iPLA2β-Null Mice Show HCC Protection by an Induction of Cell-Cycle Arrest after Diethylnitrosamine Treatment
Abstract
1. Introduction
2. Results and Discussion
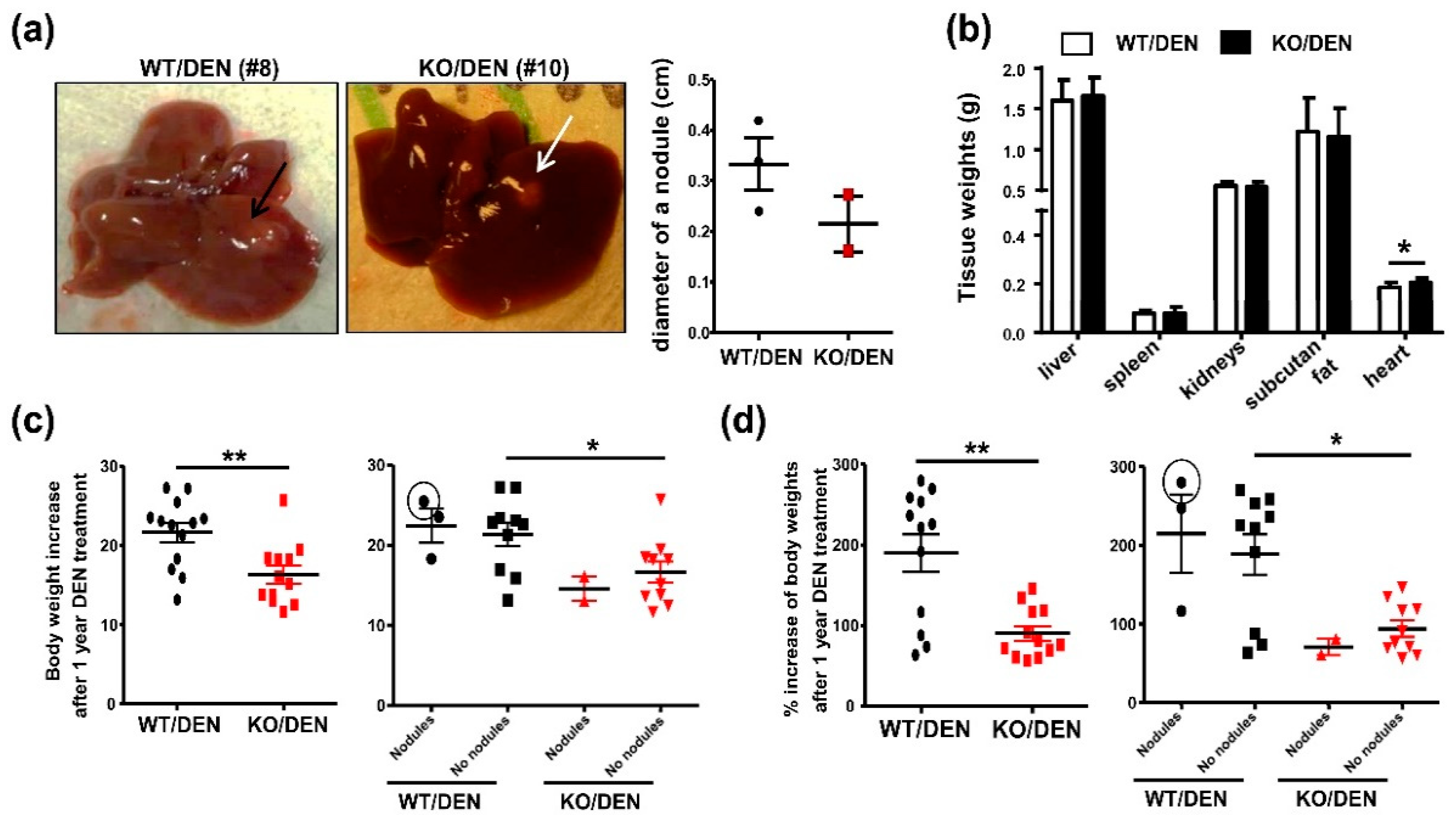
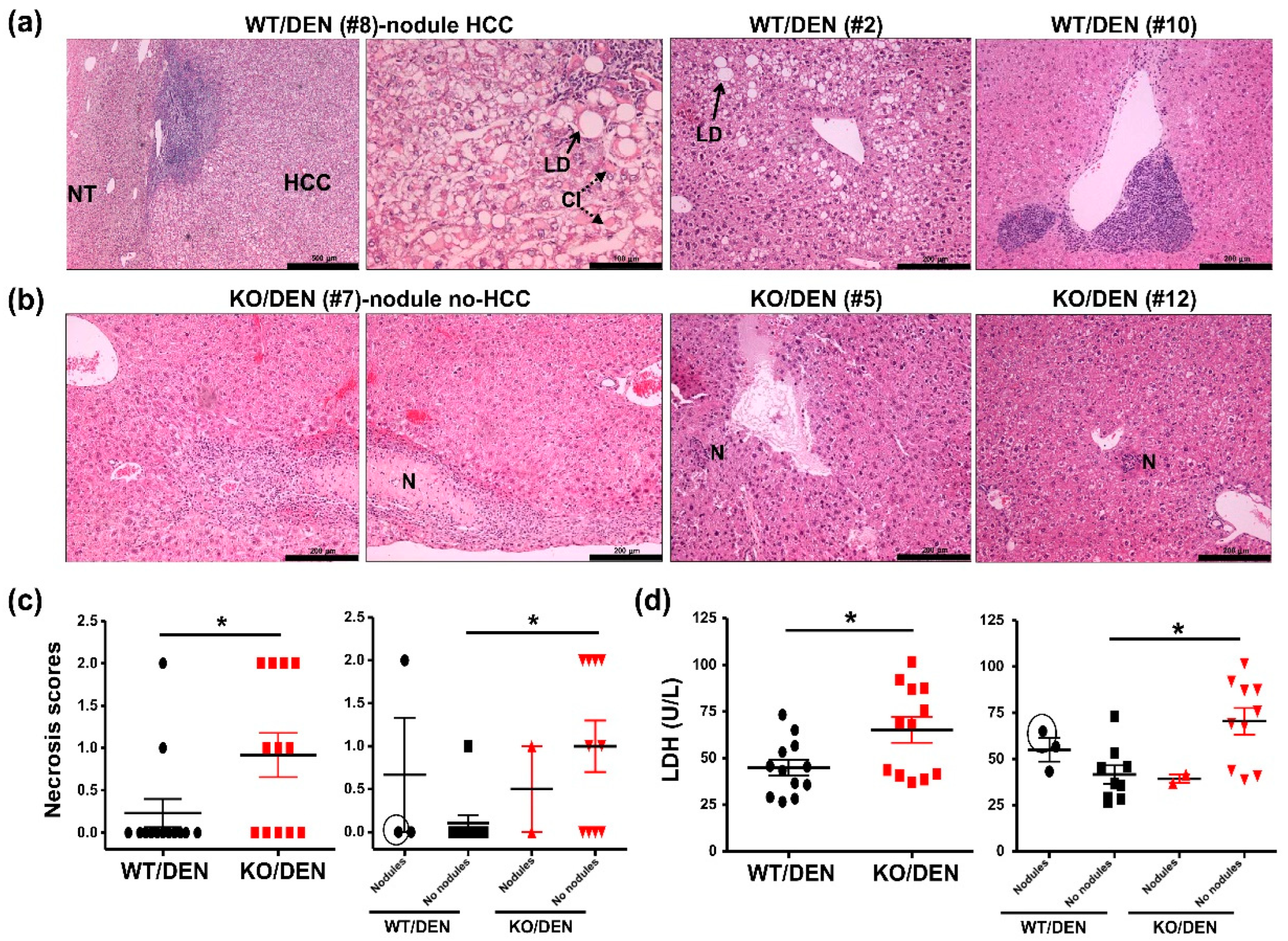
2.1. iPLA2β-Null Mice Show Reduced Body-Weight Gains, No HCC Detectable, and Enhanced Hepatic Necrosis after DEN Treatment
2.2. DEN-Treated iPLA2β-Null Mice Show Attenuated Cell-Cycle and Inflammatory Markers
3. Materials and Methods
3.1. Animals and Treatment
3.2. Biochemical Assays
3.3. Histology
3.4. ELISA
3.5. Gene Expression
3.6. Statistics
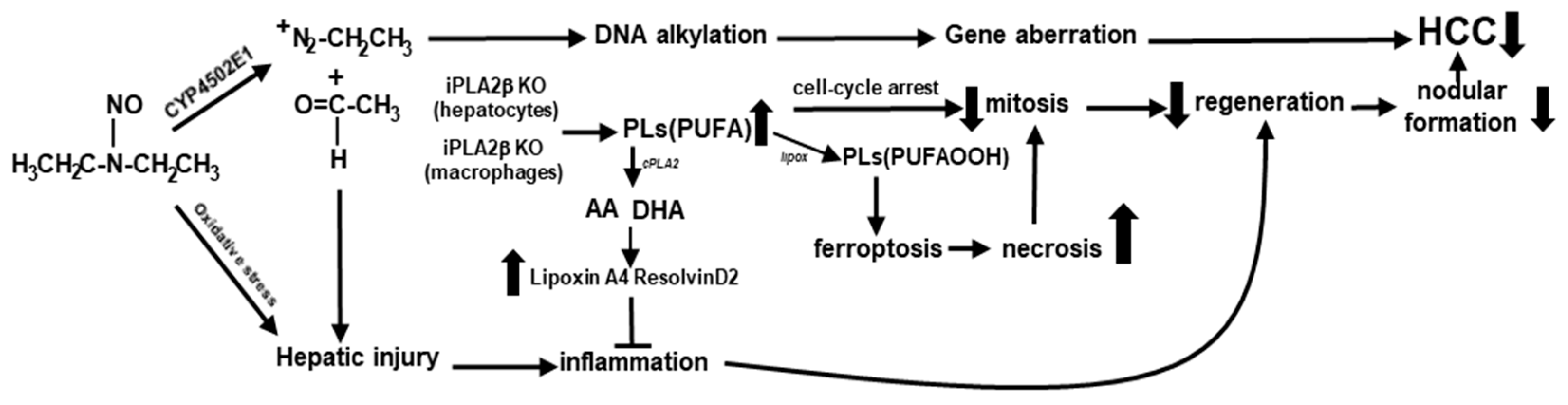
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Karin, M. Inflammation and liver tumorigenesis. Front. Med. 2013, 7, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Cotte, A.K.; Vanessa, C.; Aires, V.; Mouillot, T.; Rizk, M.; Vinault, S.; Binquet, C.; Pais de Barros, J.-P.; Hillon, P.; Delmas, D. Phospholipid profiles and hepatocellular carcinoma risk and prognosis in cirrhotic patients. Oncotarget 2019, 10, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-F.; Zhang, K.-L.; Zhang, X.-J.; Hu, Y.-J.; Li, P.; Shang, C.-Z.; Wan, J.-B. Abnormalities in plasma phospholipid fatty acid profiles of patients with hepatocellular carcinoma. Lipids 2015, 50, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, E.B.; Kwee, S.A.; Sato, M.M.; Wang, L.; Rettenmeier, C.; Xie, G.; Jia, W.; Wong, L.L. Phospholipids are a potentially important source of tissue biomarkers for hepatocellular carcinoma: Results of a pilot study involving targeted metabolomics. Diagnostics 2019, 9, 167. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Mei, H.; Mao, J.; Zhou, X. Distribution and clinical relevance of phospholipids in hepatocellular carcinoma. Hepatol. Int. 2020, 14, 544–555. [Google Scholar] [CrossRef]
- Krautbauer, S.; Meier, E.M.; Rein-Fischboeck, L.; Pohl, R.; Weiss, T.S.; Sigruener, A.; Aslanidis, C.; Liebisch, G.; Buechler, C. Ceramide and polyunsaturated phospholipids are strongly reduced in human hepatocellular carcinoma. Biochim. Biophys. Acta 2016, 1861, 1767–1774. [Google Scholar] [CrossRef]
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid. Res. 2011, 50, 240–257. [Google Scholar] [CrossRef]
- Holthuis, J.C.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef]
- Teng, Y.-W.; Mehedint, M.G.; Garrow, T.A.; Zeisel, S.H. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J. Biol. Chem. 2011, 286, 36258–36267. [Google Scholar] [CrossRef]
- Morita, Y.; Sakaguchi, T.; Ikegami, K.; Goto-Inoue, N.; Hayasaka, T.; Hang, V.T.; Tanaka, H.; Harada, T.; Shibasaki, Y.; Suzuki, A.; et al. Lysophosphatidylcholine acyltransferase 1 altered phospholipid composition and regulated hepatoma progression. J. Hepatol. 2013, 59, 292–299. [Google Scholar] [CrossRef]
- Turk, J.; White, T.D.; Nelson, A.J.; Lei, X.; Ramanadham, S. iPLA2β and its role in male fertility, neurological disorders, metabolic disorders, and inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019, 1864, 846–860. [Google Scholar] [CrossRef]
- Hooks, S.B.; Cummings, B.S. Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem. Pharmacol. 2008, 76, 1059–1067. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Liu, W.-X.; Liu, C.; Cui, J.; Li, Q.; Ni, H.; Yang, Y.; Wu, C.; Chen, C.; et al. Dysfunction of PLA2G6 and CYP2C44-associated network signals imminent carcinogenesis from chronic inflammation to hepatocellular carcinoma. J. Mol. Cell Biol. 2017, 9, 489–503. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Wei, G.; Yan, L.; Wang, D.; Zhang, H.; Sandusky, G.E.; Turk, J.; Xu, Y. Group VIA phospholipase A2 in both host and tumor cells is involved in ovarian cancer development. FASEB J. 2010, 24, 4103–4116. [Google Scholar] [CrossRef]
- Inhoffen, J.; Tuma-Kellner, S.; Straub, B.; Stremmel, W.; Chamulitrat, W. Deficiency of iPLA₂β primes immune cells for proinflammation: Potential involvement in age-related mesenteric lymph node lymphoma. Cancers 2015, 7, 2427–2442. [Google Scholar] [CrossRef]
- Zhu, X.; Gan-Schreier, H.; Otto, A.C.; Cheng, Y.; Staffer, S.; Tuma-Kellner, S.; Ganzha, A.; Liebisch, G.; Chamulitrat, W. iPla2β deficiency in mice fed with MCD diet does not correct the defect of phospholipid remodeling but attenuates hepatocellular injury via an inhibition of lipid uptake genes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 677–687. [Google Scholar] [CrossRef]
- George, J.; Tsuchishima, M.; Tsutsumi, M. Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis. Cell Death Dis. 2019, 10, 18–27. [Google Scholar] [CrossRef]
- Vesselinovitch, S.D.; Mihailovich, N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983, 43, 4253–4259. [Google Scholar]
- Tolba, R.; Kraus, T.; Liedtke, C.; Schwarz, M.; Weiskirchen, R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 2015, 49 (Suppl. 1), 59–69. [Google Scholar] [CrossRef]
- Deng, X.; Wang, J.; Jiao, L.; Utaipan, T.; Tuma-Kellner, S.; Schmitz, G.; Liebisch, G.; Stremmel, W.; Chamulitrat, W. iPLA2β deficiency attenuates obesity and hepatic steatosis in ob/ob mice through hepatic fatty-acyl phospholipid remodeling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Gurses, S.A.; Banskar, S.; Stewart, C.; Trimoski, B.; Dziarski, R.; Gupta, D. Nod2 protects mice from inflammation and obesity-dependent liver cancer. Sci. Rep. 2020, 10, 20519–20536. [Google Scholar] [CrossRef] [PubMed]
- Thoolen, B.; Maronpot, R.R.; Harada, T.; Nyska, A.; Rousseaux, C.; Nolte, T.; Malarkey, D.E.; Kaufmann, W.; Küttler, K.; Deschl, U.; et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010, 38 (Suppl. 7), 5S–81S. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, C.; Feng, R.; Bi, S. The TNF-α, IL-1B and IL-10 polymorphisms and risk for hepatocellular carcinoma: A meta-analysis. J. Cancer Res. Clin. Oncol. 2011, 137, 947–952. [Google Scholar] [CrossRef]
- Dinant, S.; Veteläinen, R.L.; Florquin, S.; van Vliet, A.K.; van Gulik, T.M. IL-10 attenuates hepatic I/R injury and promotes hepatocyte proliferation. J. Surg. Res. 2007, 141, 176–182. [Google Scholar] [CrossRef]
- Wadkin, J.C.R.; Patten, D.A.; Kamarajah, S.K.; Shepherd, E.L.; Novitskaya, V.; Berditchevski, F.; Adams, D.H.; Weston, C.J.; Shetty, S. CD151 supports VCAM-1-mediated lymphocyte adhesion to liver endothelium and is upregulated in chronic liver disease and hepatocellular carcinoma. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G138–G149. [Google Scholar] [CrossRef]
- Afford, S.C.; Humphreys, E.H.; Reid, D.T.; Russell, C.L.; Banz, V.M.; Oo, Y.; Vo, T.; Jenne, C.; Adams, D.H.; Eksteen, B. Vascular cell adhesion molecule 1 expression by biliary epithelium promotes persistence of inflammation by inhibiting effector T-cell apoptosis. Hepatology 2014, 59, 1932–1943. [Google Scholar] [CrossRef]
- EI-Emshaty, H.M.; Saad, E.A.; Toson, E.A.; Malak, C.A.; Gadelhak, N.A. Apoptosis and cell proliferation: Correlation with BCL-2 and P53 oncoprotein expression in human hepatocellular carcinoma. Hepatogastroenterology 2014, 61, 1393–1401. [Google Scholar]
- Zhao, N.; Sun, B.-C.; Zhao, X.-l.; Liu, Z.-Y.; Sun, T.; Qiu, Z.-Q.; Gu, Q.; Che, N.; Dong, X.-Y. Coexpression of Bcl-2 with epithelial-mesenchymal transition regulators is a prognostic indicator in hepatocellular carcinoma. Med. Oncol. 2012, 29, 2780–2792. [Google Scholar] [CrossRef]
- Wang, G.X.; Tu, H.-C.; Dong, Y.; Skanderup, A.J.; Wang, Y.; Takeda, S.; Ganesan, Y.T.; Han, S.; Liu, H.; Hsieh, J.J.; et al. ΔNp63 inhibits oxidative stress-induced cell death, including ferroptosis, and cooperates with the BCL-2 family to promote clonogenic survival. Cell Rep. 2017, 21, 2926–2939. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-Y.; Tyurin, V.A.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Anthonymuthu, T.S.; Zhai, Y.-J.; Pan, M.-H.; Gong, H.-B.; Lu, D.-H.; Sun, J.; et al. Phospholipase iPLA2β averts ferroptosis by eliminating a redox lipid death signal. Nat. Chem. Biol. 2021, 17, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Louandre, C.; Ezzoukhry, Z.; Godin, C.; Barbare, J.-C.; Mazière, J.-C.; Chauffert, B.; Galmiche, A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer 2013, 133, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Kato, Y.; van Diest, P.J.; Masuda, M.; Mitomi, H.; Okayasu, I. Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases. Am. J. Pathol. 2000, 156, 585–594. [Google Scholar] [CrossRef]
- Lee, V.M.; Cameron, R.G.; Archer, M.C. Zonal location of compensatory hepatocyte proliferation following chemically induced hepatotoxicity in rats and humans. Toxicol. Pathol. 1998, 26, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhao, C.; Seleznev, K.; Song, K.; Manfredi, J.J.; Ma, Z.A. Disruption of G1-phase phospholipid turnover by inhibition of Ca2+-independent phospholipase A2 induces a p53-dependent cell-cycle arrest in G1 phase. J. Cell Sci. 2006, 119 Pt 6, 1005–1015. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhao, C.; Ma, Z.A. The increase of cell-membranous phosphatidylcholines containing polyunsaturated fatty acid residues induces phosphorylation of p53 through activation of ATR. J. Cell Sci. 2007, 120 Pt 23, 4134–4143. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Cao, W.; Li, M.; Liu, J.; Zhang, S.; Noordam, L.; Verstegen, M.M.A.; Wang, L.; Ma, B.; Li, S.; Wang, W.; et al. LGR5 marks targetable tumor-initiating cells in mouse liver cancer. Nat. Commun. 2020, 11, 1961. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 772–783. [Google Scholar] [CrossRef]
- Murakami, M.; Kambe, T.; Shimbara, S.; Kudo, I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999, 274, 3103–3115. [Google Scholar] [CrossRef]
- Al-Kharusi, M.R.A.; Smartt, H.J.M.; Greenhough, A.; Collard, T.J.; Emery, E.D.; Williams, A.C.; Paraskeva, C. LGR5 promotes survival in human colorectal adenoma cells and is upregulated by PGE2: Implications for targeting adenoma stem cells with NSAIDs. Carcinogenesis 2013, 34, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Han, C.; Dai, Y.; Shen, M.; Wu, T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol. Cancer Ther. 2009, 8, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Wang, L.; Yao, B.; Chen, T.; Li, Q.; Liu, Z.; Liu, R.; Niu, Y.; Song, T.; et al. Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J. Exp. Clin. Cancer Res. 2019, 38, 170. [Google Scholar] [CrossRef]
- Nelson, A.J.; Stephenson, D.J.; Cardona, C.L.; Lei, X.; Almutairi, A.; White, T.D.; Tusing, Y.G.; Park, M.A.; Barbour, S.E.; Chalfant, C.E.; et al. Macrophage polarization is linked to Ca2+-independent phospholipase A2β-derived lipids and cross-cell signaling in mice. J. Lipid Res. 2020, 61, 143–158. [Google Scholar] [CrossRef]
- Jansakun, C.; Chunglok, W.; Altamura, S.; Muckenthaler, M.; Staffer, S.; Tuma-Kellner, S.; Merle, U.; Chamulitrat, W. Myeloid- and hepatocyte-specific deletion of group VIA calcium-independent phospholipase A2 leads to dichotomous opposing phenotypes during MCD diet-induced NASH. BBA Mol. Basis Dis. 2022, in press. [Google Scholar] [CrossRef]
- Hao, H.; Liu, M.; Wu, P.; Cai, L.; Tang, K.; Yi, P.; Li, Y.; Chen, Y.; Ye, D. Lipoxin A4 and its analog suppress hepatocellular carcinoma via remodeling tumor microenvironment. Cancer Lett. 2011, 309, 85–94. [Google Scholar] [CrossRef]
- Klement, L.J.; Jansakun, C.; Tuma-Kellner, S.; Staffer, S.; Altamura, S.; Muckenthaler, M.; Merle, U.; Chamulitrat, W. Macrophage-specific PLA2g6 deficiency exacerbates liver injury during bacterial sepsis via myelopoiesis in male mice. In Proceedings of the German Association of the Study of the Liver, Mannheim, Germany, 28–29 January 2022. [Google Scholar]
- Mrad, M.; Imbert, C.; Garcia, V.; Rambow, F.; Therville, N.; Carpentier, S.; Ségui, B.; Levade, T.; Azar, R.; Marine, J.-C.; et al. Downregulation of sphingosine kinase-1 induces protective tumor immunity by promoting M1 macrophage response in melanoma. Oncotarget 2016, 7, 71873–71886. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Nakamoto, Y.; Sakai, Y.; Mukaida, N.; Kaneko, S. Optimal amount of monocyte chemoattractant protein-1 enhances antitumor effects of suicide gene therapy against hepatocellular carcinoma by M1 macrophage activation. Cancer Sci. 2008, 99, 2075–2082. [Google Scholar] [CrossRef]
- Yuan, A.; Hsiao, Y.-J.; Chen, H.-Y.; Chen, H.-W.; Ho, C.-C.; Chen, Y.-Y.; Liu, Y.-C.; Hong, T.-H.; Yu, S.-L.; Chen, J.J.W.; et al. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci. Rep. 2015, 5, 14273–14285. [Google Scholar] [CrossRef]
- Calderon, L.E.; Liu, S.; Arnold, N.; Breakall, B.; Rollins, J.; Ndinguri, M. Bromoenol lactone attenuates nicotine-induced breast cancer cell proliferation and migration. PLoS ONE 2015, 10, e0143277. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, X.; Yonz, C.; Cummings, B.S. Inhibition of calcium-independent phospholipase A2 activates p38 MAPK signaling pathways during cytostasis in prostate cancer cells. Biochem. Pharmacol. 2010, 79, 1727–1735. [Google Scholar] [CrossRef]
- Liou, J.-Y.; Aleksic, N.; Chen, S.-F.; Han, T.-J.; Shyue, S.-K.; Wu, K.K. Mitochondrial localization of cyclooxygenase-2 and calcium-independent phospholipase A2 in human cancer cells: Implication in apoptosis resistance. Exp. Cell Res. 2005, 306, 75–84. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Antalis, C.; Zhao, Z.; Emerson, R.; Wei, G.; Zhang, S.; Zhang, Z.-Y.; Xu, Y. Combination therapy of an inhibitor of group VIA phospholipase A2 with paclitaxel is highly effective in blocking ovarian cancer development. Am. J. Pathol. 2011, 179, 452–461. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, A.; Poth, T.; Brobeil, A.; Merle, U.; Chamulitrat, W. iPLA2β-Null Mice Show HCC Protection by an Induction of Cell-Cycle Arrest after Diethylnitrosamine Treatment. Int. J. Mol. Sci. 2022, 23, 13760. https://doi.org/10.3390/ijms232213760
Andrade A, Poth T, Brobeil A, Merle U, Chamulitrat W. iPLA2β-Null Mice Show HCC Protection by an Induction of Cell-Cycle Arrest after Diethylnitrosamine Treatment. International Journal of Molecular Sciences. 2022; 23(22):13760. https://doi.org/10.3390/ijms232213760
Chicago/Turabian StyleAndrade, Adriana, Tanja Poth, Alexander Brobeil, Uta Merle, and Walee Chamulitrat. 2022. "iPLA2β-Null Mice Show HCC Protection by an Induction of Cell-Cycle Arrest after Diethylnitrosamine Treatment" International Journal of Molecular Sciences 23, no. 22: 13760. https://doi.org/10.3390/ijms232213760
APA StyleAndrade, A., Poth, T., Brobeil, A., Merle, U., & Chamulitrat, W. (2022). iPLA2β-Null Mice Show HCC Protection by an Induction of Cell-Cycle Arrest after Diethylnitrosamine Treatment. International Journal of Molecular Sciences, 23(22), 13760. https://doi.org/10.3390/ijms232213760






