articular cartilage and functions in osteoarthritis. Int. J. Mol. Sci. 2022, 23, 4672. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Ashrafizadeh, M.; Pardakhty, A.; Uzieliene, I.; Denkovskij, J.; Bernotiene, E.; Janssen, L.; Lorite, G.S.; Saarakkala, S.; Mobasheri, A. Nanotechnological strategies for osteoarthritis diagnosis, monitoring, clinical management, and regenerative medicine: Recent advances and tuture opportunities. Curr. Rheumatol. Rep. 2020, 22, 12. [Google Scholar] [CrossRef]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioact. Mater. 2021, 9, 221–238. [Google Scholar] [CrossRef]
- Zelinka, A.; Roelofs, A.J.; Kandel, R.A.; De Bari, C. Cellular therapy and tissue engineering for cartilage repair. Osteoarthr. Cartil. 2022, 30, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L. Of mice and men: Converging on a common molecular understanding of osteoarthritis. Lancet Rheumatol. 2020, 2, e633–e645. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, A.T.; Zhang, Q.F.; Liu, R.M.; Xiao, J.H. RASL11B gene enhances hyaluronic acid-mediated chondrogenic differentiation in human amniotic mesenchymal stem cells via the activation of Sox9/ERK/smad signals. Exp. Biol. Med. 2020, 245, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Galla, R.; Ruga, S.; Aprile, S.; Ferrari, S.; Brovero, A.; Grosa, G.; Molinari, C.; Uberti, F. New hyaluronic acid from plant origin to improve joint protection-An in vitro atudy. Int. J. Mol. Sci. 2022, 23, 8114. [Google Scholar] [CrossRef]
- Salerno, A.; Brady, K.; Rikkers, M.; Li, C.; Caamaño-Gutierrez, E.; Falciani, F.; Blom, A.W.; Whitehouse, M.R.; Hollander, A.P. MMP13 and TIMP1 are functional markers for two different potential modes of action by mesenchymal stem/stromal cells when treating osteoarthritis. Stem Cells 2020, 38, 1438–1453. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, S.Z.; Sun, H.; Sun, L.; Zhou, D.; Yan, J. miR-199b-5p promoted chondrogenic differentiation of C3H10T1/2 cells by regulating JAG1. J. Tissue Eng. Regen. Med. 2020, 14, 1618–1629. [Google Scholar] [CrossRef]
- Casari, G.; Resca, E.; Giorgini, A.; Candini, O.; Petrachi, T.; Piccinno, M.S.; Foppiani, E.M.; Pacchioni, L.; Starnoni, M.; Pinelli, M.; et al. Microfragmented adipose tissue is associated with improved ex vivo performance linked to HOXB7 and b-FGF expression. Stem Cell Res. Ther. 2021, 12, 481. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, H.; Wang, Y.; Du, B.; Yang, J.; Liu, L.; Zhang, Q. Hyaluronic acid hydrogel with adjustable stiffness for mesenchymal stem cell 3D culture via related molecular mechanisms to maintain stemness and induce cartilage differentiation. ACS Appl. Bio Mater. 2021, 4, 2601–2613. [Google Scholar] [CrossRef]
- Norouzi-Barough, L.; Shirian, S.; Gorji, A.; Sadeghi, M. Therapeutic potential of mesenchymal stem cell-derived exosomes as a cell-free therapy approach for the treatment of skin, bone, and cartilage defects. Connect. Tissue Res. 2022, 63, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xuezhen, S.; Haipeng, L.; Jingbin, Z.; Bangtuo, Y.; Chunbao, L.; Wei, Q.; Yujie, L.; Mingzhu, Z. Effect of N-cadherin on chondrogenic differentiation of bone marrow-derived mesenchymal stem cells through Wnt signaling pathway. Cell Mol. Biol. 2022, 67, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Niibe, K.; Kondo, T.; Limraksasin, P.; Okawa, H.; Miao, X.; Kamano, Y.; Yamada, M.; Jiang, X.; Egusa, H. Rapid and efficient generation of cartilage pellets from mouse induced pluripotent stem cells by transcriptional activation of BMP-4 with shaking culture. J. Tissue Eng. 2022, 13, 20417314221114616. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.K.; Coutinho de Almeida, R.; Ramos, Y.F.M.; Li, J.; Meulenbelt, I.; Guilak, F. The miRNA-mRNA interactome of murine induced pluripotent stem cell-derived chondrocytes in response to inflammatory cytokines. FASEB J. 2020, 34, 11546–11561. [Google Scholar] [CrossRef]
- Li, M.; Fang, F.; Sun, M.; Zhang, Y.; Hu, M.; Zhang, J. Extracellular vesicles as bioactive nanotherapeutics: An emerging paradigm for regenerative medicine. Theranostics 2022, 12, 4879–4903. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, Y.; Zhang, Q.; Lin, J.; Yu, Y.; Pan, Z.; Sun, H.; Yuan, C.; Yu, D.; Wu, H.; et al. Ezh2 ameliorates osteoarthritis by activating TNFSF13B. J. Bone Miner. Res. 2020, 35, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qian, Y.; Chen, C.; Feng, F.; Pan, L.; Yang, L.; Wang, C. Hesperetin exhibits anti-inflammatory effects on chondrocytes via the AMPK pathway to attenuate anterior cruciate ligament transection-induced osteoarthritis. Front. Pharmacol. 2021, 12, 735087. [Google Scholar] [CrossRef]
- van Schaik, T.J.A.; Gaul, F.; Dorthé, E.W.; Lee, E.E.; Grogan, S.P.; D’Lima, D.D. Development of an ex vivo murine osteochondral repair model. Cartilage 2021, 12, 112–120. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.C.; Liu, Y.; Chen, Y.R.; Deng, R.H.; Zhang, Z.N.; Yu, J.K.; Yuan, F.Z. Function and mechanism of RGD in bone and cartilage tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 773636. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Zhang, Y.; Dai, K.; Wei, Y. 3D-bioprinted gradient-structured scaffold generates anisotropic cartilage with vascularization by pore-size-dependent activation of HIF1α/FAK signaling axis. Nanomedicine 2021, 37, 102426. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, F.; Yao, H.; Li, H.; Tuan, R.S. Histone modifications and chondrocyte fate: Regulation and therapeutic implications. Front. Cell Dev. Biol. 2021, 9, 626708. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ma, Z.; Yang, M.; Cong, L.; Zhao, R.; Cheng, L.; Sun, J.; Wang, Y.; Yang, R.; Wei, X.; et al. The level of histone deacetylase 4 is associated with aging cartilage degeneration and chondrocyte hypertrophy. J. Inflamm. Res. 2022, 15, 3547–3560. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; van der Windt, A.E.; Timur, U.T.; Baart, E.B.; Lian, W.S.; Rolauffs, B.; Wang, F.S.; Pufe, T. Physosmotic induction of chondrogenic maturation is TGF-β dependent and enhanced by calcineurin inhibitor FK506. Int. J. Mol. Sci. 2022, 23, 5110. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.P.; Mastbergen, S.C. Joint distraction for osteoarthritis: Clinical evidence and molecular mechanisms. Nat. Rev. Rheumatol. 2022, 18, 35–46. [Google Scholar] [CrossRef]
- Zhao, R.; Wei, X.; Zhang, C.; Wu, H.; Xiang, C.; Li, H.; Duan, W.; Duan, Z.; Li, C.; Zhao, Y.; et al. α2-macroglobulin-rich serum as a master inhibitor of inflammatory factors attenuates cartilage degeneration in a mini pig model of osteoarthritis induced by "idealized" anterior cruciate ligament reconstruction. Front. Pharmacol. 2022, 13, 849102. [Google Scholar] [CrossRef]
- Khezri, K.; Dizaj, S.M.; Saadat, Y.R.; Sharifi, S.; Shahi, S.; Ahmadian, E.; Eftekhari, A.; Abdolahinia, E.D.; Lotfipour, F. Osteogenic differentiation of mesenchymal stem cells via curcumin-containing nanoscaffolds. Stem Cells Int. 2021, 2021, 1520052. [Google Scholar] [CrossRef]
- Chodari, L.; Aytemir, M.D.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting mitochondrial biogenesis with polyphenol compounds. Oxid. Med. Cell Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Fahey, M.J.; Pugliese, B.R.; Irwin, R.M.; Antonyak, M.A.; Delco, M.L. Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front. Bioen. Biotechnol. 2022, 10, 870193. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, P.; Moghaddamshahabi, R.; Webster, T.J.; Koyuncu, A.C.C.; Ahmadian, E.; Khan, W.S.; Mohamed, A.J.; Eftekhari, A. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: A review. Int. J. Mol. Sci. 2021, 22, 9215. [Google Scholar] [CrossRef]

| Authors Reference | Year | Methods and Results | Conclusicons |
|---|---|---|---|
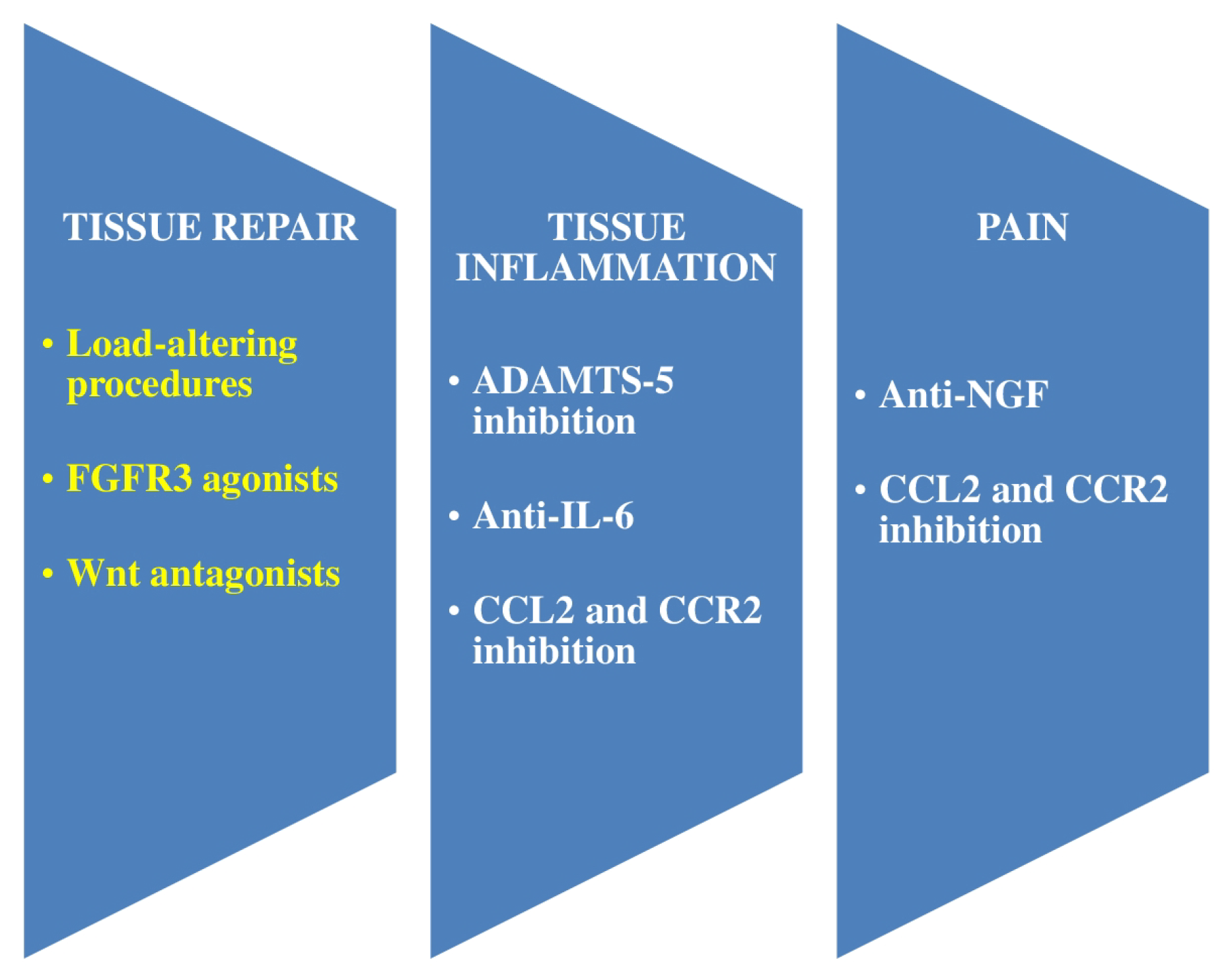
| Vincent [14] | 2020 | Genome-wide studies pointed to defects in repair pathways, which agreed well with contemporary potential utilizing growth factor treatment or Wnt pathway antagonism. | Nerve growth factor appeared as a good objective in OA pain in phase 2–3 trials. |
| Salerno et al. [17] | 2020 | In a try at developing functional markers of MSCs potency, Salerno et al. recognized loss of mRNA expression for MMP13 as correlating with loss of chondrogenic potential of MSCs and continued secretion of elevated levels of TIMP1 protein as correlating with the maintenance of trophic repair ability. | Early passage MMP13+, TIMP1-secretinghigh MSCs should be utilized for autologous OA treatment, while later passage MMP13−, TIMP1-secretinghigh MSCs could be used for allogeneic OA treatment. |
| Luo et al. [15] | 2020 | Microarray analysis demonstrated that RASL11B played an essential role in the process of hyaluronic acid-mediated chondrogenesis of hAMSCs. | Hyaluronic acid triggered the RASL11B gene to potentiate the chondrogenic differentiation of hAMSCs through the activation of Sox9 and ERK/Smad signaling. |
| Ross et al. [24] | 2020 | These authors carried out miRNA and mRNA sequencing to establish the temporal and dynamic reactions of genes to specific inflammatory cytokines as well as miRNAs that are differentially expressed (DE) in reaction to both cytokines or exclusively to IL-1β or TNF-α. | This study utilized an integrative approach to establish the miRNA interactome controlling the reaction to inflammatory cytokines. |
| Lu et al. [5] | 2020 | This study showed that SIRT1 could facilitate BMP2-induced chondrogenic differentiation of MSCs and diminish the apoptosis and decomposition of ECM under oxidative stress. | SIRT1 played an important coordination role in BMP2-induced chondrogenic differentiation of stem cells and cartilage maintenance under oxidative stress. |
| Zhang et al. [18] | 2020 | To facilitate comprehension of the molecular regulation of chondrogenesis differentiation in MSCs, these authors compared the modifications in miRNAs during in vitro chondrogenesis process of hBMSCs. MiR-199b-5p was upregulated substantially during this process. | miR-199b-5p was the positive regulator to modulate chondrogenic differentiation of C3H10T1/2 cells by aiming JAG1. |
| Mohammadinejad et al. [11] | This review article discussed the possible utilization of nanotechnological approaches for the diagnosis, follow-up, and treatment of OA and analyzed how nanotechnology was being integrated quickly into regenerative medicine for OA. | Nanotechnology platforms might be combined with cell, gene, and biological treatment for the discovery of a new generation of future OA drugs. | |
| Travascio et al. [3] | 2020 | This study analyzed the functions of solute size and tissue composition on molecular diffusion in knee AC. Diffusivity was influenced by molecular size, with the magnitude of the diffusion coefficients diminishing as the Stokes radius of the probe augmented. | This study rendered new data on the processes of diffusion in AC. The results of the study can be used to further examine OA and to design approaches for AC restoration or replacement. |
| Du et al. [26] | 2020 | These authors studied the expression of EZH2, an H3K27me3 transferase, in human OA cartilages and its functions in controlling OA pathogenesis. | Utilizing histological analysis and RNA sequencing (RNA-Seq), it was noticed that EZH2 was highly expressed in both mice and human OA cartilage. |
| Wu et al. [27] | 2021 | This study evaluated the potential chondroprotective effects of hesperetin (HPT) against the TNF-α-induced inflammatory reaction of chondrocytes and related processes, and elucidated the influence of HPT on OA induced by anterior cruciate ligament transection (ACLT). | HPT had significant protective and anti-inflammatory impact on chondrocytes via the AMPK signaling pathway, efficaciously averting cartilage degeneration. |
| Casari et al. [19] | These authors compared adipose tissue (AT) processed by centrifugation (C-AT) to microfragmentation (MF-AT). They evaluated the impact of synovial fluid (SF) alone on both MF-AT and isolated AT-MSC to better comprehend their cartilage repair processes. | MF had a positive impact on the maintenance of AT histology and might initiate the expression of trophic factors that ameliorate tissue repair by processed AT. | |
| Keppie et al. [1] | 2021 | This study described three pro-regenerative factors, fibroblast growth factor 2 (FGF2), connective tissue growth factor, bound to transforming growth factor-beta (CTGF-TGFβ), and hepatoma-derived growth factor (HDGF), that were quickly liberated from the pericellular matrix (PCM) of AC upon mechanical injury. | Loss of aggrecan in late-stage OA averted growth factor liberation and likely contributed to illness progression. |
| Zhong et al. [6] | 2021 | These authors explored the possible function and process of circadian gene clock in OA pathology. | Clock knockdown attenuated ROS-mediated senescence of chondrocytes via reestablishing autophagic flux in noncircadian mode. |
| Liu et al. [4] | 2021 | This experimental study aimed to determine the proliferative reactions of chondrocytes to negative pressure and explored the possible molecular processes. | HMGB2 facilitated chondrocyte proliferation under negative pressure via the phosphorylation of AKT. |
| van Schaik et al. [28] | 2021 | In this study, the authors used an ex vivo murine osteochondral repair model utilizing human infrapatellar fat pad (IPFP) progenitor cells. | These authors showed the regenerative potential of OA human IPFP-derived progenitors in mouse femurs. |
| Yang et al. [29] | 2021 | These authors reviewed the role of RGD stated Arginine–Glycine–Aspartic acid (Arg–Gly–Asp, RGD) peptide family in osseous and cartilaginous tissues engineering. | RGD peptide via integrin was introduced in the discipline of osseous and cartilaginous tissues engineering. |
| Sun et al. [30] | 2021 | In this study, Sun et al. constructed an anisotropic gradient-structured cartilage scaffold by three-dimensional (3D) bioprinting, in which bone marrow stromal cell (BMSC)-laden anisotropic hydrogel micropatterns were utilized for heterogeneous chondrogenic differentiation and physically gradient synthetic PCL to achieve mechanical strength. | The results of this study rendered a theoretical basis for using 3D bioprinting gradient-structured constructs for anisotropic cartilage regeneration. |
| Shi et al. [7] | 2021 | This study explored the function of long noncoding RNA (lncRNA)-colorectal neoplasia differentially expressed gene (CRNDE) in the chondrogenic differentiation of BMSCs and the intrinsic molecular process, seeking to create a new therapeutic approach for OA. | Overexpression of lncRNA-CRNDE augmented the binding capacity of SOX9 and col2α1 promoter, which was reversed by the concomitant transfection of CRNDE overexpression (pcDNA-CRNDE) and SIRT1 small interfering RNA (si-SIRT1). |
| Wan et al. [31] | 2021 | These authors stated that comprehending the molecular processes and impact of histone modification enzymes in cartilage development, homeostasis, and pathology will render essential and precise prospects to understand the biological behavior of chondrocytes during skeletal development and the pathogenesis of several cartilage-related illnesses. | These authors emphasized the importance of using small molecules and drugs to manipulate histone signals to treat cartilage lesions and OA. |
| Ren et al. [20] | 2021 | In this study, Ren et al. stated that the stemness and differentiation characteristics of BMSCs in 3D culture were of great importance for stem cell treatment and cartilage tissue engineering repair. Furthermore, because of their mechanical sensitivity, scaffold materials played relevant functions in various cell behaviors in 3D culture. | This research could be valuable for designing biomaterials for BMSCs’ delivery in vivo, as well as for developing cartilage repair drug delivery programs based on molecular processes. |
| Yan et al. [2] | 2021 | This study showed that the DNA supramolecular hydrogel can improve formation of quality cartilage, diminish bone spurs, and normalize subchondral osseous tissue in OA. | DNA supramolecular hydrogel could be a good cell delivery system for MSCs treatment. |
| Zhu et al. [12] | 2021 | These authors summarized the contemporary data in the designs of the chondroinductive/chondroconductive peptides, their intrinsic molecular processes, and their functionalized biomaterials for cartilage tissue engineering. | Peptides constitute a remarkable group of bioactive drugs for chondrogenic functionalization. |
| Khezari et al. [36] | 2021 | This article has reviewed the pharmacological effects of curcumin nanoformulations. | Nanocurcumin may benefit the osteogenic differentiation of MSCs in the scaffolds. |
| Chodari et al. [37] | 2021 | These authors reviewed effects of different natural polyphenol compounds from various plant kingdoms on modulating signaling pathways of mitochondrial biogenesis. | The use of polyphenol compounds is a promising alternative for the treatment of OA. |
| Vehadi et al. [39] | 2021 | These authors stated that the infrapatellar fat pad IPFP is a rich source of MSCs. | IPFP-derived MSCs are promising in the treatment of articular cartilage damage |
| Thomas et al. [38] | 2022 | This study suggested that intercellular transfer of healthy mitochondria to chondrocytes could represent a new, acellular approach to increase mitochondrial content and function in cartilage. | The intercellular transfer of healthy mitochondria to chondrocytes is a promising alternative for the treatment of OA. |
| Yan et al. [8] | 2022 | This study assessed the anti-OA effectiveness of adipose-derived stem cells (ADSCs) and explored the intrinsic mechanism of action. | The anti-OA efficacy, safety, and a paracrine-based mechanism of ADSCs was shown. |
| Zhao et al. [35] | 2022 | In this study, Zhao et al. assessed the protective role of of alpha2MRS against post-surgery cartilage degradation. | This study highlighted the chondroprotective impact of alpha2MRS and rendered a basis for the clinical translation of alpha2MRS. |
| Norouzi-Barough et al. [21] | 2022 | MSC-derived exosomes can imitate the biological activity of MSCs by horizontal transfer of many molecules including mRNAs, miRNAs, proteins, and lipids to the local microenvironment and recipient cells. | Exosomes could be deemed as an alternative approach for cell-based treatments in regenerative medicine. |
| Dong et al. [32] | 2022 | In transgenic and control mice, these authors tried to define the function of histone deacetylase 4 (HDAC4)-controlled chondrocyte hypertrophy in the start and appearance of age-related OA. | HDAC4 expression controlled the start and appearance of age-related OA by regulating chondrocyte hypertrophy. |
| Jahr et al. [33] | 2022 | This investigation aimed at clarifying the function of osmolarity in chondrogenic differentiation. | Physiological osmolarity improved terminal chondrogenic differentiation of progenitor cells. |
| Feng et al. [22] | 2022 | This study compared and analyzed the impact of N-cadherin on chondrogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) and explored the related mechanism. | N-cadherin can facilitate chondrogenic differentiation of BMSCs by restraining the Wnt signaling pathway. |
| Zhang et al. [23] | 2022 | This study described a tetracycline (tet)-controlled bone morphogenetic protein-4 (BMP-4) expressing the iPSC (iPSC-Tet/BMP-4) line in which transcriptional activation of BMP-4 was associated with improved chondrogenesis. | The culture system employed could be a valuable instrument for further research of the mechanism of BMP-4 in controlling iPSC differentiation toward the chondrogenic lineage. |
| Galla et al. [16] | 2022 | In this study, Galla et al. confirmed the effectiveness of a new high molecular weight hyaluronic acid of plant origin (called GreenIuronic®) in preserving the homeostasis of the joints and averting the detrimental mechanisms of OA. | The new form of HA reported in this study appeared to be well-absorbed and distributed to chondrocytes. |
| Li et al. [25] | 2022 | These authors provided a systematic review of the latest progress of extracellular vesicles (EVs) for regenerative applications. | The bioactive EV-based nanotherapeutics have opened new horizons for clinicians, making feasible strong tools and treatment for regenerative medicine. |
| Jaabar et al. [9] | 2022 | In this study, Jaabar et al. used X-ray photoelectron spectroscopy (XPS), and created a new method that rendered the molecular composition of the ECM. | Aiming the homeostatic equilibrium of chondrocyte metabolism via the control of enzymatic reactions implicated in catabolic processes could be a new therapeutic approach against OA. |
| Takahata et al. [10] | 2022 | This review article showed that proteoglycan 4 (Prg4)/lubricin protects against OA. | Molecular targeting of Prg4 and GDF5 could be a potential treatment of OA. |
| Zelinka et al. [13] | 2022 | These authors stated that cell-based techniques have entered clinical practice in orthopedic surgery and that some tissue engineering strategies to repair AC are about to be clinically employed. | Comprehending the intrinsic mechanisms of action of cell therapy and tissue engineering techniques will be helpful in the future. |
| Jansen et al. [34] | 2022 | Several mechanisms are implicated in the effect of joint distraction. | Joint distraction is a joint-preserving management for end-stage OA. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Merchán, E.C. Molecular Mechanisms of Cartilage Repair and Their Possible Clinical Uses: A Review of Recent Developments. Int. J. Mol. Sci. 2022, 23, 14272. https://doi.org/10.3390/ijms232214272
Rodríguez-Merchán EC. Molecular Mechanisms of Cartilage Repair and Their Possible Clinical Uses: A Review of Recent Developments. International Journal of Molecular Sciences. 2022; 23(22):14272. https://doi.org/10.3390/ijms232214272
Chicago/Turabian StyleRodríguez-Merchán, Emérito Carlos. 2022. "Molecular Mechanisms of Cartilage Repair and Their Possible Clinical Uses: A Review of Recent Developments" International Journal of Molecular Sciences 23, no. 22: 14272. https://doi.org/10.3390/ijms232214272
APA StyleRodríguez-Merchán, E. C. (2022). Molecular Mechanisms of Cartilage Repair and Their Possible Clinical Uses: A Review of Recent Developments. International Journal of Molecular Sciences, 23(22), 14272. https://doi.org/10.3390/ijms232214272






