Electrospun Azithromycin-Laden Gelatin Methacryloyl Fibers for Endodontic Infection Control
Abstract
1. Introduction
2. Results
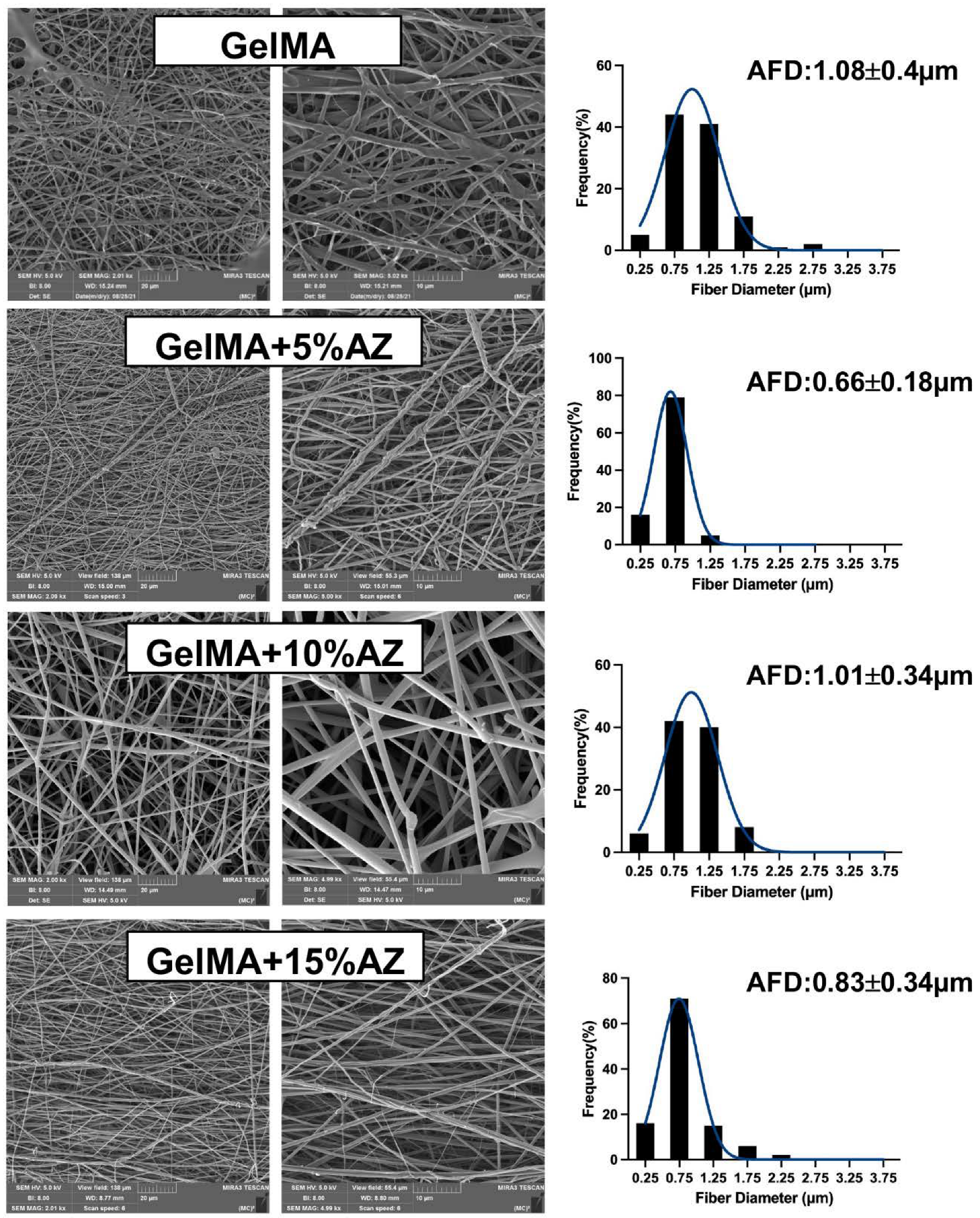
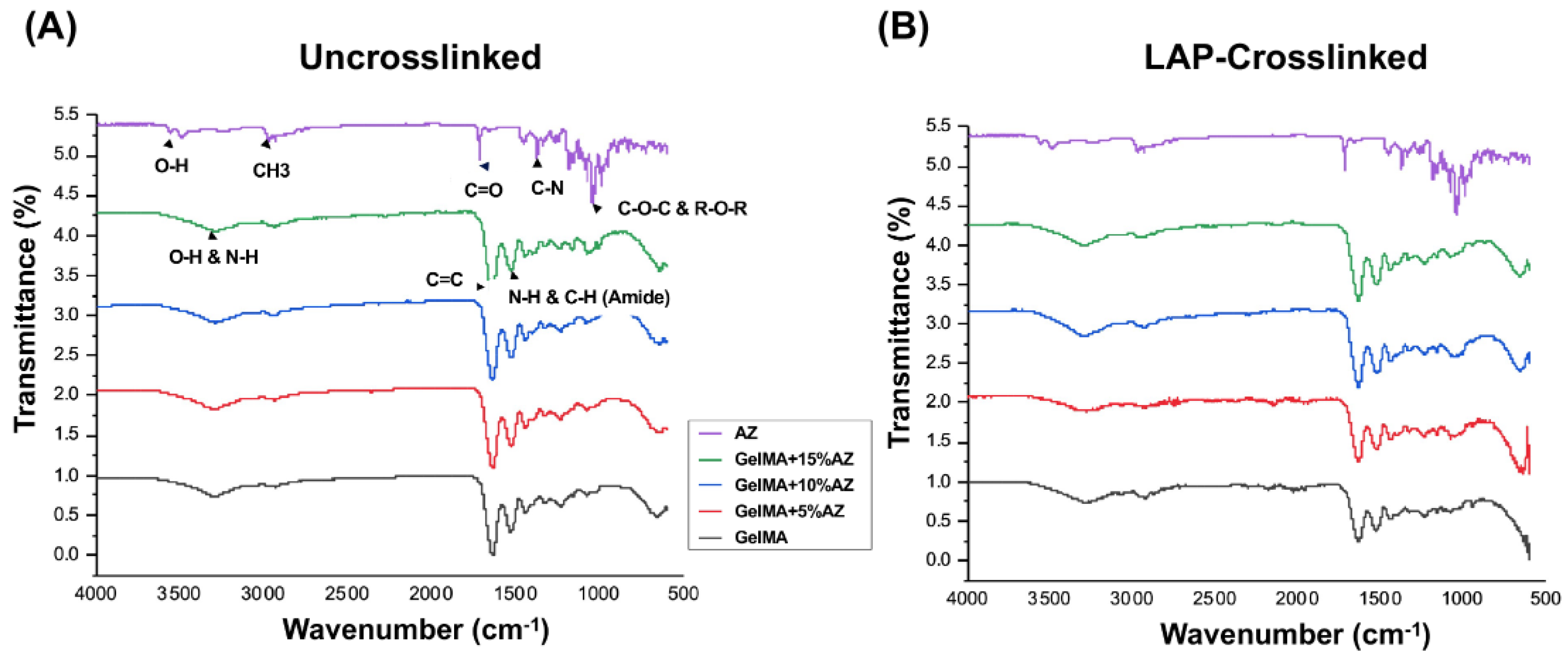
2.1. Morphological and Chemical Characteristics
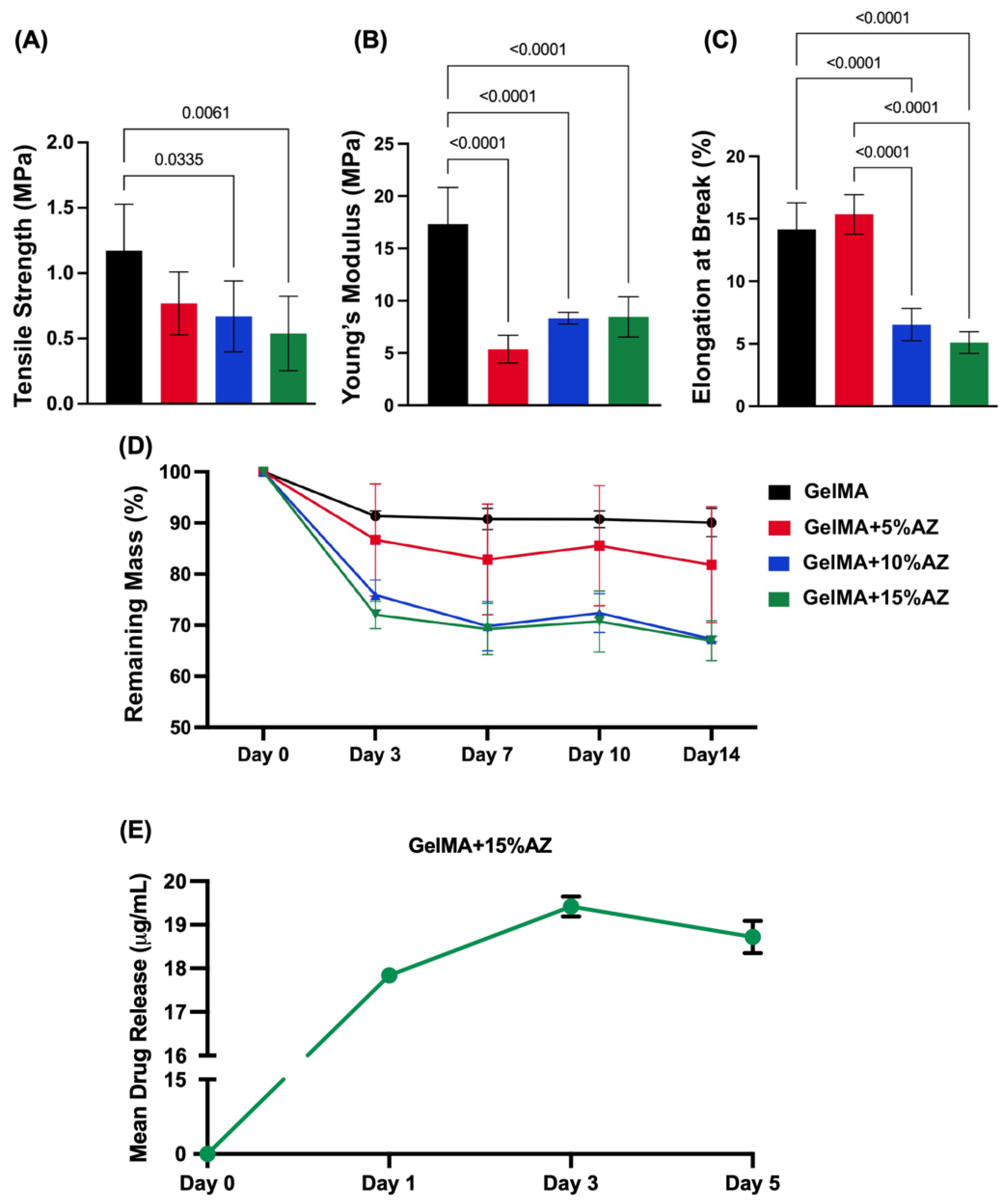
2.2. Mechanical Properties and Degradation Profile
2.3. Drug Release
2.4. Cytocompatibility
2.5. Antimicrobial Assessment
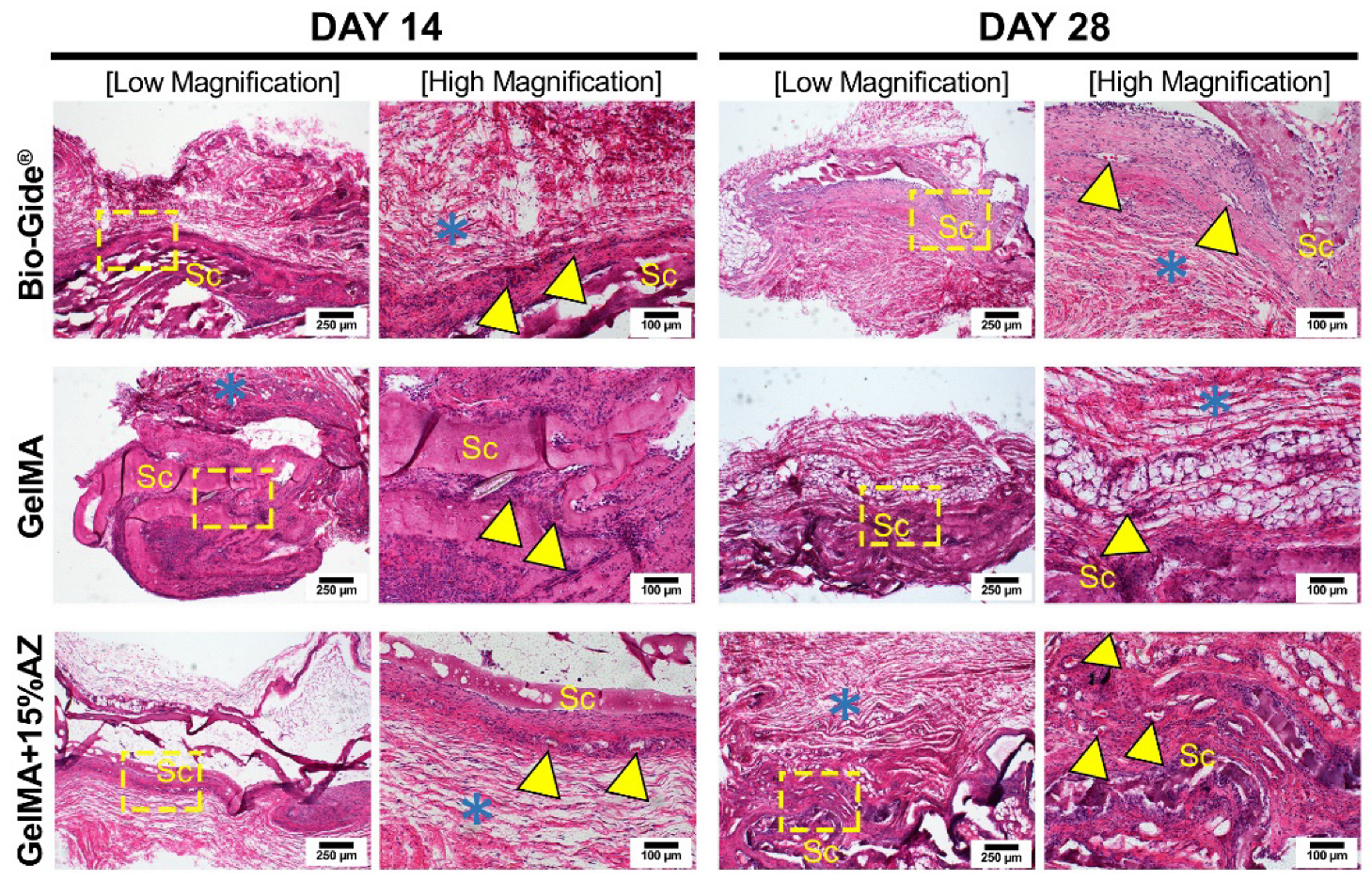
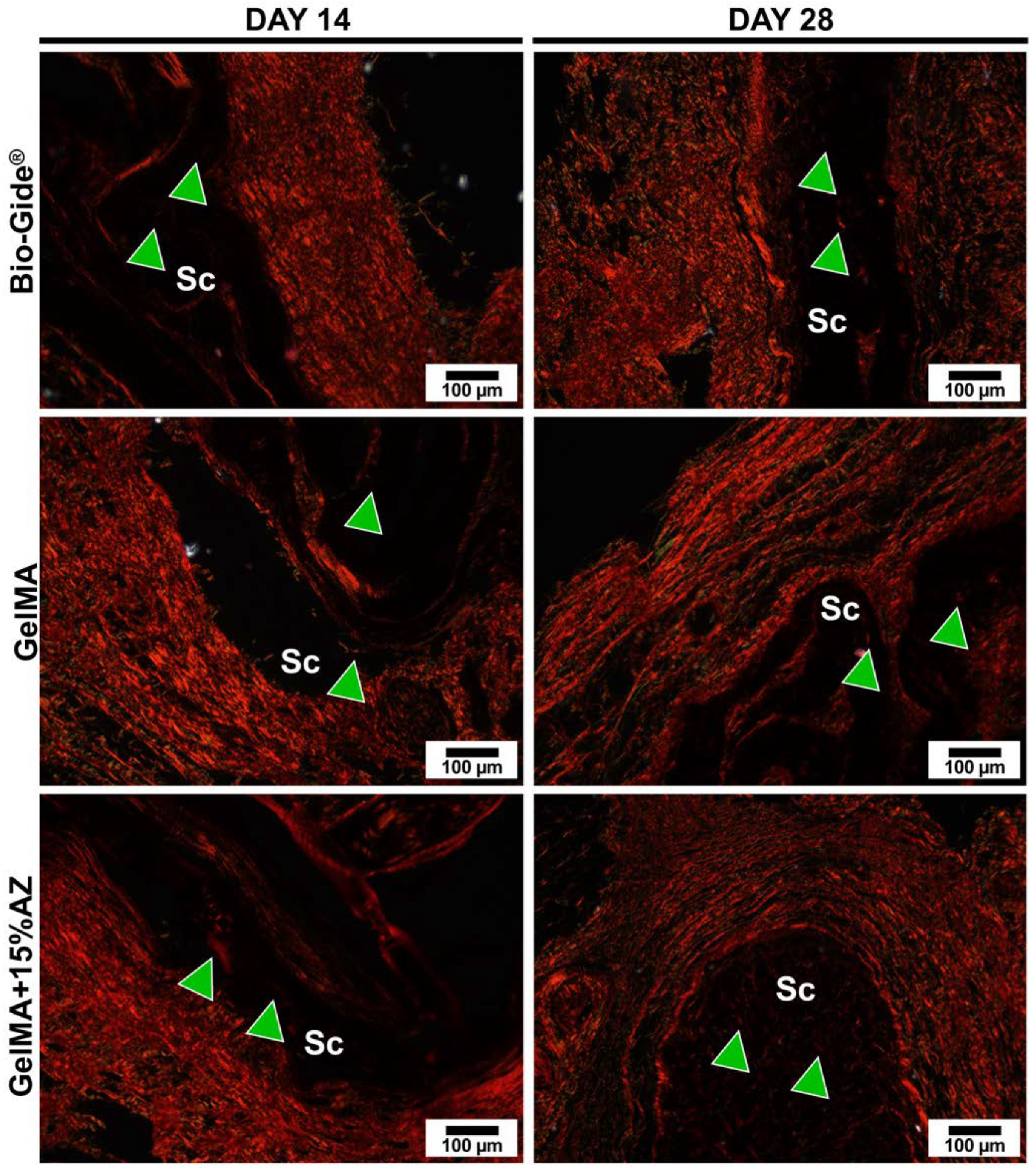
2.6. In Vivo Biocompatibility
3. Discussion
4. Materials and Methods
4.1. Gelatin Methacryloyl Synthesis
4.2. Electrospinning and Nanofibers Preparation
4.3. Morphological and Chemical Characteristics
4.4. Mechanical Properties
4.5. Degradation Profile
4.6. Drug Release
4.7. Cytocompatibility
4.8. Antimicrobial Assessment
4.9. In Vivo Biocompatibility
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Songtrakul, K.; Azarpajouh, T.; Malek, M.; Sigurdsson, A.; Kahler, B.; Lin, L.M. Modified Apexification Procedure for Immature Permanent Teeth with a Necrotic Pulp/Apical Periodontitis: A Case Series. J. Endod. 2020, 46, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Cehreli, Z.C.; Isbitiren, B.; Sara, S.; Erbas, G. Regenerative Endodontic Treatment (Revascularization) of Immature Necrotic Molars Medicated with Calcium Hydroxide: A Case Series. J. Endod. 2011, 37, 1327–1330. [Google Scholar] [CrossRef]
- Smith, A.J.; Cooper, P.R. Regenerative Endodontics: Burning Questions. J. Endod. 2017, 43, S1–S6. [Google Scholar] [CrossRef]
- Verma, P.; Nosrat, A.; Kim, J.R.; Price, J.B.; Wang, P.; Bair, E.; Xu, H.H.; Fouad, A.F. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J. Dent. Res. 2017, 96, 100–106. [Google Scholar] [CrossRef]
- Soares, D.G.; Bordini, E.A.F.; Swanson, W.B.; de Souza Costa, C.A.; Bottino, M.C. Platform technologies for regenerative endodontics from multifunctional biomaterials to tooth-on-a-chip strategies. Clin. Oral Investig. 2021, 25, 4749–4779. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Münchow, E.A.; Bordini, E.A.F.; de Oliveira da Rosa, W.L.; Bottino, M.C. Antimicrobial Therapeutics in Regenerative Endodontics: A Scoping Review. J. Endod. 2020, 46, S115–S127. [Google Scholar] [CrossRef]
- Murray, P.E. Review of guidance for the selection of regenerative endodontics, apexogenesis, apexification, pulpotomy, and other endodontic treatments for immature permanent teeth. Int. Endod. J. 2022. [Google Scholar] [CrossRef]
- Albuquerque, M.T.P.; Nagata, J.Y.; Diogenes, A.R.; Azabi, A.A.; Gregory, R.L.; Bottino, M.C. Clinical Perspective of Electrospun Nanofibers as a Drug Delivery Strategy for Regenerative Endodontics. Curr. Oral Health. Rep. 2016, 3, 209–220. [Google Scholar] [CrossRef]
- Bottino, M.C.; Albuquerque, M.T.P.; Azabi, A.; Munchow, E.A.; Spolnik, K.J.; Nör, J.E.; Edwards, P.C. A novel patient-specific three-dimensional drug delivery construct for regenerative endodontics. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Sharifi, H.; Akbari, A.; Chodosh, J. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Sci. Rep. 2021, 11, 23276. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Xiao, W.; Sun, J.; Zhong, M.; Guo, L.; Fan, H.; Zhang, X. A biocompatible hydrogel with improved stiffness and hydrophilicity for modular tissue engineering assembly. J. Mater. Chem. B 2015, 3, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dubey, N.; Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Xu, J.; Castilho, R.M.; Bottino, M.C. Metformin-loaded nanospheres-laden photocrosslinkable gelatin hydrogel for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2021, 116, 104293. [Google Scholar] [CrossRef]
- Aldana, A.A.; Malatto, L.; Rehman, M.A.U.; Boccaccini, A.R.; Abraham, G.A. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomater. Basel 2019, 9, 120. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Mei, L.; Dubey, N.; Fenno, J.C.; Piva, E.; Lund, R.G.; Schwendeman, A.; Bottino, M.C. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl. Mater. Interfaces 2020, 12, 16006–16017. [Google Scholar] [CrossRef]
- Sato, R.; Harada, R.; Shigeta, Y. Theoretical analyses on a flipping mechanism of UV-induced DNA damage. Biophys. Phys. 2016, 13, 311–319. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Goering, P.L.; Reipa, V.; Narayan, R.J. Toxicity and photosensitizing assessment of gelatin methacryloyl-based hydrogels photoinitiated with lithium phenyl-2,4,6-trimethylbenzoylphosphinate in human primary renal proximal tubule epithelial cells. Biointerphases 2019, 14, 021007. [Google Scholar] [CrossRef]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef]
- Andrada, A.C.; Azuma, M.M.; Furusho, H.; Hirai, K.; Xu, S.; White, R.R.; Sasaki, H. Immunomodulation Mediated by Azithromycin in Experimental Periapical Inflammation. J. Endod. 2020, 46, 1648–1654. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Yang, X.; Li, C. Preparation of azithromycin microcapsules by a layer-by-layer self-assembly approach and release behaviors of azithromycin. Colloids Surf. A Physicochem. Eng. Asp. 2010, 362, 135–139. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N. Diversity of Endodontic Microbiota Revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Daghrery, A.; Dubey, N.; Li, C.; Mei, L.; Fenno, J.C.; Schwendeman, A.; Aytac, Z.; Bottino, M.C. Hybrid Antimicrobial Hydrogel as Injectable Therapeutics for Oral Infection Ablation. Biomacromolecules 2020, 21, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.S.; Das, A.C.; Sahoo, S.K.; Bhardwaj, S.S.; Babaji, P.; Varghese, J.G. Evaluation of role of periodontal pathogens in endodontic periodontal diseases. J. Fam. Med. Prim. Care 2020, 9, 239–242. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Soares, A.J.; Souza-Filho, F.J.; Zaia, A.A.; Ferraz, C.C.; Almeida, J.F.; Gomes, B.P. Microbial Evaluation of Traumatized Teeth Treated with Triple Antibiotic Paste or Calcium Hydroxide with 2% Chlorhexidine Gel in Pulp Revascularization. J. Endod. 2014, 40, 778–783. [Google Scholar] [CrossRef]
- Sassone, L.M.; Fidel, R.; Faveri, M.; Fidel, S.; Figueiredo, L.; Feres, M. Microbiological evaluation of primary endodontic infections in teeth with and without sinus tract. Int. Endod. J. 2008, 41, 508–515. [Google Scholar] [CrossRef]
- Kulik, E.M.; Thurnheer, T.; Karygianni, L.; Walter, C.; Sculean, A.; Eick, S. Antibiotic Susceptibility Patterns of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis Strains from Different Decades. Antibiot. Basel 2019, 8, 253. [Google Scholar] [CrossRef]
- Narita, M.; Shibahara, T.; Takano, N.; Fujii, R.; Okuda, K.; Ishihara, K. Antimicrobial Susceptibility of Microorganisms Isolated from Periapical Periodontitis Lesions. Bull. Tokyo Dent. Coll. 2016, 57, 133–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, P.-C.; Schibler, M.R.; Walters, J.D. Azithromycin Enhances Phagocytic Killing of Aggregatibacter actinomycetemcomitansY4 by Human Neutrophils. J. Periodontol. 2015, 86, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Cosme-Silva, L.; Benetti, F.; Dal-Fabbro, R.; Gomes Filho, J.E.; Sakai, V.T.; Cintra, L.T.A.; Alvarez, N.; Ervolino, E.; Viola, N.V. Biocompatibility and biomineralization ability of Bio-C Pulpecto. A histological and immunohistochemical study. Int. J. Paediatr. Dent. 2019, 29, 352–360. [Google Scholar] [CrossRef]
- Cigana, C.; Assael, B.M.; Melotti, P. Azithromycin Selectively Reduces Tumor Necrosis Factor Alpha Levels in Cystic Fibrosis Airway Epithelial Cells. Antimicrob. Agents Chemother. 2007, 51, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, S.; Rubin, B.K. Mechanisms of Action and Clinical Application of Macrolides as Immunomodulatory Medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Haydar, D.; Cory, T.J.; Birket, S.E.; Murphy, B.S.; Pennypacker, K.R.; Sinai, A.P.; Feola, D.J. Azithromycin Polarizes Macrophages to an M2 Phenotype via Inhibition of the STAT1 and NF-kappaB Signaling Pathways. J. Immunol. 2019, 203, 1021–1030. [Google Scholar] [CrossRef]
- Letsiou, E.; Kitsiouli, E.; Nakos, G.; Lekka, M.E. Mild stretch activates cPLA2 in alveolar type II epithelial cells independently through the MEK/ERK and PI3K pathways. Biochim. Biophys. Acta 2011, 1811, 370–376. [Google Scholar] [CrossRef]
- Ahlstrand, T.; Kovesjoki, L.; Maula, T.; Oscarsson, J.; Ihalin, R. Aggregatibacter actinomycetemcomitans LPS binds human interleukin-8. J. Oral Microbiol. 2018, 11, 1549931. [Google Scholar] [CrossRef]
- Daghrery, A.; Ferreira, J.A.; de Souza Araújo, I.J.; Clarkson, B.H.; Eckert, G.J.; Bhaduri, S.B.; Malda, J.; Bottino, M.C. A Highly Ordered, Nanostructured Fluorinated CaP-Coated Melt Electrowritten Scaffold for Periodontal Tissue Regeneration. Adv. Health Mater. 2021, 10, e2101152. [Google Scholar] [CrossRef]
- Cosme-Silva, L.; Dal-Fabbro, R.; Gonçalves, L.D.O.; Prado, A.S.D.; Plazza, F.A.; Viola, N.V.; Cintra, L.T.A.; Gomes Filho, J.E. Hypertension affects the biocompatibility and biomineralization of MTA, High-plasticity MTA, and Biodentine®. Braz. Oral Res. 2019, 33, e060. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, A.A.; Mahmoud, A.H.; Ribeiro, J.S.; Daghrery, A.; Xu, J.; Fenno, J.C.; Schwendeman, A.; Sasaki, H.; Dal-Fabbro, R.; Bottino, M.C. Electrospun Azithromycin-Laden Gelatin Methacryloyl Fibers for Endodontic Infection Control. Int. J. Mol. Sci. 2022, 23, 13761. https://doi.org/10.3390/ijms232213761
Ayoub AA, Mahmoud AH, Ribeiro JS, Daghrery A, Xu J, Fenno JC, Schwendeman A, Sasaki H, Dal-Fabbro R, Bottino MC. Electrospun Azithromycin-Laden Gelatin Methacryloyl Fibers for Endodontic Infection Control. International Journal of Molecular Sciences. 2022; 23(22):13761. https://doi.org/10.3390/ijms232213761
Chicago/Turabian StyleAyoub, Afzan A., Abdel H. Mahmoud, Juliana S. Ribeiro, Arwa Daghrery, Jinping Xu, J. Christopher Fenno, Anna Schwendeman, Hajime Sasaki, Renan Dal-Fabbro, and Marco C. Bottino. 2022. "Electrospun Azithromycin-Laden Gelatin Methacryloyl Fibers for Endodontic Infection Control" International Journal of Molecular Sciences 23, no. 22: 13761. https://doi.org/10.3390/ijms232213761
APA StyleAyoub, A. A., Mahmoud, A. H., Ribeiro, J. S., Daghrery, A., Xu, J., Fenno, J. C., Schwendeman, A., Sasaki, H., Dal-Fabbro, R., & Bottino, M. C. (2022). Electrospun Azithromycin-Laden Gelatin Methacryloyl Fibers for Endodontic Infection Control. International Journal of Molecular Sciences, 23(22), 13761. https://doi.org/10.3390/ijms232213761








