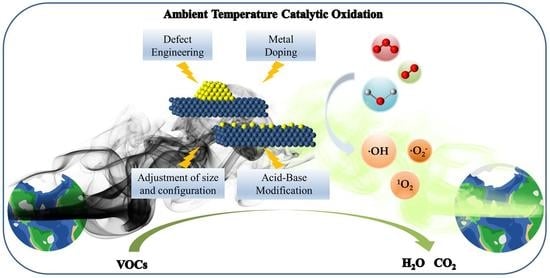
Review on Catalytic Oxidation of VOCs at Ambient Temperature
Abstract
1. Introduction
2. Catalyst Sorts and Catalytic Elimination of VOCs
2.1. Aromatic Hydrocarbon Elimination
2.2. Aliphatic Hydrocarbon Elimination
2.3. Oxygen-Containing Elimination
2.4. Chlorine/Sulfur-Containing Elimination
| Catalyst | Reaction Mixture | Reaction Conditions | Reaction Temperature | Conversion | CO2 Selectivity | Ref. | |
|---|---|---|---|---|---|---|---|
| Aromatics | |||||||
| Benzene | MnO2/ZSM-5 | C6H6: 30 ppm O3: 450 ppm | GHSV 1: 48,000 h−1 RH 3: 50% | 25 °C | 100% | - | [38] |
| MnOx/AC | C6H6: 30 ppm O3: 300 ppm | GHSV: 28,000 h−1 RH: 50% | 25 ± 1 °C | 100% | 61.9% | [39] | |
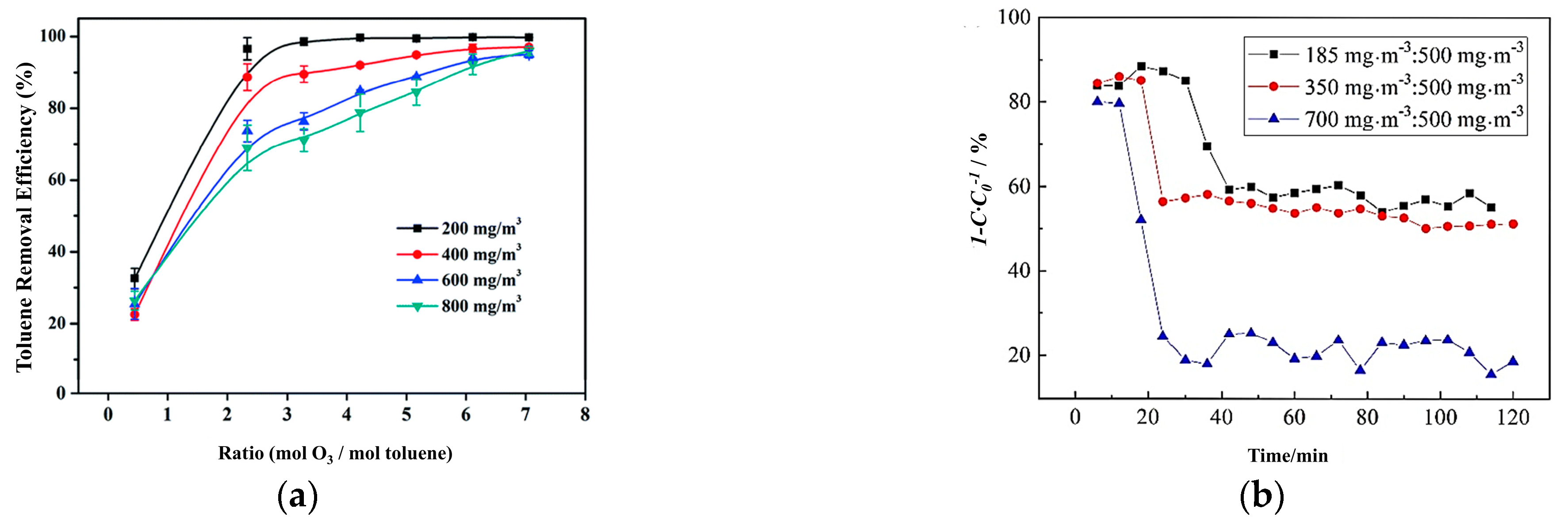
| Toluene | Pt/MnOx-T | C7H8: 30 ppm O3: 300 ppm | WHSV 2: 60 L g−1 h−1 | RT 4 | 98% | 90% | [40] |
| Y/La-MnO2 | C7H8: 10 ppm | WHSV: 60 L g−1 h−1 | 40 °C | 100% | 100% | [4] | |
| Pt/TiO2 | C7H8: 250 mg m−3 O3: 2600 mg m−3 | GHSV: 30,000 h−1 | RT | 65% | 100% | [41] | |
| Mn/Al2O3 | C7H8: 50 ppm O3: 1000 ppm | WHSV: 120 L g−1 h−1 | RT | 98.94% | ~47% | [42] | |
| MnOx/Al2O3 | C7H8: 120 ppm O3: 1000 ppm | WHSV: 300 L g−1 h−1 | 25 °C | 80% | - | [43] | |
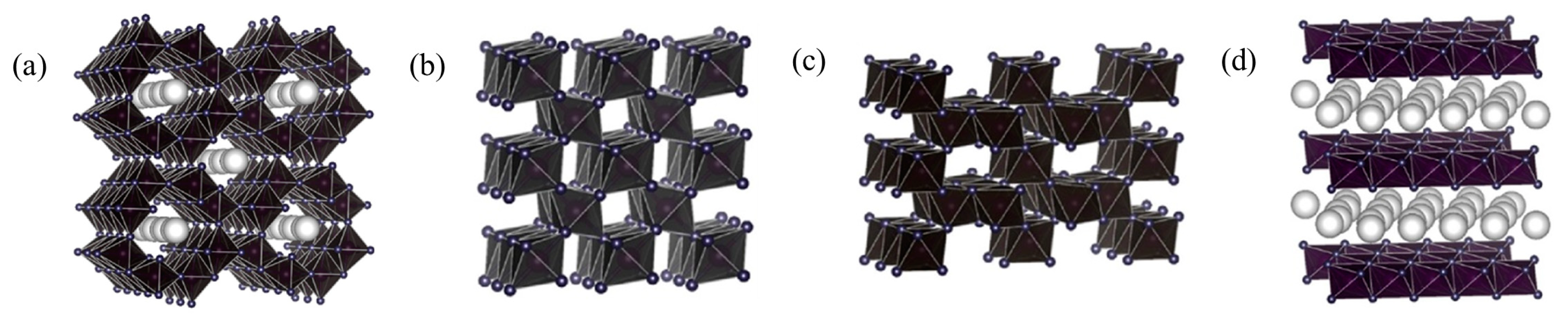
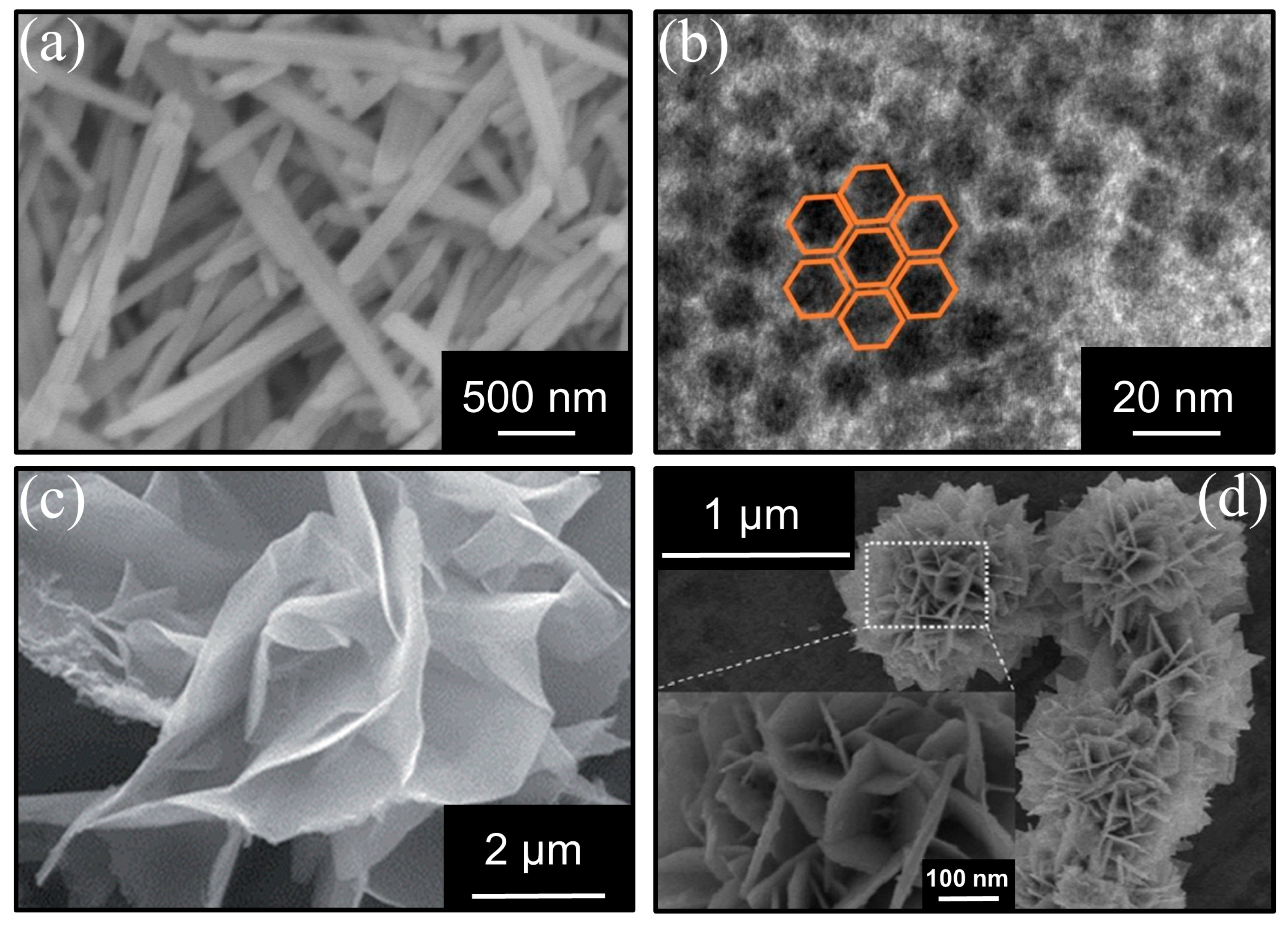
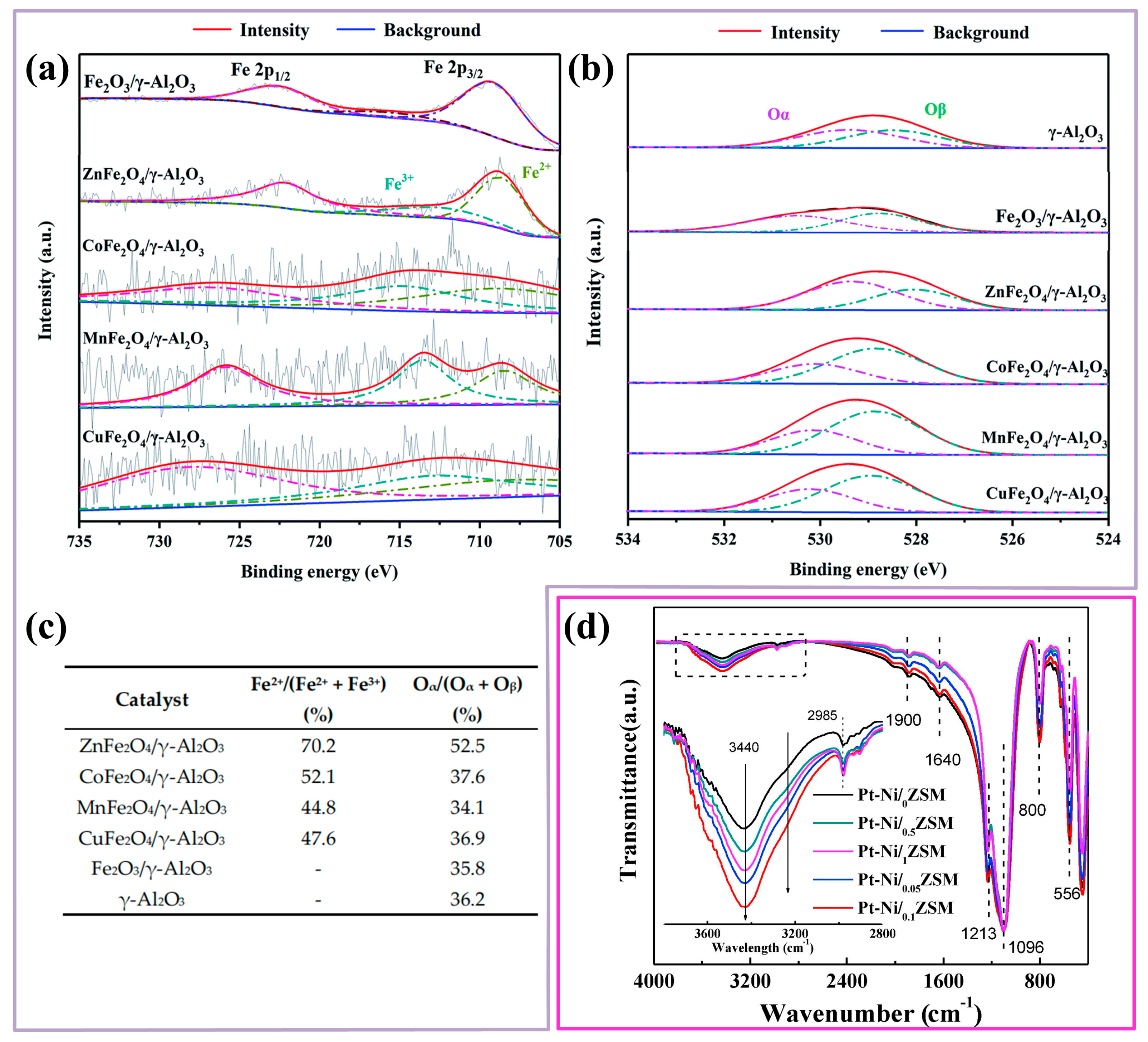
| ZnFe2O4/ɣ-Al2O3 | C7H8: 49 ppm O3: 97 ppm | GHSV: 1500 h−1 | 20 °C | ~100% | 100% | [44] | |
| MnOx/MCM-41 | C7H8: 100 ppm O3: 1000 ppm | - | 20 °C | 100% | 73% | [45] | |
| Pt-Ce/BEA | C7H8: 22 ppm O3: 300 ppm | WHSV: 60 L g−1 h−1 | 30 °C | 95% | 56% | [46] | |
| 3D-NiO1-δ/NF | C7H8: 100 ppm O3: 350 ppm | GHSV: 10,000 h−1 RH: 50% | 25 °C | 100% | 70.6% | [47] | |
| Mn/AC-0.43FN | C7H8: 100 ppm O3: 2100 ppm | WHSV: 300 L g−1 h−1 | 30 °C | ~87% | 36% | [48] | |
| Mn(OH)F/Ni | C7H8: 100 ppm O3: 1000 ppm | GHSV: 6000 h−1 RH: 50% | 25 °C | 100% | - | [49] | |
| Aliphatic hydrocarbons | |||||||
| Ethylene | Ag/ZSM-5 | C2H2: 100 ppm | WHSV: 7500 mL g−1 h−1 | RT | 100% | 100% | [50] |
| 5%Ag/ZSM-5 | C2H2: 100 ppm | WHSV: 7500 mL g−1 h−1 | RT | 100% | 100% | [51] | |
| Pt/F-ZSM-5 | C2H2: 100 ppm | WHSV: 7500 mL g−1 h−1 | 25 °C | 100% | 100% | [52] | |
| OVOCs | |||||||
| Methanol | Pt/FeOx | CH4O: 380 ppm O3: 200 ppm | GHSV: 24,000 h−1 RH: 30% | 30 °C | 100% | 100% | [53] |
| Isopropanol | Na-Pt/TiO2 | C3H8O: 75.00 ppm O3: 105.30 ppm | WHSV: 16,920 mL g−1 h−1 | 35 °C | 100% | 91.34% | [54] |
| Oxalic acid | Mn-C@Fe | H2C2O4: 40 mg L−1 O3: 0.36 mM | WHSV: 120 L g−1 h−1 | 25 ± 0.5 °C | 90.9% | - | [55] |
| Ethyl acetate | Pd/ACF-fiber | C4H8O2: 350 mg/m3 O3: 1000 mg/m3 | GHSV: 30,000 h−1 | 30~50 °C | 60% | 92% | [56] |
| β-MnO2 | C4H8O2: 500 ppm O3: 1000 ppm | GHSV: 10,000 h−1 | RT | 100 | 70% | [57] | |
| Formaldehyde | Pd/TiO2 | HCHO: 140 ppm | GHSV: 95,000 h−1 | RT | 100% | - | [58] |
| α-MnO2 | HCHO: 60 ppm O3: 230 ppm | WHSV: 120 L g−1 h−1 RH: 50% | RT | 90% | 100% | [59] | |
| 3D-MnO2 | HCHO: 100 ppm | WHSV: 180 L g−1 h−1 RH: 65% | RT | 45% | 100% | [60] | |
| MnOx | HCHO: 80 ppm | GHSV: 180,000 h−1 RH 33% | RT | 93% | - | [61] | |
| MnO@C | HCHO: 60 ppm O3: 180 ppm | WHSV: 60 L g−1 h−1 RH: 50% | 30 °C | 100% | 100% | [62] | |
| MnCeOx | HCHO: 55 ppm O3: 165 ppm | WHSV: 210,000 h−1 RH: 60% | 25 °C | 100 | 70% | [63] | |
| 3D-NiCo2O4 | HCHO: 200 ppm | GHSV: 60,000 h−1 | 25 °C | 95.30% | 100% | [64] | |
| MnOx/CVC-TiO2 | HCHO: 45 ppm O3: 225 ppm | GHSV: 20,000 h−1 RH: 50~80% | 25 °C | 100% | 100% | [65] | |
| Au/Co-MgAl LDH | HCHO: 80 ppm | WHSV: 18 L g−1 h−1 | RT | 96.2% | 100.00% | [66] | |
| Pt/NiAl-LDHs | HCHO: 223 ppm | - | RT | 92.40% | 100% | [67] | |
| Pt/SnOx | HCHO: 172 ppm | - | RT | 87% | 100% | [68] | |
| K-Pt/NaY | HCHO: 300 ppm | RH: 35~51% | RT | 98% | 100% | [69] | |
| Pt/NiO | HCHO: 200 ppm | - | RT | 89% | - | [70] | |
| NiO | HCHO: 60 ppm O3: 180 ppm | WHSV: 60 L g−1 h−1 RH: 50% | 30 °C | 100% | 100% | [71] | |
| Au-Ce3Co/GA | HCHO: 50 ppm | WHSV: 20 L g−1 h−1 | 60 °C | 100% | 100% | [72] | |
| Pt/Co3O4 | HCHO: 210 ppm | - | RT | 91.40% | 100% | [73] | |
| Pt-Ni/ZSM-5 | HCHO: 50 ppm | WHSV: 30 L g−1 h−1 RH: 35% | 30 °C | 90% | 100% | [74] | |
| Pt/Al2O3 | HCHO: 160 ppm | - | RT | 62.50% | 100% | [75] | |
| Pt/NiO | HCHO: 200 ppm | RH: 50% | RT | 90% | 100% | [76] | |
| Acetone | MnOx/Al2O3 | C3H6O: 120 ppm O3: 1150 ppm | WHSV: 600 L g−1 h−1 | RT | 95% | - | [77] |
| Mn-Co/γ-Al2O3 | C3H6O: 150 ppm O3: 1200 ppm | WHSV: 231 L g−1 h−1 | RT | 84% | - | [78] | |
| CoO/γ-Al2O3 | C3H6O: 150 ppm O3: 1200 ppm | WHSV: 230 L g−1 h−1 | RT | 85% | - | [79] | |
| Cl, S-containing VOCs | |||||||
| Chlorobenzene | MnOx/CNT | C6H5Cl: 50 ppm O3: 2300 ppm | WHSV: 24 L g−1 h−1 | 80 °C | 95% | 100% | [80] |
| Methyl mercaptan | α-MnO2 | CH3SH: 70 ppm O3: ~933 ppm | WHSV: 120 L g−1 h−1 | RT | 100% | - | [81] |
| Ag/MnO2 | CH3SH: 70 ppm O3: 700 ppm | WHSV: 60 L g−1 h−1 | RT | 95% | - | [82] | |
| CuO/Vo-MnO2 | CH3SH: 80 ppm O3: 200 ppm | GHSV: 60,000 h−1 | RT | 99% | - | [83] | |
3. Reaction Mechanism for VOC Catalytic Oxidation
4. Optimization of Catalytic Activity
4.1. Nanoparticle Catalysts
4.1.1. Nanoscale Metal Oxide Catalysts
4.1.2. Supported Metal Nanoparticle Catalyst
- Support effect
- Metal size effect
- Metal loading
- Polymetallic doping
4.2. Metal Cluster Catalysts
4.3. Single-Atom Catalysts
4.4. Regulation of Reaction Conditions
4.4.1. Initial Concentration
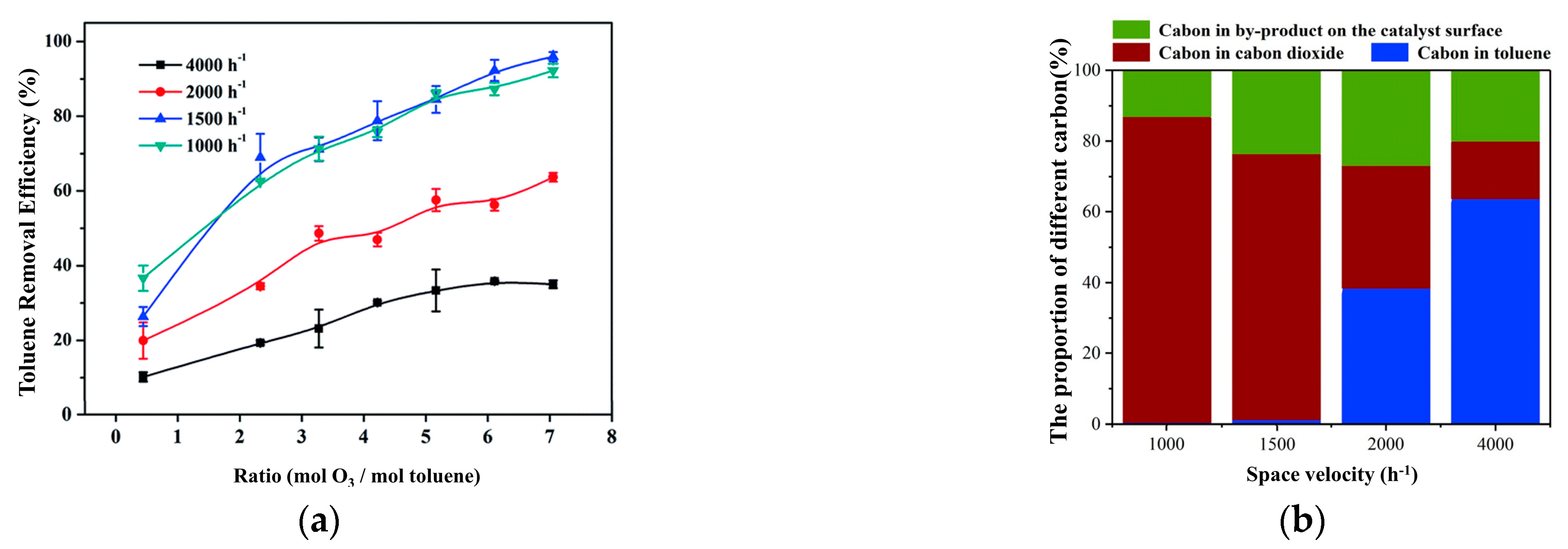
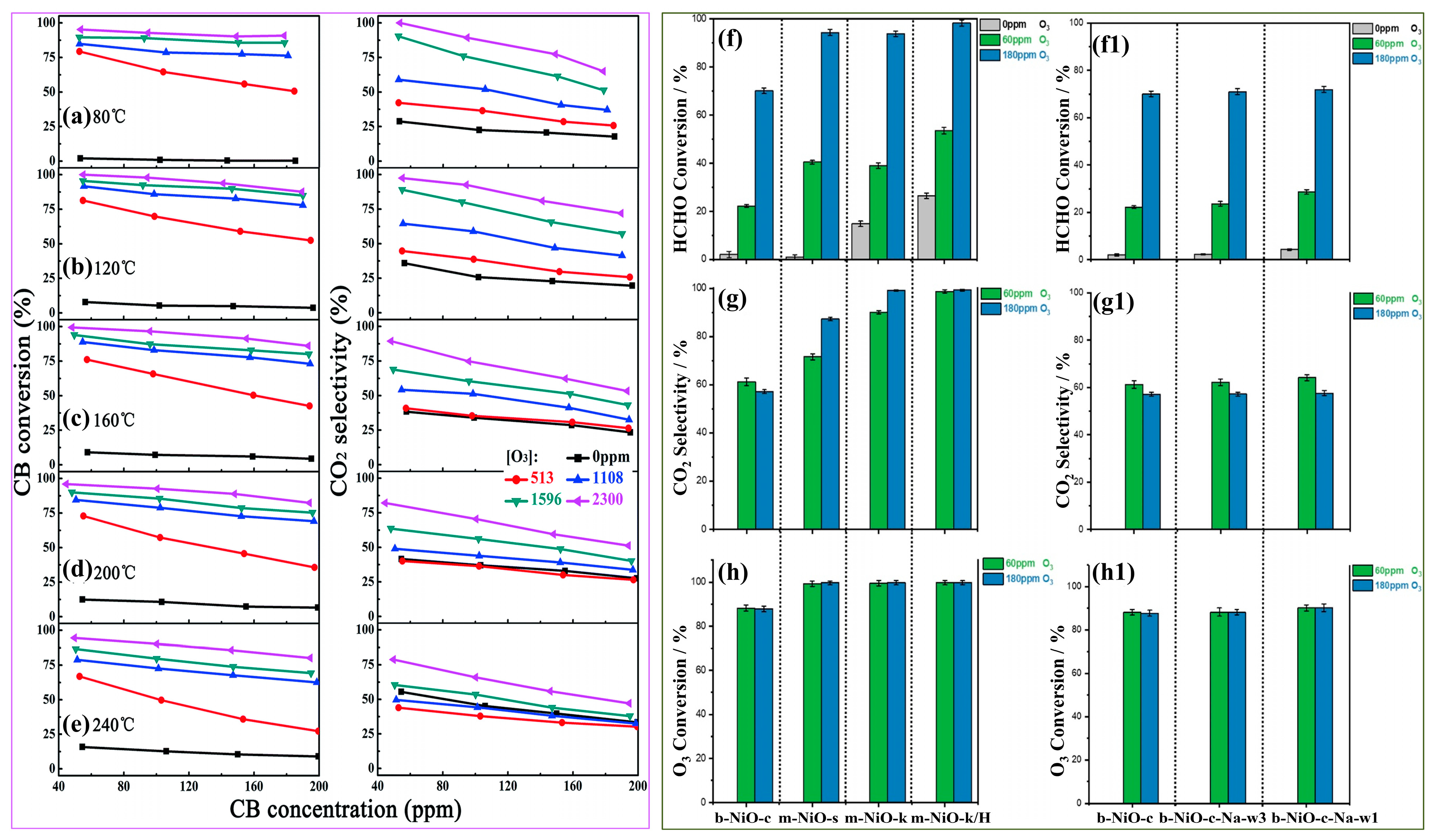
4.4.2. Space Velocity
4.4.3. Oxidation Atmosphere
4.4.4. Humidity
5. Conclusions and Outlook
- Nanoparticle catalysts are widely used in ambient temperature catalytic oxidation, which often uses a variety of silicon-based materials, activated carbon, and metal oxides as catalyst supports, and noble metals, transition metals, and transition metal oxides as active components.
- The oxidation activity of the ambient temperature catalytic reaction mainly comes from the reactive oxygen species (·O2−,·OH, 1O2), which are mainly formed by the activation of ozone, oxygen, and water in metal active sites, defects, and acid/base sites.
- Configuration optimization, metal doping, defect engineering, acid/base modification, and other control methods are common strategies for preparing catalysts. Configuration optimization can provide a larger specific surface area, more active facets, more unsaturated coordination sites, and higher electron migration ability. In addition, metal doping, defect engineering, and acid/base modification can effectively adjust the coordination environment of catalysts and affect the metal charge of the active centers, thereby improving the dispersion, stability, and catalytic activity of the active sites.
- The catalytic oxidation reaction at ambient temperature is an extremely complex process. The initial concentration of pollutants, reaction space velocity, oxidation atmosphere, and relative humidity are important factors affecting the catalytic reaction efficiency. The space velocity and the initial concentration of pollutants in the reaction conditions are mainly limited by the activation performance of catalysts. In contrast, the oxidation atmosphere and humidity affect the activity of catalysts during the reaction of ambient temperature catalytic oxidation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.K.; Shaikh, A.R.; Mohammad, M.H. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar]
- Kwok, W.S.; Wenxin, L. A Review on Catalytic Nanomaterials for Volatile Organic Compounds VOC Removal and Their Applications for Healthy Buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Xu, T.; Zhang, P. Atomically Dispersed Y or La on Birnessite-Type MnO2 for the Catalytic Decomposition of Low-Concentration Toluene at Room Temperature. ACS Appl. Mater. Interfaces 2021, 13, 17532–17542. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Suh, S.S. Effects of Operating Conditions on Adsorption and Desorption of Benzene in TSA Process Using Activated Carbon and Zeolite 13X. Appl. Chem. Eng. 2018, 29, 594–603. [Google Scholar]
- Ali Rangkooy, H.; Pour, M.N.; Dehaghi, B.F. Efficiency evaluation of the photocatalytic degradation of zinc oxide nanoparticles immobilized on modified zeolites in the removal of styrene vapor from air. Korean J. Chem. Eng. 2017, 34, 3142–3149. [Google Scholar] [CrossRef]
- Xie, H.; Du, Q.; Li, H.; Zhou, G.; Chen, S.; Jiao, Z.; Ren, J. Catalytic combustion of volatile aromatic compounds over CuO-CeO2 catalyst. Korean J. Chem. Eng. 2017, 34, 1944–1951. [Google Scholar] [CrossRef]
- Shim, W.G.; Nah, J.W.; Jung, H.-Y.; Park, Y.-K.; Jung, S.C.; Kim, S.C. Recycling of red mud as a catalyst for complete oxidation of benzene. J. Ind. Eng. Chem. 2018, 60, 259–267. [Google Scholar] [CrossRef]
- Ali, A.M.; Daous, M.A.; Arafat, A.; AlZahrani, A.A.; Alhamed, Y.; Tuerdimaimaiti, A.; Petrov, L.A. Effect of Au Precursor and Support on the Catalytic Activity of the Nano-Au-Catalysts for Propane Complete Oxidation. J. Nanomater. 2015, 2015, 901439. [Google Scholar] [CrossRef]
- Lou, B.Z.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.L.; Zhao, Y.W.; Zhao, W.C.; Jiang, Y.Q.; Rui, Y.K. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: Current advancement and future prospectives. J. Clean Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- Liu, B.; Ji, J.; Zhang, B.; Huang, W.; Gan, Y.; Leung, D.Y.C.; Huang, H. Catalytic ozonation of VOCs at low temperature: A comprehensive review. J. Hazard. Mater. 2021, 422, 126847. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, Y.; Deng, J.; Zhang, K.; Hou, Z.; Zhao, X.; Zhang, X.; Zhang, K.; Wei, R.; Dai, H. Coupled Palladium–Tungsten Bimetallic Nanosheets/TiO2 Hybrids with Enhanced Catalytic Activity and Stability for the Oxidative Removal of Benzene. Environ. Sci. Technol. 2019, 53, 5926–5935. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Tang, W.; Guo, Y.; Binder, A.; Kyriakidou, E.A.; Toops, T.J.; Wang, S.; Ren, Z.; Hoang, S.; Gao, P.-X. Understanding low temperature oxidation activity of nanoarray-based monolithic catalysts: From performance observation to structural and chemical insights. Emission Control Sci. Tech. 2017, 3, 18–36. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.L.; Zhang, P.Y. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Guo, J.H.; Lin, C.X.; Jiang, C.J.; Zhang, P.Y. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Vellingiri, K.; Vikrant, K.; Kumar, V.; Kim, K.H. Advances in thermocatalytic and photocatalytic techniques for the room/low temperature oxidative removal of formaldehyde in air. Chem. Eng. J. 2020, 399, 125759. [Google Scholar] [CrossRef]
- Wu, P.; Jin, X.J.; Qiu, Y.C.; Ye, D.Q. Recent Progress of Thermocatalytic and Photo/Thermocatalytic Oxidation for VOCs Purification over Manganese-based Oxide Catalysts. Environ. Sci. Technol. 2021, 55, 4268–4286. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B 2021, 281, 119447. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Zhixiang, Z.; Zheng, J.; Wenfeng, S. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal Today 2016, 264, 270–278. [Google Scholar]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, S.; Mehl, M.; Ranzi, E.; Schweiger, D.; Schedler, J. Improve efficiency of thermal regenerators and VOCs abatement systems: An experimental and modeling study. Exp. Therm. Fluid Sci. 2007, 31, 403–411. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Tang, W.; Xiao, W.; Wang, S.; Ren, Z.; Ding, J.; Gao, P.-X. Boosting catalytic propane oxidation over PGM-free Co3O4 nanocrystal aggregates through chemical leaching: A comparative study with Pt and Pd based catalysts. Appl. Catal. B 2018, 226, 585–595. [Google Scholar] [CrossRef]
- Hartikainen, A.; Yli-Pirilä, P.; Tiitta, P.; Leskinen, A.; Kortelainen, M.; Orasche, J.; Schnelle-Kreis, J.; Lehtinen, K.E.J.; Zimmermann, R.; Jokiniemi, J.; et al. Volatile Organic Compounds from Logwood Combustion: Emissions and Transformation under Dark and Photochemical Aging Conditions in a Smog Chamber. Environ. Sci. Technol. 2018, 52, 4979–4988. [Google Scholar] [CrossRef]
- Fang, Y.; Li, L.; Yang, J.; Hoang, S.; Wang, L.; Xu, J.; Yang, W.; Pan, C.; Zhu, Y.; Deng, H.; et al. Engineering the Nucleophilic Active Oxygen Species in CuTiOx for Efficient Low-Temperature Propene Combustion. Environ. Sci. Technol. 2020, 54, 15476–15488. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.M.; Chen, M.Q.; Shi, C. CoMnxOy nanosheets with molecular-scale homogeneity: An excellent catalyst for toluene combustion. Catal. Sci. Technol. 2018, 8, 459–471. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Drozdek, M.; Michalik, M.; Gac, W.; Gajewska, M.; Kuśtrowski, P. Catalytic activity of Pt species variously dispersed on hollow ZrO2 spheres in combustion of volatile organic compounds. Appl. Surf. Sci. 2020, 513, 145788. [Google Scholar] [CrossRef]
- Losch, P.; Huang, W.; Vozniuk, O.; Goodman, E.D.; Schmidt, W.; Cargnello, M. Modular Pd/Zeolite Composites Demonstrating the Key Role of Support Hydrophobic/Hydrophilic Character in Methane Catalytic Combustion. ACS Catal. 2019, 9, 4742–4753. [Google Scholar] [CrossRef]
- Legreid, G.; Reimann, S.; Steinbacher, M.; Staehelin, J.; Young, D.; Stemmler, K. Measurements of OVOCs and NMHCs in a Swiss Highway Tunnel for Estimation of Road Transport Emissions. Environ. Sci. Technol. 2007, 41, 7060–7066. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, B.; Li, X.; Shao, M.; Lu, S.; Li, Y.; Chang, C.C.; Wang, Z.; Hu, W.; Huang, X.; et al. Impact of pollution controls in Beijing on atmospheric oxygenated volatile organic compounds (OVOCs) during the 2008 Olympic Games: Observation and modeling implications. Atmos. Chem. Phys. 2015, 15, 3045–3062. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Hao, H.; Chen, D.; Liu, J.; Yu, J.; Lu, J.; Liu, F.; He, S.; Li, K.; Luo, Y. Effects of rare-earth (Nd, Er and Y) doping on catalytic performance of HZSM-5 zeolite catalysts for methyl mercaptan (CH3SH) decomposition. Appl. Catal. A-Gen. 2017, 533, 66–74. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Lago, R.M.; Fabris, J.D.; Sapag, K. Catalytic oxidation of aromatic VOCs with Cr or Pd-impregnated Al-pillared bentonite: Byproduct formation and deactivation studies. Appl. Clay Sci. 2008, 39, 218–222. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, W.; Wang, X.; Lu, G. Sandwich-structured CeO2@ZSM-5 hybrid composites for catalytic oxidation of 1,2-dichloroethane: An integrated solution to coking and chlorine poisoning deactivation. Appl. Catal. B 2017, 203, 31–42. [Google Scholar] [CrossRef]
- He, D.; Wan, G.; Hao, H.; Chen, D.; Lu, J.; Zhang, L.; Liu, F.; Zhong, L.; He, S.; Luo, Y. Microwave-assisted rapid synthesis of CeO2 nanoparticles and its desulfurization processes for CH3SH catalytic decomposition. Chem. Eng. J. 2016, 289, 161–169. [Google Scholar] [CrossRef]
- Lu, J.; Hao, H.; Zhang, L.; Xu, Z.; Zhong, L.; Zhao, Y.; He, D.; Liu, J.; Chen, D.; Pu, H.; et al. The investigation of the role of basic lanthanum (La) species on the improvement of catalytic activity and stability of HZSM-5 material for eliminating methanethiol-(CH3SH). Appl. Catal. B 2018, 237, 185–197. [Google Scholar] [CrossRef]
- Aghbolaghy, M.; Soltan, J.; Chen, N. Role of Surface Carboxylates in the Gas Phase Ozone-Assisted Catalytic Oxidation of Toluene. Catal. Lett. 2017, 147, 2421–2433. [Google Scholar] [CrossRef]
- Ruimei, F.; Haibao, H.; Wenjun, H.; Jian, J.; Qiuyu, F.; Yajie, S.; Yujie, Z.; Gaoyuan, L.; Ruijie, X. Influence of peracetic acid modification on the physicochemical properties of activated carbon and its performance in the ozone-catalytic oxidation of gaseous benzene. Appl. Surf. Sci. 2017, 420, 905–910. [Google Scholar]
- Xu, Z.; Mo, S.; Li, Y.; Zhang, Y.; Wu, J.; Fu, M.; Niu, X.; Hu, Y.; Ye, D. Pt/MnOx for toluene mineralization via ozonation catalysis at low temperature: SMSI optimization of surface oxygen species. Chemosphere 2022, 286, 131754. [Google Scholar] [CrossRef]
- Lui, R.; Tian, M.; Shang, W.; Cui, J.; Zhao, D.; Liu, S.; Zhao, Y.; Ding, H.; Fu, J. Normal Temperature Catalytic Degradation of Toluene over Pt/TiO2. Environ. Technol. 2020, 43, 2047–2058. [Google Scholar]
- Ryu, H.W.; Song, M.Y.; Park, J.S.; Kim, J.M.; Jung, S.-C.; Song, J.; Kim, B.-J.; Park, Y.-K. Removal of toluene using ozone at room temperature over mesoporous Mn/Al2O3 catalysts. Environ. Res. 2019, 172, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, M.; Soltan, J.; Chen, N. Synthesis of MnOx/Al2O3 Catalyst by Polyol Method and Its Application in Room Temperature Ozonation of Toluene in Air. Catal. Lett. 2021, 151, 1418–1432. [Google Scholar] [CrossRef]
- Hongbin, J.; Xiaochen, X.; Rao, Z.; Yun, Z.; Jie, C.; Fenglin, Y. Nano ferrites (AFe2O4, A = Zn, Co, Mn, Cu as efficient catalysts for catalytic ozonation of toluene. RSC Adv. 2020, 10, 5116–5128. [Google Scholar]
- Dong, Y.; Sun, J.; Ma, X.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Li, W. Study on the synergy effect of MnOx and support on catalytic ozonation of toluene. Chemosphere 2022, 303, 134991. [Google Scholar] [CrossRef] [PubMed]
- Hailin, X.; Junliang, W.; Xueqing, W.; Jialing, W.; Shengpeng, M.; Mingli, F.; Limin, C.; Daiqi, Y. Ozone-enhanced deep catalytic oxidation of toluene over a platinum-ceria-supported BEA zeolite catalyst. Mol. Catal. 2018, 460, 7–15. [Google Scholar] [CrossRef]
- Tian, S.; Zhan, S.; Lou, Z.; Zhu, J.; Feng, J.; Xiong, Y. Electrodeposition synthesis of 3D-NiO1-δ flowers grown on Ni foam monolithic catalysts for efficient catalytic ozonation of VOCs. J. Catal. 2021, 398, 1–13. [Google Scholar] [CrossRef]
- Xu, P.-L.; Wei, T.; Yue, H.-Y.; Wen, Y.-C.; Wei, Y.; Guo, T.-J.; Li, S.-J.; Li, W.; Wang, X.-Q. Effect of different nitric acid concentrations on manganese/activated carbon-modified catalysts for the catalytic ozonation of toluene. Catal. Sci. Technol. 2020, 1, 6729–6737. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Sun, J.X.; Tian, S.H.; Yang, J.; Feng, J.X.; Xiong, Y. Catalytic activity and mechanism of fluorinated MgO film supported on 3D nickel mesh for ozonation of gaseous toluene. Environ. Sci.-Nano 2020, 7, 2723–2734. [Google Scholar] [CrossRef]
- Yang, H.; Ma, C.; Zhang, X.; Li, Y.; Cheng, J.; Hao, Z. Understanding the Active Sites of Ag/Zeolites and Deactivation Mechanism of Ethylene Catalytic Oxidation at Room Temperature. ACS Catal. 2018, 8, 1248–1258. [Google Scholar] [CrossRef]
- Hongling, Y.; Chunyan, M.; Yang, L.; Junhui, W.; Xin, Z.; Gang, W.; Nanli, Q.; Yonggang, S.; Jie, C.; Zhengping, H. Synthesis, characterization and evaluations of the Ag/ZSM-5 for ethylene oxidation at room temperature: Investigating the effect of water and deactivation. Chem. Eng. J. 2018, 347, 808–818. [Google Scholar] [CrossRef]
- Yang, H.L.; Ma, C.Y.; Wang, G.; Sun, Y.G.; Cheng, J.; Zhang, Z.S.; Zhang, X.; Hao, Z.P. Fluorine-enhanced Pt/ZSM-5 catalysts for low-temperature oxidation of ethylene. Catal. Sci. Technol. 2018, 8, 1988–1996. [Google Scholar] [CrossRef]
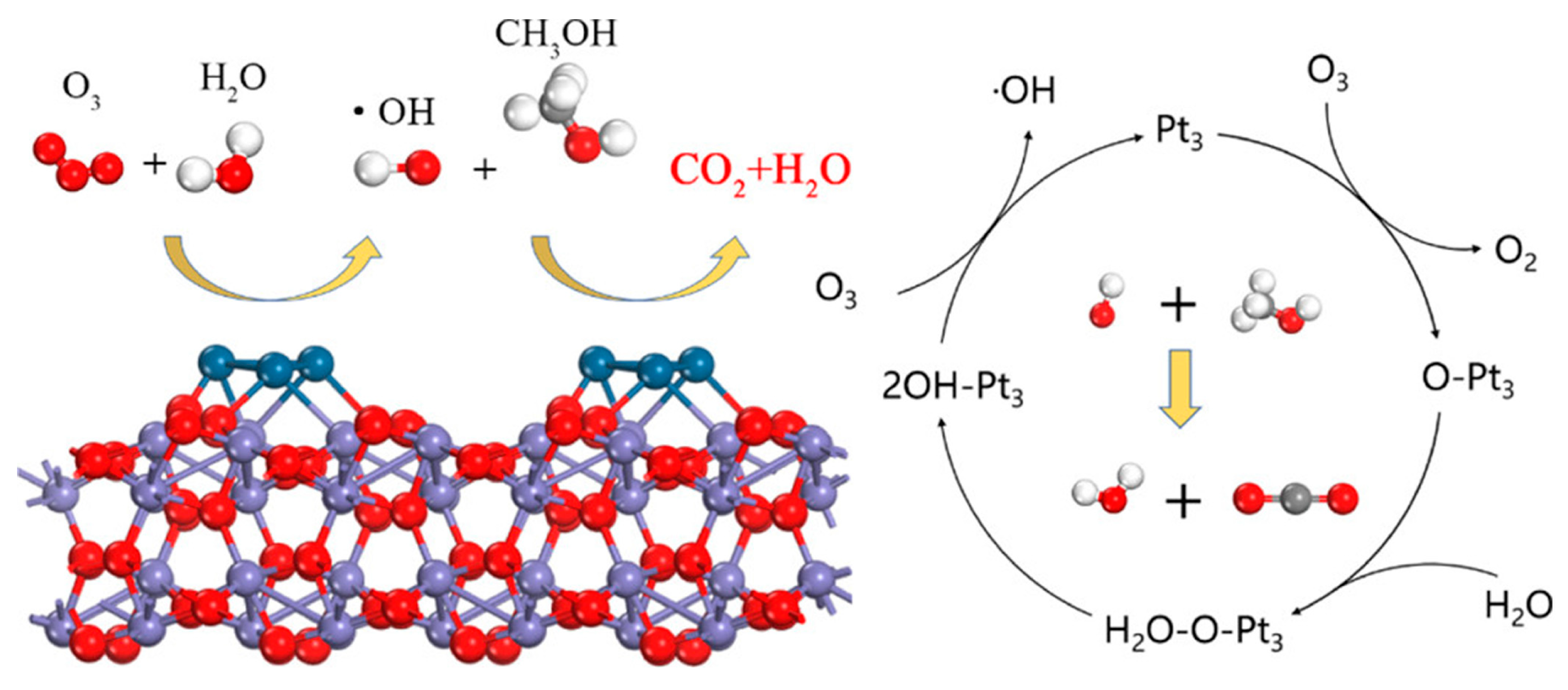
- Tian, M.; Liu, S.; Wang, L.; Ding, H.; Zhao, D.; Wang, Y.; Cui, J.; Fu, J.; Shang, J.; Li, G.K. Complete Degradation of Gaseous Methanol over Pt/FeOx Catalysts by Normal Temperature Catalytic Ozonation. Environ. Sci. Technol. 2020, 54, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hao, Z.; Wang, Y.; Xue, L.; Xue, H.; Tu, L.; Hao, L.; Tian, M.; Guo, J.; Zhao, D.; et al. Mechanism of ozone-assisted catalytic oxidation of isopropanol over single-atom platinum catalysts at ambient temperature. Chem. Eng. J. 2022, 446, 136989. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Wang, D. A core-shell Mn–C@Fe nanocatalyst under ozone activation for efficient organic degradation: Surface-mediated non-radical oxidation. Chemosphere 2021, 281, 130895. [Google Scholar] [CrossRef]
- Jiahao, C.; Shejiang, L.; Hua, X.; Xianqin, W.; Ziquan, H.; Rui, L.; Wei, S.; Dan, Z.; Hui, D. Catalytic ozonation of volatile organic compounds(ethyl acetate)at normal temperature. Chin. J. Chem. Eng. 2021, 32, 159–167. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Z.; Zhang, T.; Chen, X. Catalytic ozonation of ethyl acetate over mesoporous manganese oxides synthesized by a sonochemical method. Asia-Pac. J. Chem. Eng. 2020, 16, e2605. [Google Scholar] [CrossRef]
- Yaobin, L.; Changbin, Z.; Jinzhu, M.; Min, C.; Hua, D.; Hong, H. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation. Appl. Catal. B 2017, 217, 560–569. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Fang, W.; Chen, M.; Zhang, Z.; Jiang, Z.; Shangguan, W.; Einaga, H. Simultaneous catalytic elimination of formaldehyde and ozone over one-dimensional rod-like manganese dioxide at ambient temperature. J. Chem. Technol. Biotechnol. 2019, 94, 2305–2317. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, P.; Yang, Y.; Zhu, L.; Wang, J.; Liu, F. MnO2 Framework for Instantaneous Mineralization of Carcinogenic Airborne Formaldehyde at Room Temperature. ACS Catal. 2017, 7, 1057–1067. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Li, J.; Jiang, C.; Yunus, R.; Kim, J. Room-Temperature Oxidation of Formaldehyde by Layered Manganese Oxide: Effect of Water. Environ. Sci. Technol. 2015, 49, 12372–12379. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Z.; Jiang, Z.; Jiang, Z.; Zhang, Y.; Zhang, Z.; Shangguan, W. Trifunctional C@MnO Catalyst for Enhanced Stable Simultaneously Catalytic Removal of Formaldehyde and Ozone. ACS Catal. 2018, 8, 3164–3180. [Google Scholar] [CrossRef]
- Yi, Z.; Minxia, C.; Zhixiang, Z.; Zhi, J.; Wenfeng, S.; Hisahiro, E. Simultaneously catalytic decomposition of formaldehyde and ozone over manganese cerium oxides at room temperature: Promotional effect of relative humidity on the MnCeOx solid solution. Catal Today 2019, 327, 323–333. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Qiu, W.; Zhao, F.; Tong, Y.; Ji, H. Alkali-modified non-precious metal 3D-NiCo2O4 nanosheets for efficient formaldehyde oxidation at low temperature. J. Mater. Chem. A 2016, 4, 3648–3654. [Google Scholar] [CrossRef]
- Kim, M.; Park, E.; Jurng, J. Oxidation of gaseous formaldehyde with ozone over MnOx/TiO2 catalysts at room temperature (25 °C). Powder Technol. 2018, 325, 368–372. [Google Scholar] [CrossRef]
- Shuping, L.; Chizoba, I.E.; Shuping, Z.; Ya, X.; Shengwei, L. Co-doped MgAl-LDHs nanosheets supported Au nanoparticles for complete catalytic oxidation of HCHO at room temperature. Appl. Surf. Sci. 2019, 487, 260–271. [Google Scholar] [CrossRef]
- Zhaoxiong, Y.; Zhihua, X.; Lin, Y.; Ling, S.; Linyong, H. Hierarchical Ni−Al hydrotalcite supported Pt catalyst for efficient catalytic oxidation of formaldehyde at room temperature. Chin. J. Catal. 2018, 39, 1919–1928. [Google Scholar] [CrossRef]
- Yuanyuan, D.; Shaoqing, S.; Bei, C.; Jiaguo, Y.; Chuanjia, J. Effects of hierarchical structure on the performance of tin oxide-supported platinum catalyst for room-temperature formaldehyde oxidation. Chin. J. Catal. 2017, 38, 199–206. [Google Scholar] [CrossRef]
- Shaoqing, S.; Xi, W.; Changhai, L.; Meicheng, W.; Zhanggao, L.; Shujuan, J. Solid strong base K-Pt/NaY zeolite nano-catalytic system for completed elimination of formaldehyde at room temperature. Appl. Surf. Sci. 2018, 442, 195–203. [Google Scholar] [CrossRef]
- Lifang, Q.; Bei, C.; Wingkei, H.; Gang, L.; Jiaguo, Y. Hierarchical Pt/NiO Hollow Microspheres with Enhanced Catalytic Performance. ChemNanoMat 2015, 1, 58–67. [Google Scholar] [CrossRef]
- Hongchao, W.; Weiqi, G.; Zhi, J.; Ruoou, Y.; Zheng, J.; Yang, P.; Wenfeng, S. New insight into the enhanced activity of ordered mesoporous nickel oxide in formaldehyde catalytic oxidation reactions. J. Catal. 2018, 361, 370–383. [Google Scholar] [CrossRef]
- Qu, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. 3D Gold-Modified Cerium and Cobalt Oxide Catalyst on a Graphene Aerogel for Highly Efficient Catalytic Formaldehyde Oxidation. Small 2018, 15, 1804415. [Google Scholar] [CrossRef] [PubMed]
- Zhaoxiong, Y.; Zhihua, X.; Bei, C.; Chuanjia, J. Co3O4 nanorod-supported Pt with enhanced performance for catalytic HCHO oxidation at room temperature. Appl. Surf. Sci. 2017, 404, 426–434. [Google Scholar] [CrossRef]
- Junjie, D.; Zebao, R.; Pintian, L.; Yilang, L.; Xikun, L.; Hongbing, J. Enhanced formaldehyde oxidation performance over Pt/ZSM-5 through a facile nickel cation modification. Appl. Surf. Sci. 2018, 457, 670–675. [Google Scholar] [CrossRef]
- Xiaofeng, Z.; Jiaguo, Y.; Chuanjia, J.; Bei, C. Enhanced room-temperature HCHO decomposition activity of highly-dispersed Pt/Al2O3 hierarchical microspheres with exposed {110} facets. J. Ind. Eng. Chem. 2016, 45, 197–205. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Cheng, B.; Yu, J.; Jiang, C. Flexible nickel foam decorated with Pt/NiO nanoflakes with oxygen vacancies for enhanced catalytic formaldehyde oxidation at room temperature. Environ. Sci. Nano 2017, 4, 2215–2224. [Google Scholar] [CrossRef]
- Ghavami, M.; Soltan, J. Kinetics and mechanism of catalytic ozonation of acetone in air over MnOx/Al2O3 catalyst. React. Kinet. Mech. Catal. 2021, 133, 953–970. [Google Scholar] [CrossRef]
- Ghavami, M.; Aghbolaghy, M.; Soltan, J.; Chen, N. Room temperature oxidation of acetone by ozone over alumina-supported manganese and cobalt mixed oxides. Front. Chem. Sci. Eng. 2020, 14, 937–947. [Google Scholar] [CrossRef]
- Aghbolaghy, M.; Ghavami, M.; Soltan, J.; Chen, N. Effect of active metal loading on catalyst structure and performance in room temperature oxidation of acetone by ozone. J. Ind. Eng. Chem. 2019, 77, 118–127. [Google Scholar] [CrossRef]
- Jin, D.; Ren, Z.; Ma, Z.; Liu, F.; Yang, H. Low temperature chlorobenzene catalytic oxidation over MnOx/CNTs with the assistance of ozone. RSC Adv. 2015, 5, 15103–15109. [Google Scholar] [CrossRef]
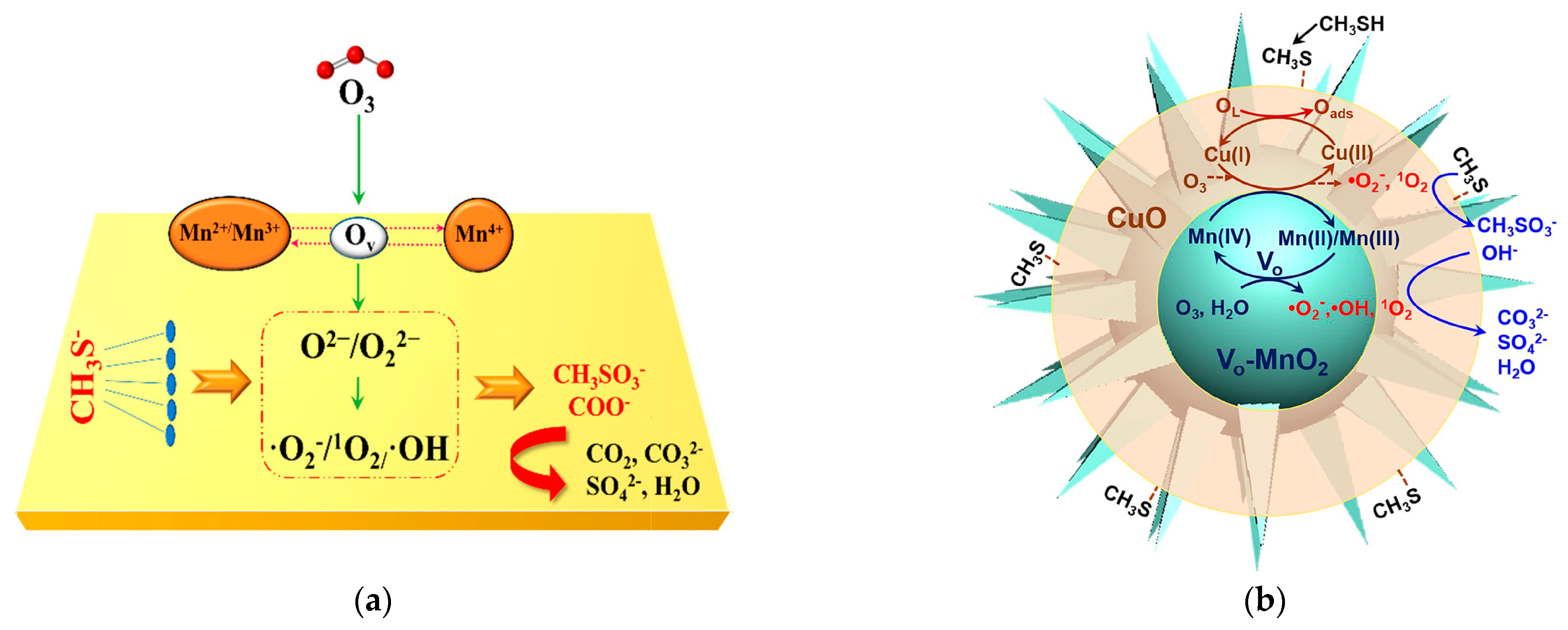
- He, C.; Wang, Y.; Li, Z.; Huang, Y.; Liao, Y.; Xia, D.; Lee, S. Facet Engineered α-MnO2 for Efficient Catalytic Ozonation of Odor CH3SH: Oxygen Vacancy-Induced Active Centers and Catalytic Mechanism. Environ. Sci. Technol. 2020, 54, 12771–12783. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Xu, W.; Wang, Y.; Yang, J.; Huang, Y.; Hu, L.; He, C.; Shu, D.; Dennis, Y.C.L.; Pang, Z. Enhanced Performance and Conversion Pathway for Catalytic Ozonation of Methyl Mercaptan on Single-Atom Ag Deposited Three-Dimensional Ordered Mesoporous MnO2. Environ. Sci. Technol. 2018, 52, 13399–13409. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, Y.; Chen, Y.W.; Xia, D.; Mou, C.Y.; Hu, L.; Zeng, J.; He, C.; Wong, P.K.; Zhu, H.Y. Active site-directed tandem catalysis on CuO/VO-MnO2 for efficient and stable catalytic ozonation of S-VOCs under mild condition. Nano Today 2020, 35, 100944. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Hui, K.N.; Hui, K.S. Novel mechanistic view of catalytic ozonation of gaseous toluene by dual-site kinetic modelling. Chem. Eng. J. 2017, 308, 710–718. [Google Scholar] [CrossRef]
- Reed, C.; Xi, Y.; Oyama, S.T. Distinguishing between reaction intermediates and spectators: A kinetic study of acetone oxidation using ozone on a silica-supported manganese oxide catalyst. J. Catal. 2005, 235, 378–392. [Google Scholar] [CrossRef]
- Yibin, B.; Yafeng, C.; Guiming, J.; Xinmei, H.; Shun, L.; Zuotai, Z. Understanding of Au-CeO2 interface and its role in catalytic oxidation of formaldehyde. Appl. Catal. B 2020, 260, 118138. [Google Scholar]
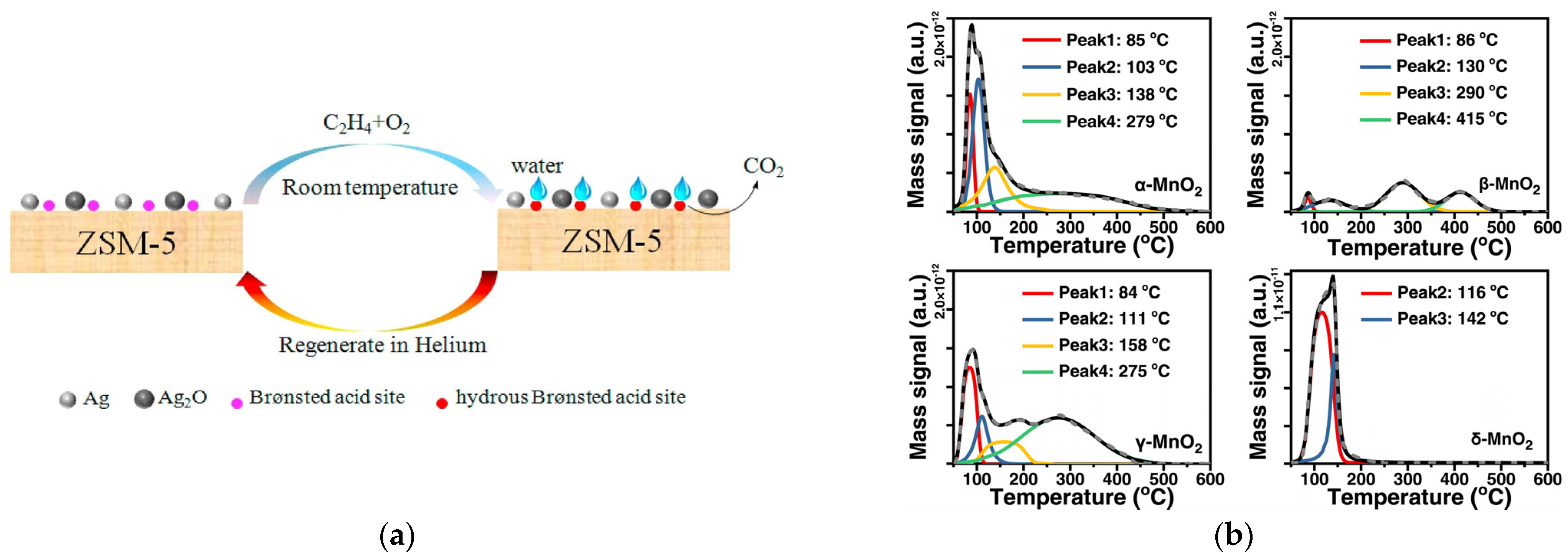
- Jingbo, J.; Pengyi, Z.; Long, C. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Tareque, O.-W.; Qun, L.; Isroil, A.; Jiale, H.; Qingbiao, L. Towards efficient Pd/Mn3O4 catalyst with enhanced acidic sites and low temperature reducibility for Benzene abatement. Mol. Catal. 2019, 477, 110558. [Google Scholar]
- Serguei, A.; Héctor, V.; Marie-Hélène, M.; Claudio, A.Z. Oxidative regeneration of toluene-saturated natural zeolite by gaseous ozone: The influence of zeolite chemical surface characteristics. J. Hazard. Mater. 2014, 274, 212–220. [Google Scholar] [CrossRef]
- Wenhao, Y.; Zi, A.S.; Zhenghao, X.; Weinan, Y.; Yue, P.; Junhua, L. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B 2020, 260, 118150. [Google Scholar] [CrossRef]
- Rong, S.P.; Li, K.Z.; Zhang, P.Y.; Liu, F.; Zhang, J.Y. Potassium associated manganese vacancy in birnessite-type manganese dioxide for airborne formaldehyde oxidation. Catal. Sci. Technol. 2018, 8, 1799–1812. [Google Scholar] [CrossRef]
- Qi, L.; Ho, W.; Wang, J.; Zhang, P.; Yu, J. Enhanced catalytic activity of hierarchically macro-/mesoporous Pt/TiO2 toward room-temperature decomposition of formaldehyde. Catal. Sci. Technol. 2015, 5, 2366–2377. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, W.; Lou, Y.; Ma, J.; Yao, W.; Zong, R.; Zhu, Y. Encapsulate α-MnO2 nanofiber within graphene layer to tune surface electronic structure for efficient ozone decomposition. Nat. Commun. 2021, 12, 4152. [Google Scholar] [CrossRef]
- Jianwei, F.; Xufei, N.; Wei, T.; Peng, Z.; Wei, X.Z.; Dongyuan, Z. Highly dispersed Fe-Ce Mixed Oxide Catalysts Confined in Mesochannels toward Low-Temperature Oxidation Formaldehyde. J. Mater. Chem. A 2020, 8, 17174–17184. [Google Scholar]
- Yun-Peng, X.; Jun-Ling, J.; Guang-Xiong, D.; Xing, L.; Thomas, C.W.M. High-nuclearity silver(I) chalcogenide clusters: A novel class of supramolecular assembly. Coord. Chem. Rev. 2017, 331, 54–72. [Google Scholar]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, L.; Zhang, J. Preparation and properties of polyoxo-titanium clusters. Chin. Sci. Bull. 2018, 63, 2731–2744. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Huiyu, Z.; Shanhong, S.; Xianming, Z.; Ranran, C.; Pengyi, Z. One-pot synthesis of atomically dispersed Pt on MnO2 for efficient catalytic decomposition of toluene at low temperatures. Appl. Catal. B 2019, 257, 117878. [Google Scholar]
- Cui, T.; Li, L.; Ye, C.; Li, X.; Liu, C.; Zhu, S.; Chen, W.; Wang, D. Heterogeneous Single Atom Environmental Catalysis: Fundamentals, Applications, and Opportunities. Adv. Funct. Mater. 2021, 32, 2108381. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zuo, S.; Zhou, W.; Zhang, J.; Lou, X.W.D. Isolated Cobalt Centers on W18O49 Nanowires Perform as a Reaction Switch for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2021, 143, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Jingyue, L. Catalysis by Supported Single Metal Atoms. ACS Catal. 2016, 7, 34–59. [Google Scholar]
- Lyu, J.Z.; Zhu, L.Z.; Burda, C. Considerations to improve adsorption and photocatalysis of low concentration air pollutants on TiO2. Catal Today 2014, 225, 24–33. [Google Scholar] [CrossRef]
- Yuan; Wang; He; Xie; Lin; Zhu; Cen, Ozone Production with Dielectric Barrier Discharge from Air: The Influence of Pulse Polarity. Ozone Sci. Eng. 2018, 40, 494–502. [CrossRef]
- Machniewski, P.; Biń, A.; Kłosek, K. Effectiveness of toluene mineralization by gas-phase oxidation over Co(II)/SiO2 catalyst with ozone. Environ. Technol. 2020, 42, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
- Cuiting, Y.; Guang, M.; Yunhong, P.; Qibin, X.; Junliang, W.; Zhong, L.; Jing, X. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, G.; Sun, Y.; Li, N.; Zhang, Z.; Cheng, J.; Ma, C.; Hao, Z. The positive effect of water on acetaldehyde oxidation depended on the reaction temperature and MnO2 structure. Appl. Catal. B 2022, 303, 120886. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Wang, H.; Zhao, D.; Liu, R.; Liu, S.; Fu, J.; Zhang, Y.; Ding, H. Review on Catalytic Oxidation of VOCs at Ambient Temperature. Int. J. Mol. Sci. 2022, 23, 13739. https://doi.org/10.3390/ijms232213739
Zhao R, Wang H, Zhao D, Liu R, Liu S, Fu J, Zhang Y, Ding H. Review on Catalytic Oxidation of VOCs at Ambient Temperature. International Journal of Molecular Sciences. 2022; 23(22):13739. https://doi.org/10.3390/ijms232213739
Chicago/Turabian StyleZhao, Rui, Han Wang, Dan Zhao, Rui Liu, Shejiang Liu, Jianfeng Fu, Yuxin Zhang, and Hui Ding. 2022. "Review on Catalytic Oxidation of VOCs at Ambient Temperature" International Journal of Molecular Sciences 23, no. 22: 13739. https://doi.org/10.3390/ijms232213739
APA StyleZhao, R., Wang, H., Zhao, D., Liu, R., Liu, S., Fu, J., Zhang, Y., & Ding, H. (2022). Review on Catalytic Oxidation of VOCs at Ambient Temperature. International Journal of Molecular Sciences, 23(22), 13739. https://doi.org/10.3390/ijms232213739