Involvement of the p38 MAPK-NLRC4-Caspase-1 Pathway in Ionizing Radiation-Enhanced Macrophage IL-1β Production
Abstract
1. Introduction
2. Results
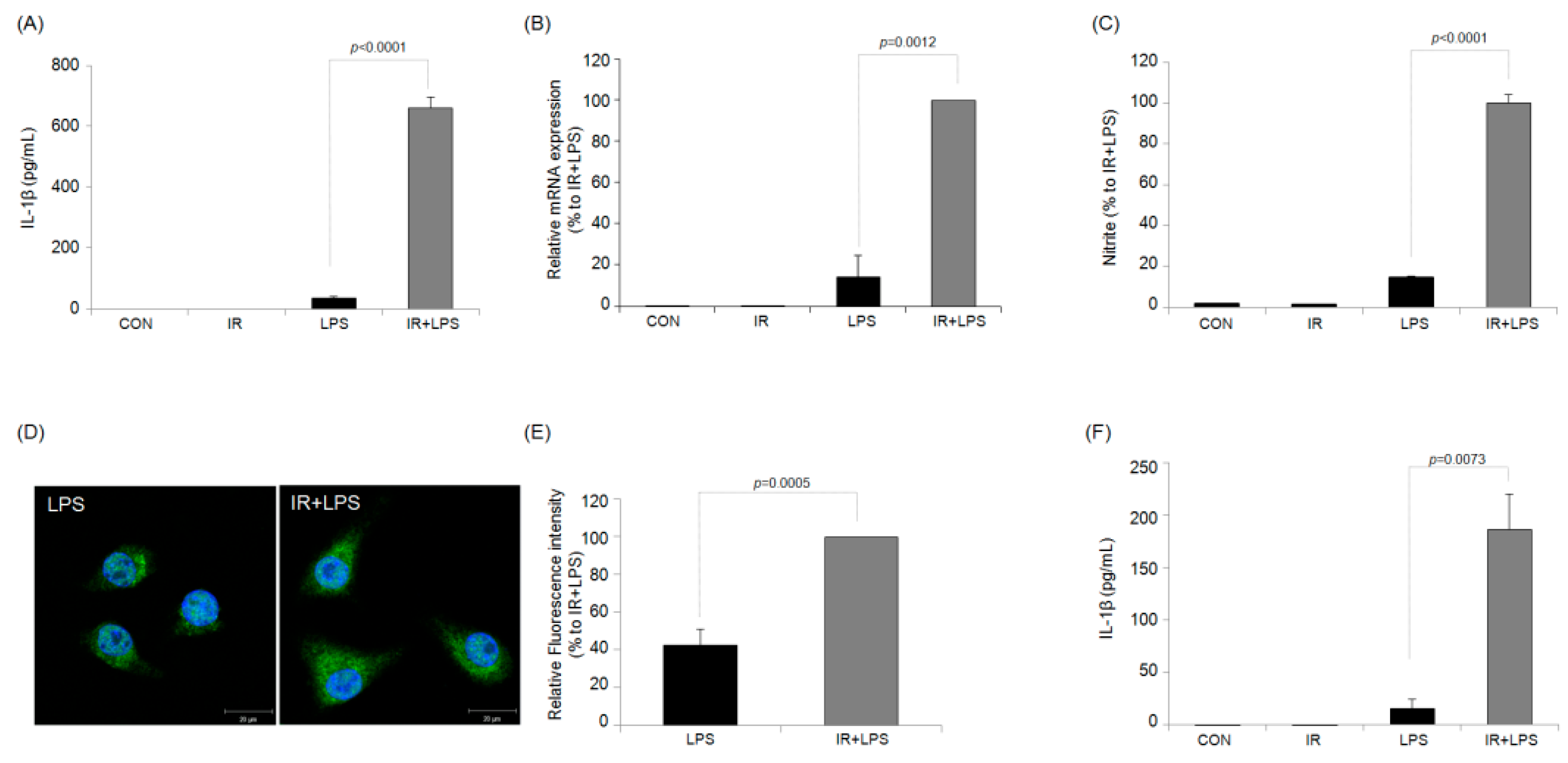
2.1. IR Enhances NO and IL-1β Production in LPS-Stimulated RAW264.7 Macrophages
2.2. IR Activates Caspase-1/Interleukin-1 Converting Enzyme (ICE) in RAW264.7 Macrophages
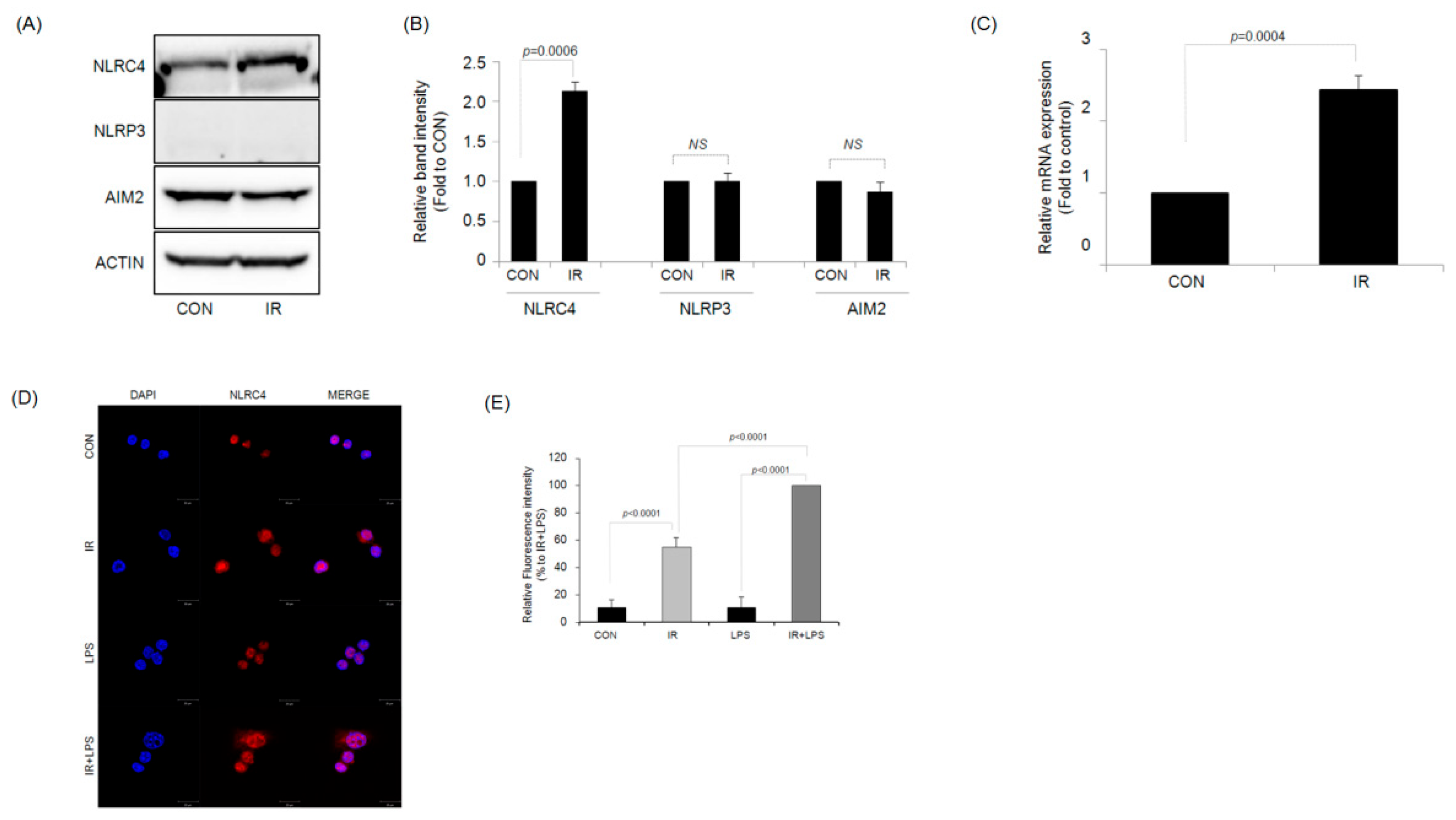
2.3. IR Activates the NLRC4 Inflammasome of RAW264.7 Macrophages
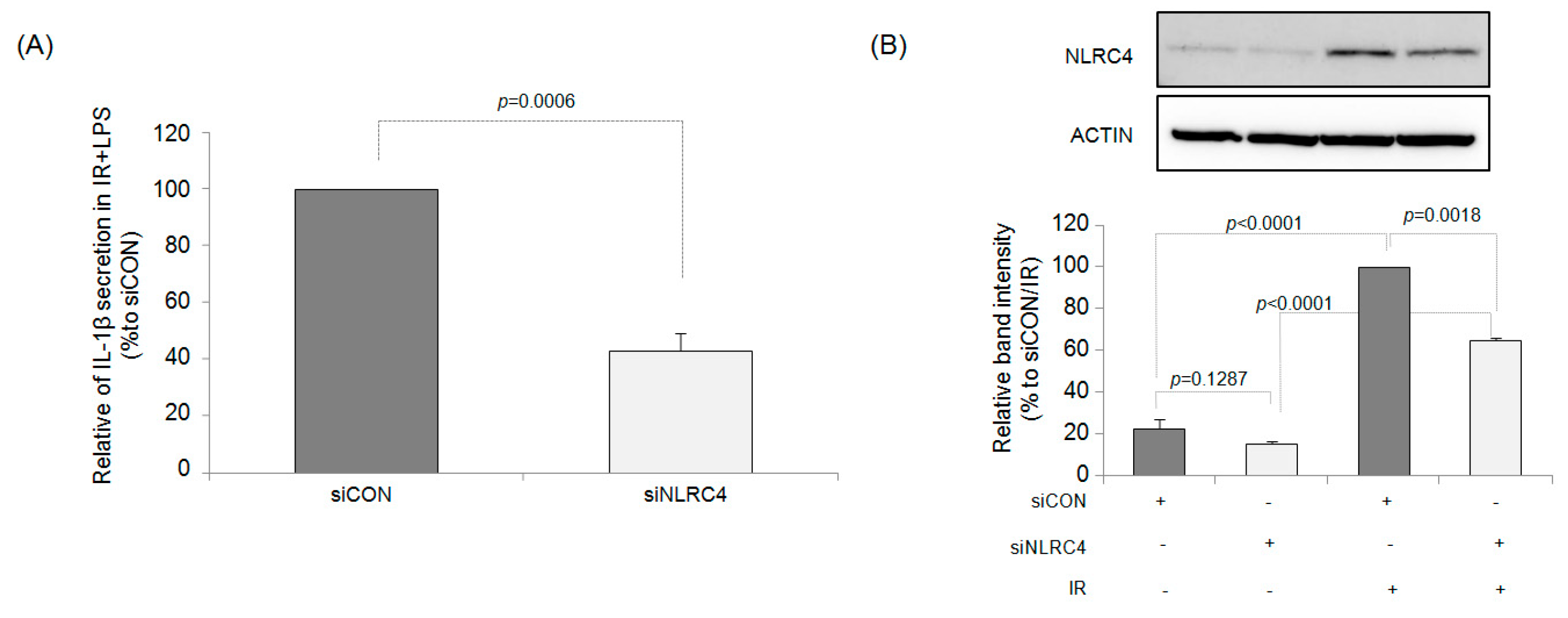
2.4. NLRC4 Plays an Important Role in IR-Induced IL-1β Production
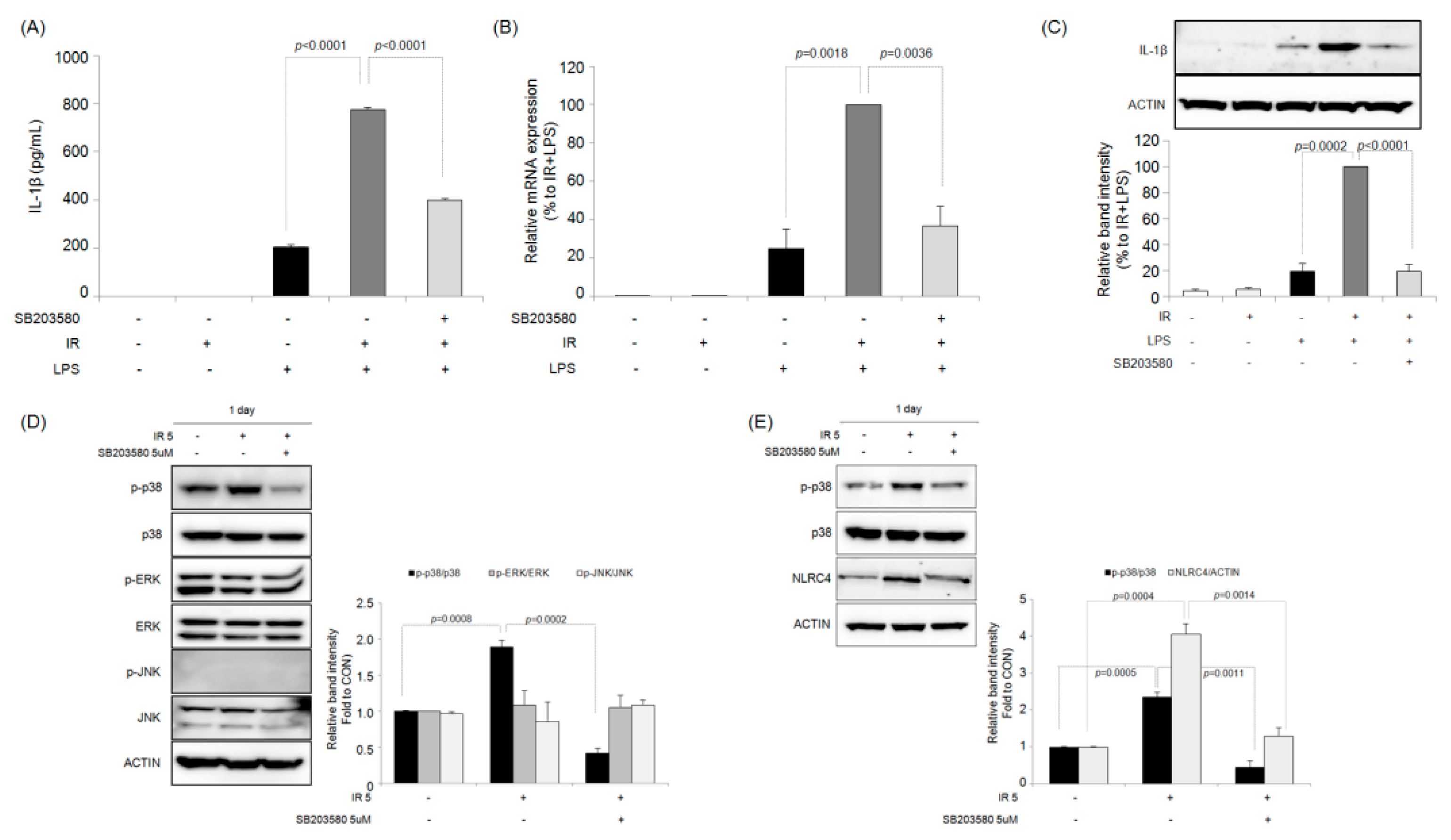
2.5. Effects of Selective MAPK Inhibitors on IR-Induced IL-1β Production in Macrophages
2.6. Effects of the Selective p38 MAPK Inhibitor on IR-Induced ROS Generation in Macrophages
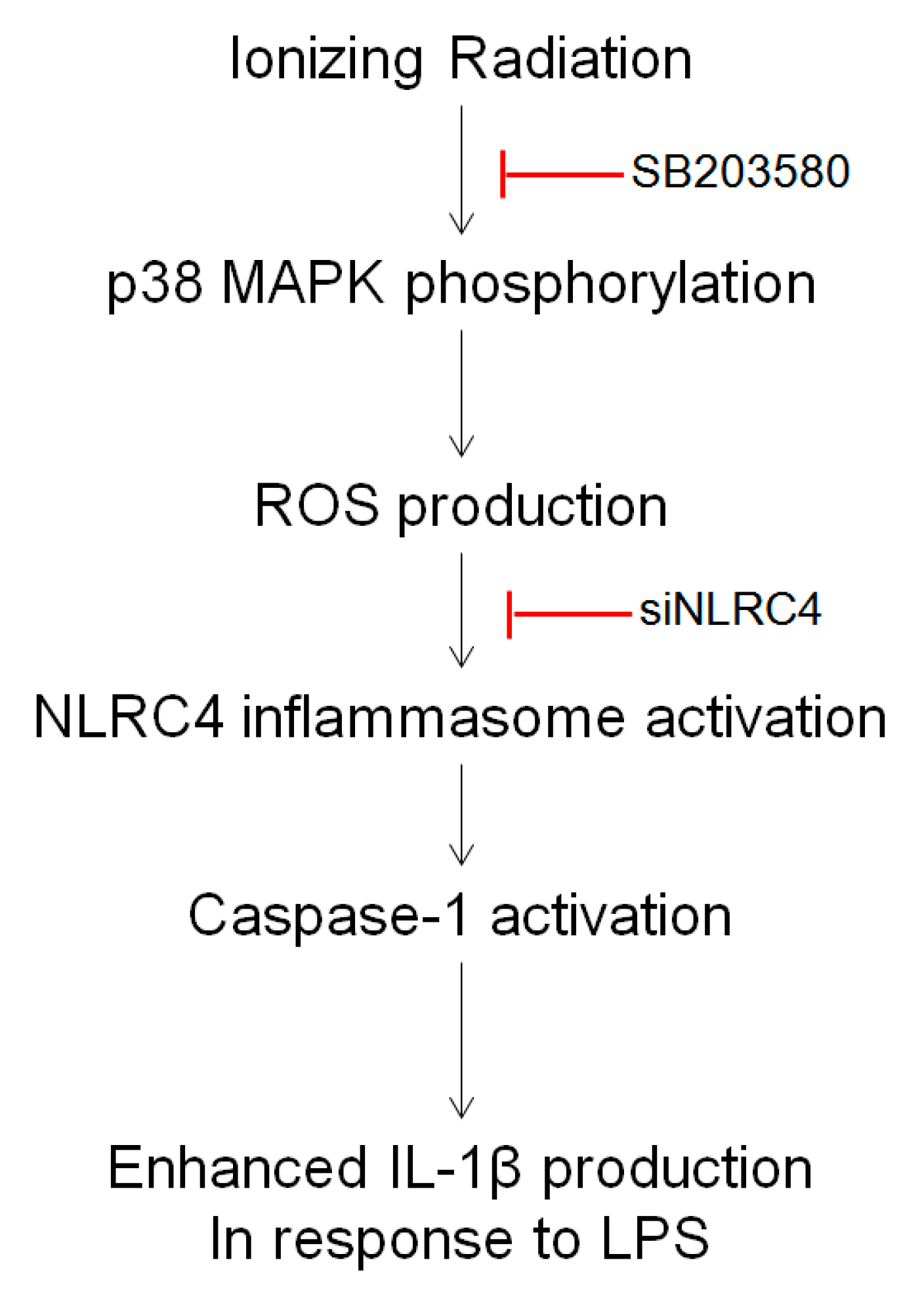
3. Discussion
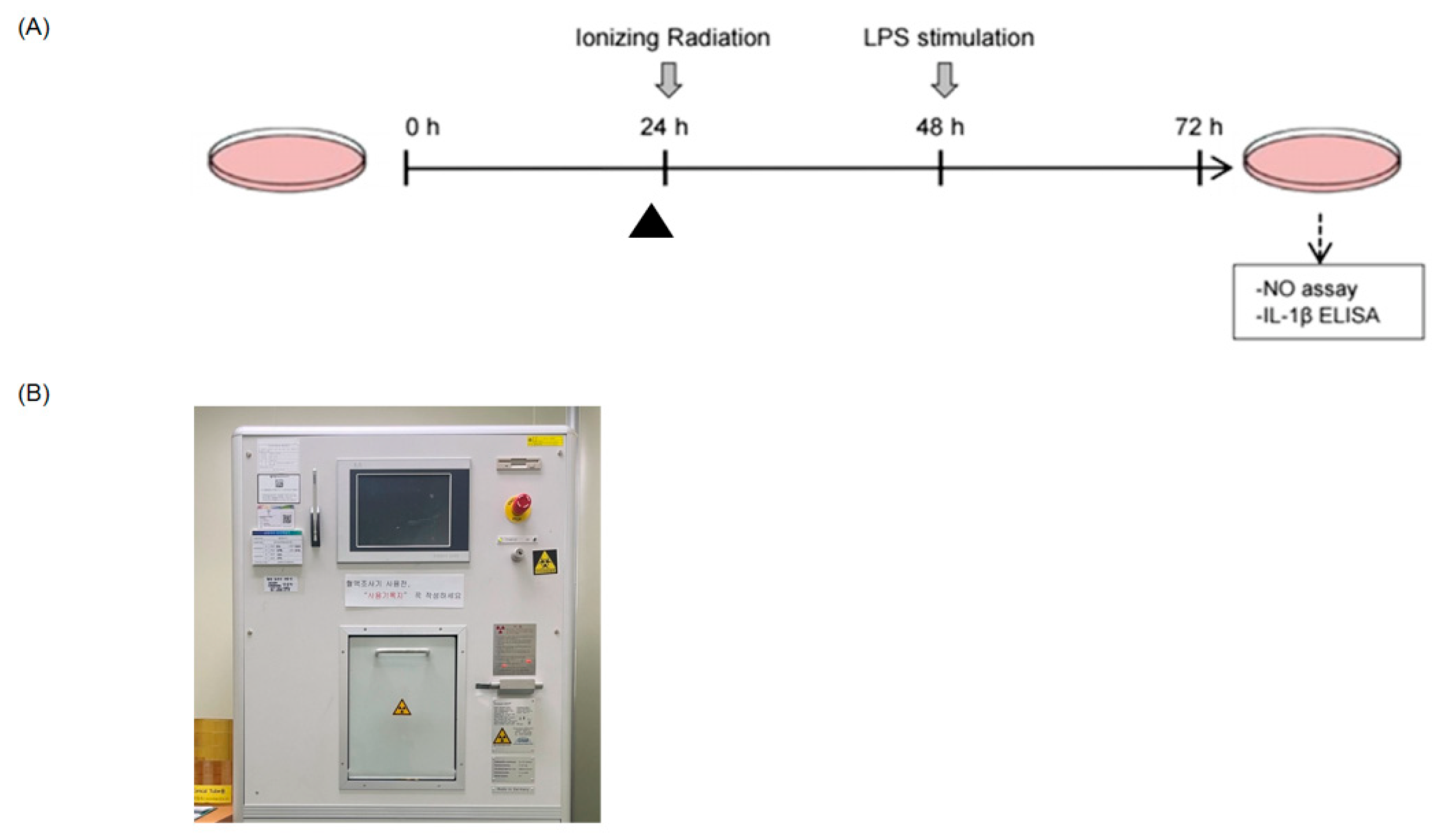
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Animals
4.3. Preparation of Peritoneal Macrophages
4.4. NO Assay
4.5. Total RNA Isolation and Quantitative PCR
4.6. Western Blotting
4.7. Immunofluorescence Staining
4.8. ELISA and Cytokine Arrays
4.9. SiRNA Transfection
4.10. Cell Transfection and Lentiviral Infection
4.11. Measurement of Direct Caspase-1 Activity Using Culture Supernatant
4.12. ROS Detection
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Beauford, S.S.; Kumari, A.; Garnett-Benson, C. Ionizing radiation modulates the phenotype and function of human CD4+ induced regulatory T cells. BMC Immunol. 2020, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Portella, L.; Scala, S. Ionizing radiation effects on the tumor microenvironment. Semin. Oncol. 2019, 46, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Impens, N.; Armengol, G.; Candéias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rödel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ. Int. 2021, 149, 106212. [Google Scholar] [CrossRef] [PubMed]
- McKinney, L.C.; Aquilla, E.M.; Coffin, D.; Wink, D.A.; Vodovotz, Y. Ionizing radiation potentiates the induction of nitric oxide synthase by IFN-gamma and/or LPS in murine macrophage cell lines: Role of TNF-alpha. J. Leukoc. Biol. 1998, 64, 459–466. [Google Scholar] [CrossRef]
- Gallin, E.K.; Green, S.W.; Sheehy, P.A. Enhanced activity of the macrophage-like cell line J774.1 following exposure to gamma radiation. J. Leukoc. Biol. 1985, 38, 369–381. [Google Scholar] [CrossRef]
- Lambert, L.E.; Paulnock, D.M. Modulation of macrophage function by gamma-irradiation. Acquisition of the primed cell intermediate stage of the macrophage tumoricidal activation pathway. J. Immunol. 1987, 139, 2834–2841. [Google Scholar]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- O’Brien-Ladner, A.; Nelson, M.E.; Kimler, B.F.; Wesselius, L.J. Release of interleukin-1 by human alveolar macrophages after in vitro irradiation. Radiat. Res. 1993, 136, 37–41. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, J.Y.; Lee, C.G.; Heo, K.; Park, S.I.; Park, Y.S.; Kim, J.S.; Yang, K.M.; Lee, K.J.; Kim, T.H.; et al. Korean Red Ginseng saponin fraction modulates radiation effects on lipopolysaccharide-stimulated nitric oxide production in RAW264.7 macrophage cells. J. Ginseng Res. 2014, 38, 208–214. [Google Scholar] [CrossRef]
- Baik, J.S.; Seo, Y.N.; Yi, J.M.; Rhee, M.H.; Park, M.T.; Kim, S.D. Ginsenoside-Rp1 inhibits radiation-induced effects in lipopolysaccharide-stimulated J774A.1 macrophages and suppresses phenotypic variation in CT26 colon cancer cells. J. Ginseng Res. 2020, 44, 843–848. [Google Scholar] [CrossRef]
- Wen, J.; Xuan, B.; Liu, Y.; Wang, L.; He, L.; Meng, X.; Zhou, T.; Wang, Y. Updating the NLRC4 Inflammasome: From Bacterial Infections to Autoimmunity and Cancer. Front. Immunol. 2021, 12, 702527. [Google Scholar] [CrossRef]
- Dowling, J.K.; O’Neill, L.A. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 424–443. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Hu, Z.; Yan, C.; Liu, P.; Huang, Z.; Ma, R.; Zhang, C.; Wang, R.; Zhang, Y.; Martinon, F.; Miao, D.; et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 2013, 341, 172–175. [Google Scholar] [CrossRef]
- Wen, H.; Miao, E.A.; Ting, J.P. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 2013, 39, 432–441. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Osuka, A.; Ishikawa, S.; Lederer, M.R.; Wanke-Jellinek, L.; Lederer, J.A. Radiation exposure induces inflammasome pathway activation in immune cells. J. Immunol. 2015, 194, 1178–1189. [Google Scholar] [CrossRef]
- Liu, Y.G.; Chen, J.K.; Zhang, Z.T.; Ma, X.J.; Chen, Y.C.; Du, X.M.; Liu, H.; Zong, Y.; Lu, G.C. NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrow-derived macrophages. Cell Death Dis. 2017, 8, e2579. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, J.D.; Bibbs, L.; Ulevitch, R.J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994, 265, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [PubMed]
- Juretic, N.; Santibáñez, J.F.; Hurtado, C.; Martínez, J. ERK 1,2 and p38 pathways are involved in the proliferative stimuli mediated by urokinase in osteoblastic SaOS-2 cell line. J. Cell. Biochem. 2001, 83, 92–98. [Google Scholar] [CrossRef]
- Liu, B.; Fang, M.; Lu, Y.; Lu, Y.; Mills, G.B.; Fan, Z. Involvement of JNK-mediated pathway in EGF-mediated protection against paclitaxel-induced apoptosis in SiHa human cervical cancer cells. Br. J. Cancer 2001, 85, 303–311. [Google Scholar] [CrossRef]
- Yosimichi, G.; Nakanishi, T.; Nishida, T.; Hattori, T.; Takano-Yamamoto, T.; Takigawa, M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). Eur. J. Biochem. 2001, 268, 6058–6065. [Google Scholar] [CrossRef]
- Wang, X.; McGowan, C.H.; Zhao, M.; He, L.; Downey, J.S.; Fearns, C.; Wang, Y.; Huang, S.; Han, J. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol. Cell. Biol. 2000, 20, 4543–4552. [Google Scholar] [CrossRef]
- Lee, S.H.; Eom, M.; Lee, S.J.; Kim, S.; Park, H.J.; Park, D. BetaPix-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling. J. Biol. Chem. 2001, 276, 25066–25072. [Google Scholar] [CrossRef]
- Taher, M.M.; Hershey, C.M.; Oakley, J.D.; Valerie, K. Role of the p38 and MEK-1/2/p42/44 MAP kinase pathways in the differential activation of human immunodeficiency virus gene expression by ultraviolet and ionizing radiation. Photochem. Photobiol. 2000, 71, 455–459. [Google Scholar] [CrossRef]
- Kim, S.J.; Ju, J.W.; Oh, C.D.; Yoon, Y.M.; Song, W.K.; Kim, J.H.; Yoo, Y.J.; Bang, O.S.; Kang, S.S.; Chun, J.S. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J. Biol. Chem. 2002, 277, 1332–1339. [Google Scholar] [CrossRef]
- Sisakht, M.; Darabian, M.; Mahmoodzadeh, A.; Bazi, A.; Shafiee, S.M.; Mokarram, P.; Khoshdel, Z. The role of radiation induced oxidative stress as a regulator of radio-adaptive responses. Int. J. Radiat. Biol. 2020, 96, 561–576. [Google Scholar] [CrossRef]
- Lauber, K.; Ernst, A.; Orth, M.; Herrmann, M.; Belka, C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front. Oncol. 2012, 2, 116. [Google Scholar] [CrossRef]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef]
- Ying, H.; Fang, M.; Hang, Q.Q.; Chen, Y.; Qian, X.; Chen, M. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-β1/Smad3 pathway. J. Cell. Mol. Med. 2021, 25, 8662–8675. [Google Scholar] [CrossRef]
- Su, L.; Dong, Y.; Wang, Y.; Wang, Y.; Guan, B.; Lu, Y.; Wu, J.; Wang, X.; Li, D.; Meng, A.; et al. Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis. 2021, 12, 527. [Google Scholar] [CrossRef]
- Coates, P.J.; Rundle, J.K.; Lorimore, S.A.; Wright, E.G. Indirect macrophage responses to ionizing radiation: Implications for genotype-dependent bystander signaling. Cancer Res. 2008, 68, 450–456. [Google Scholar] [CrossRef]
- Ibuki, Y.; Mizuno, S.; Goto, R. Gamma-Irradiation-induced DNA damage enhances NO production via NF-kappaB activation in RAW264.7 cells. Biochim. Biophys. Acta 2003, 1593, 159–167. [Google Scholar] [CrossRef][Green Version]
- Khabipov, A.; Käding, A.; Liedtke, K.R.; Freund, E.; Partecke, L.I.; Bekeschus, S. RAW 264.7 Macrophage Polarization by Pancreatic Cancer Cells—A Model for Studying Tumour-promoting Macrophages. Anticancer Res. 2019, 39, 2871–2882. [Google Scholar] [CrossRef]
- Li, P.; Hao, Z.; Wu, J.; Ma, C.; Xu, Y.; Li, J.; Lan, R.; Zhu, B.; Ren, P.; Fan, D.; et al. Comparative Proteomic Analysis of Polarized Human THP-1 and Mouse RAW264.7 Macrophages. Front. Immunol. 2021, 12, 700009. [Google Scholar] [CrossRef]
- Lee, C.; Lee, J.; Choi, S.A.; Kim, S.K.; Wang, K.C.; Park, S.H.; Kim, S.H.; Lee, J.Y.; Phi, J.H. M1 macrophage recruitment correlates with worse outcome in SHH Medulloblastomas. BMC Cancer 2018, 18, 535. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. An expanding role for interleukin-1 blockade from gout to cancer. Mol. Med. 2014, 20 (Suppl. 1), S43–S58. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Borcherding, N.; Kolb, R. IL-1 Signaling in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 1–23. [Google Scholar]
- Pei, H.P.; Tan, F.B.; Liu, L.; Yu, N.H.; Zhu, H. IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch. Biochem. Biophys. 2016, 604, 20–26. [Google Scholar]
- Li, Y.; Wang, L.; Pappan, L.; Galliher-Beckley, A.; Shi, J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer 2012, 11, 87. [Google Scholar] [CrossRef]
- Lu, L.; Wang, P.; Zou, Y.; Zha, Z.; Huang, H.; Guan, M.; Wu, Y.; Liu, G. IL-1β Promotes Stemness of Tumor Cells by Activating Smad/ID1 Signaling Pathway. Int. J. Med. Sci. 2020, 17, 1257–1268. [Google Scholar] [CrossRef]
- Kang, A.R.; Cho, J.H.; Lee, N.G.; Kwon, J.H.; Song, J.Y.; Hwang, S.G.; Jung, I.S.; Kim, J.S.; Um, H.D.; Oh, S.C.; et al. Radiation-induced IL-1β expression and secretion promote cancer cell migration/invasion via activation of the NF-κB-RIP1 pathway. Biochem. Biophys. Res. Commun. 2021, 534, 973–979. [Google Scholar] [CrossRef]
- Tulotta, C.; Lefley, D.V.; Moore, C.K.; Amariutei, A.E.; Spicer-Hadlington, A.R.; Quayle, L.A.; Hughes, R.O.; Ahmed, K.; Cookson, V.; Evans, C.A.; et al. IL-1B drives opposing responses in primary tumours and bone metastases; harnessing combination therapies to improve outcome in breast cancer. NPJ Breast Cancer 2021, 7, 95. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Dinarello, C.A. How interleukin-1β induces gouty arthritis. Arthritis Rheum. 2010, 62, 3140–3144. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Hu, B.; Jin, C.; Li, H.B.; Tong, J.; Ouyang, X.; Cetinbas, N.M.; Zhu, S.; Strowig, T.; Lam, F.C.; Zhao, C.; et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 2016, 354, 765–768. [Google Scholar] [CrossRef]
- Kolb, R.; Kluz, P.; Tan, Z.W.; Borcherding, N.; Bormann, N.; Vishwakarma, A.; Balcziak, L.; Zhu, P.; Davies, B.S.; Gourronc, F.; et al. Obesity-associated inflammation promotes angiogenesis and breast cancer via angiopoietin-like 4. Oncogene 2019, 38, 2351–2363. [Google Scholar] [CrossRef]
- Hung, S.J.; Tang, S.C.; Liao, P.Y.; Ge, J.S.; Hsiao, Y.P.; Yang, J.H. Photoprotective Potential of Glycolic Acid by Reducing NLRC4 and AIM2 Inflammasome Complex Proteins in UVB Radiation-Induced Normal Human Epidermal Keratinocytes and Mice. DNA Cell Biol. 2017, 36, 177–187. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Bull, H.G.; Calaycay, J.R.; Chapman, K.T.; Howard, A.D.; Kostura, M.J.; Miller, D.K.; Molineaux, S.M.; Weidner, J.R.; Aunins, J.; et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992, 356, 768–774. [Google Scholar] [CrossRef]
- Winter, R.N.; Rhee, J.G.; Kyprianou, N. Caspase-1 enhances the apoptotic response of prostate cancer cells to ionizing radiation. Anticancer Res. 2004, 24, 1377–1386. [Google Scholar]
- Tabraue, C.; Lara, P.C.; De Mirecki-Garrido, M.; De La Rosa, J.V.; López-Blanco, F.; Fernández-Pérez, L.; Boscá, L.; Castrillo, A. LXR Signaling Regulates Macrophage Survival and Inflammation in Response to Ionizing Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 913–923. [Google Scholar] [CrossRef]
- Provoost, S.; Maes, T.; Pauwels, N.S.; Vanden Berghe, T.; Vandenabeele, P.; Lambrecht, B.N.; Joos, G.F.; Tournoy, K.G. NLRP3/caspase-1-independent IL-1beta production mediates diesel exhaust particle-induced pulmonary inflammation. J. Immunol. 2011, 187, 3331–3337. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Barber, D.L.; Shenderov, K.; White, S.D.; Wilson, M.S.; Cheever, A.; Kugler, D.; Hieny, S.; Caspar, P.; Núñez, G.; et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 2010, 184, 3326–3330. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell. Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Neamatallah, T. Mitogen-Activated Protein Kinase Pathway: A Critical Regulator in Tumor-associated Macrophage Polarization. J. Microsc. Ultrastruct. 2019, 7, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M. MAP kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Yacoub, A.; Fisher, P.B.; Hagan, M.P.; Grant, S. MAPK pathways in radiation responses. Oncogene 2003, 22, 5885–5896. [Google Scholar] [CrossRef]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef]
- Kim, S.; Choe, J.H.; Lee, G.J.; Kim, Y.S.; Kim, S.Y.; Lee, H.M.; Jin, H.S.; Kim, T.S.; Kim, J.M.; Cho, M.J.; et al. Ionizing Radiation Induces Innate Immune Responses in Macrophages by Generation of Mitochondrial Reactive Oxygen Species. Radiat. Res. 2017, 187, 32–41. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, M.J.; Kang, C.M.; Bae, S.; Cho, C.K.; Soh, J.W.; Kim, J.H.; Kang, S.; Chung, H.Y.; Lee, Y.S.; et al. Activation of Bak and Bax through c-abl-protein kinase Cdelta-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. J. Biol. Chem. 2006, 281, 7049–7059. [Google Scholar] [CrossRef]
- Hwang, S.; Jeong, H.; Hong, E.H.; Joo, H.M.; Cho, K.S.; Nam, S.Y. Low-dose ionizing radiation alleviates Aβ42-induced cell death via regulating AKT and p38 pathways in Drosophila Alzheimer’s disease models. Biol. Open 2019, 8, bio036657. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, C. p38 MAPK in regulating cellular responses to ultraviolet radiation. J. Biomed. Sci. 2007, 14, 303–312. [Google Scholar]
- Sun, Y.; Zhang, J.; Zhai, T.; Li, H.; Li, H.; Huo, R.; Shen, B.; Wang, B.; Chen, X.; Li, N.; et al. CCN1 promotes IL-1β production in keratinocytes by activating p38 MAPK signaling in psoriasis. Sci. Rep. 2017, 7, 43310. [Google Scholar] [CrossRef]
- Yao, K.; Zhao, Y.; Jin, P.; Lou, X.; Luo, Z.; Zhang, H.; Li, F. Involvement of the NLRC4 inflammasome in promoting retinal ganglion cell death in an acute glaucoma mouse model. Exp. Eye Res. 2021, 203, 108388. [Google Scholar] [CrossRef]
- Farias-Eisner, R.; Chaudhuri, G.; Aeberhard, E.; Fukuto, J.M. The chemistry and tumoricidal activity of nitric oxide/hydrogen peroxide and the implications to cell resistance/susceptibility. J. Biol. Chem. 1996, 271, 6144–6151. [Google Scholar] [CrossRef]
- Brantley, E.C.; Guo, L.; Zhang, C.; Lin, Q.; Yokoi, K.; Langley, R.R.; Kruzel, E.; Maya, M.; Kim, S.W.; Kim, S.J.; et al. Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl. Oncol. 2010, 3, 380–388. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Y.; Nelin, L.D. Extracellular signal-regulated kinase mediates expression of arginase II but not inducible nitric-oxide synthase in lipopolysaccharide-stimulated macrophages. J. Biol. Chem. 2015, 290, 2099–2111. [Google Scholar] [CrossRef]
- Pyo, S. The mechanism of poly I:C-induced antiviral activity in peritoneal macrophage. Arch. Pharmacal Res. 1994, 17, 93–99. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baik, J.S.; Seo, Y.N.; Lee, Y.-C.; Yi, J.M.; Rhee, M.H.; Park, M.-T.; Kim, S.D. Involvement of the p38 MAPK-NLRC4-Caspase-1 Pathway in Ionizing Radiation-Enhanced Macrophage IL-1β Production. Int. J. Mol. Sci. 2022, 23, 13757. https://doi.org/10.3390/ijms232213757
Baik JS, Seo YN, Lee Y-C, Yi JM, Rhee MH, Park M-T, Kim SD. Involvement of the p38 MAPK-NLRC4-Caspase-1 Pathway in Ionizing Radiation-Enhanced Macrophage IL-1β Production. International Journal of Molecular Sciences. 2022; 23(22):13757. https://doi.org/10.3390/ijms232213757
Chicago/Turabian StyleBaik, Ji Sue, You Na Seo, Young-Choon Lee, Joo Mi Yi, Man Hee Rhee, Moon-Taek Park, and Sung Dae Kim. 2022. "Involvement of the p38 MAPK-NLRC4-Caspase-1 Pathway in Ionizing Radiation-Enhanced Macrophage IL-1β Production" International Journal of Molecular Sciences 23, no. 22: 13757. https://doi.org/10.3390/ijms232213757
APA StyleBaik, J. S., Seo, Y. N., Lee, Y.-C., Yi, J. M., Rhee, M. H., Park, M.-T., & Kim, S. D. (2022). Involvement of the p38 MAPK-NLRC4-Caspase-1 Pathway in Ionizing Radiation-Enhanced Macrophage IL-1β Production. International Journal of Molecular Sciences, 23(22), 13757. https://doi.org/10.3390/ijms232213757







