The Relationships among “STAY-GREEN” Trait, Post-Anthesis Assimilate Remobilization, and Grain Yield in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Results
2.1. Yield and Yield Components
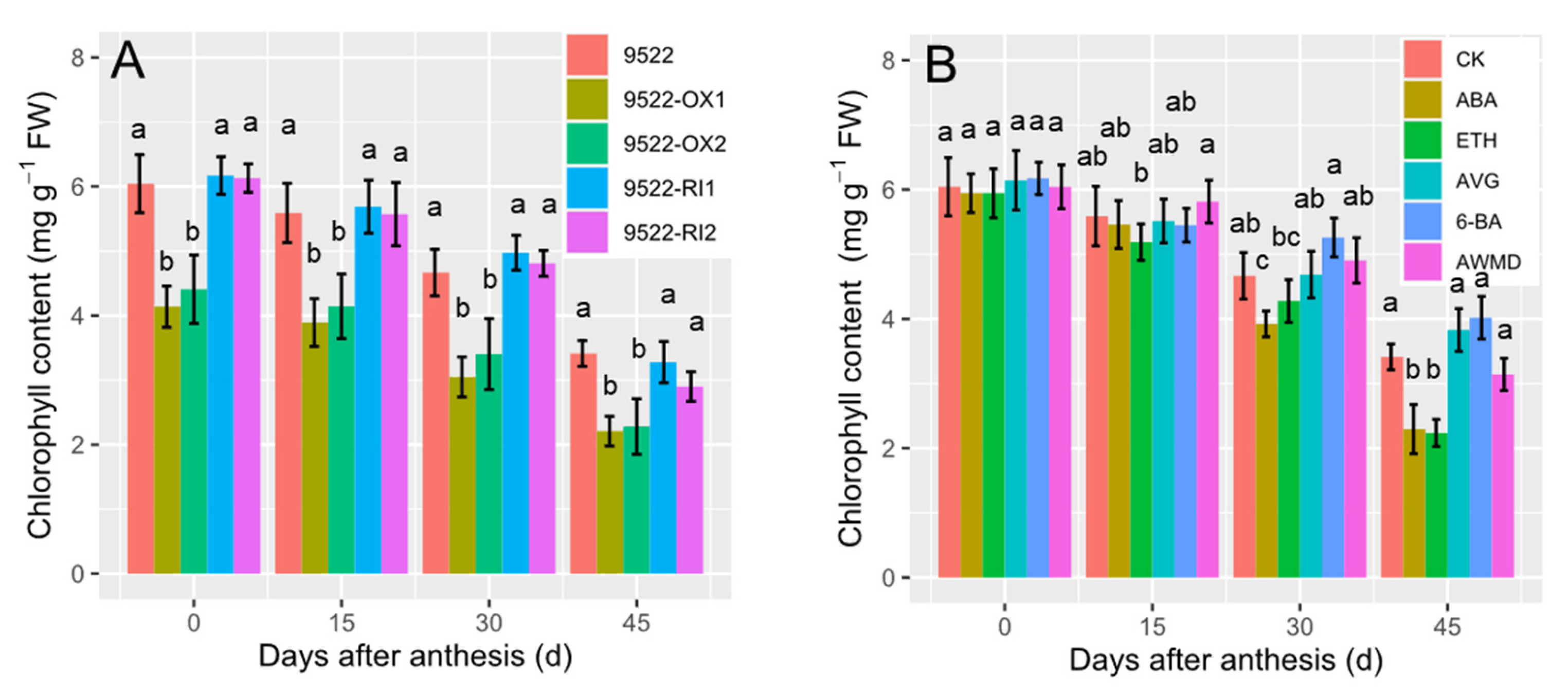
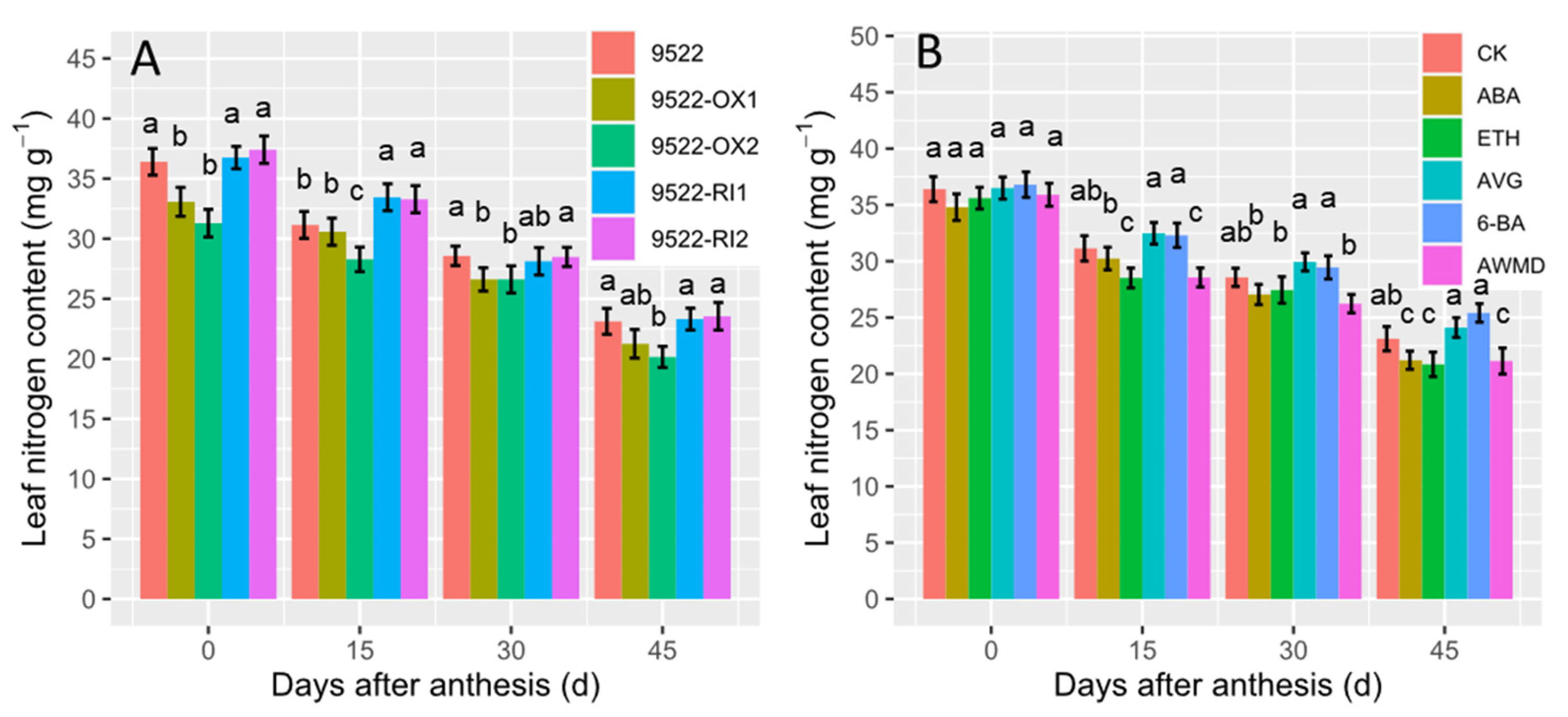
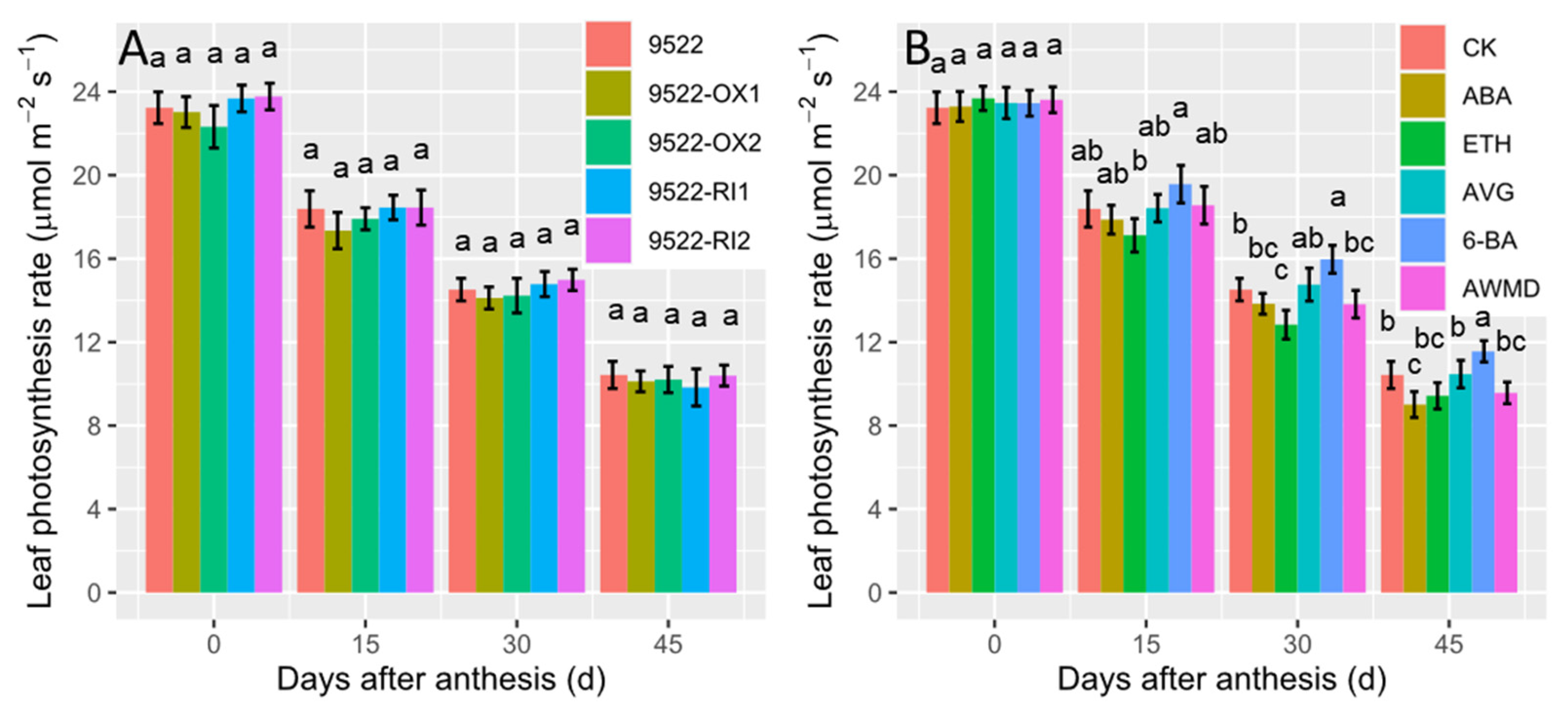
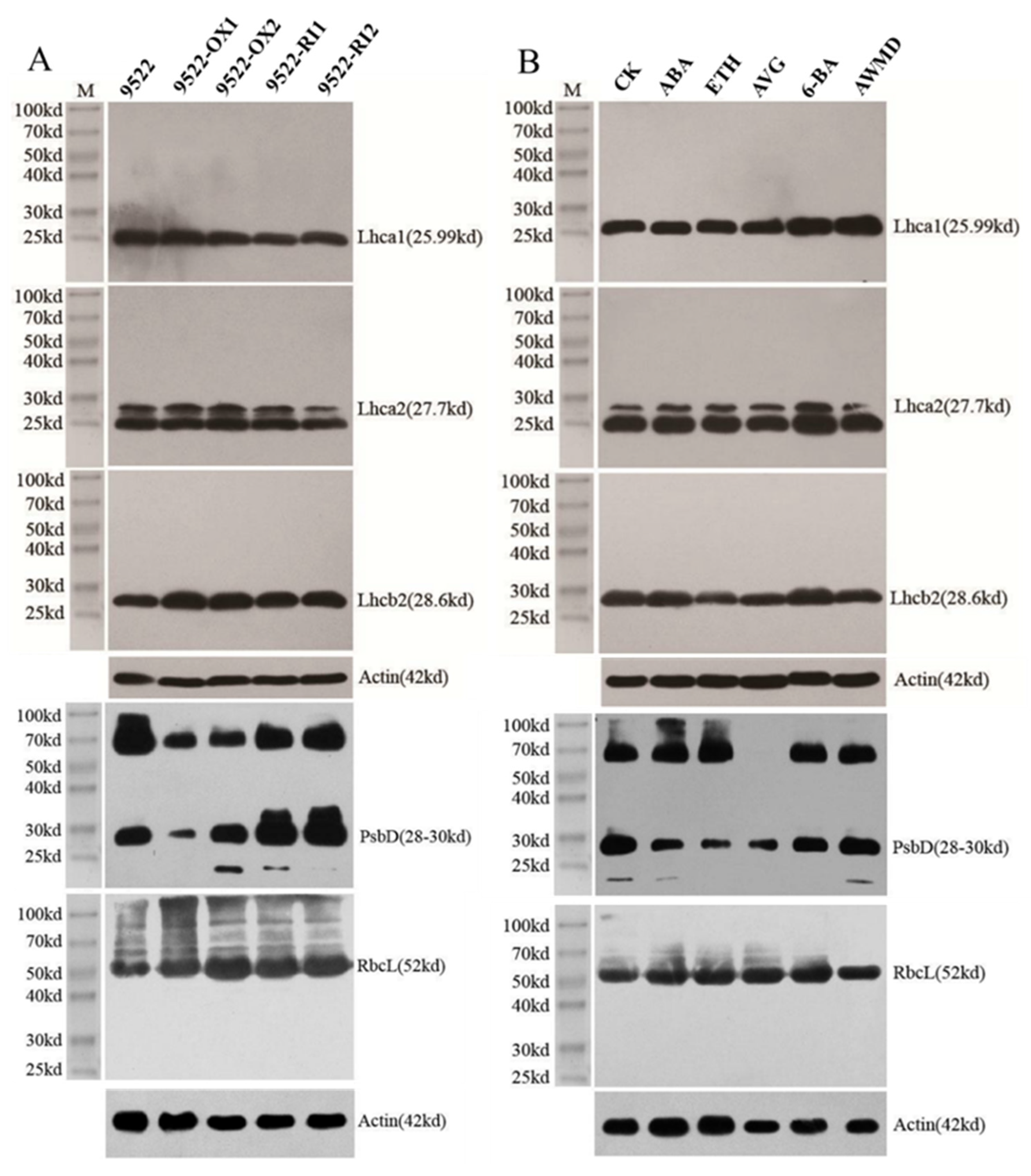
2.2. Leaf Photosynthesis-Related Traits
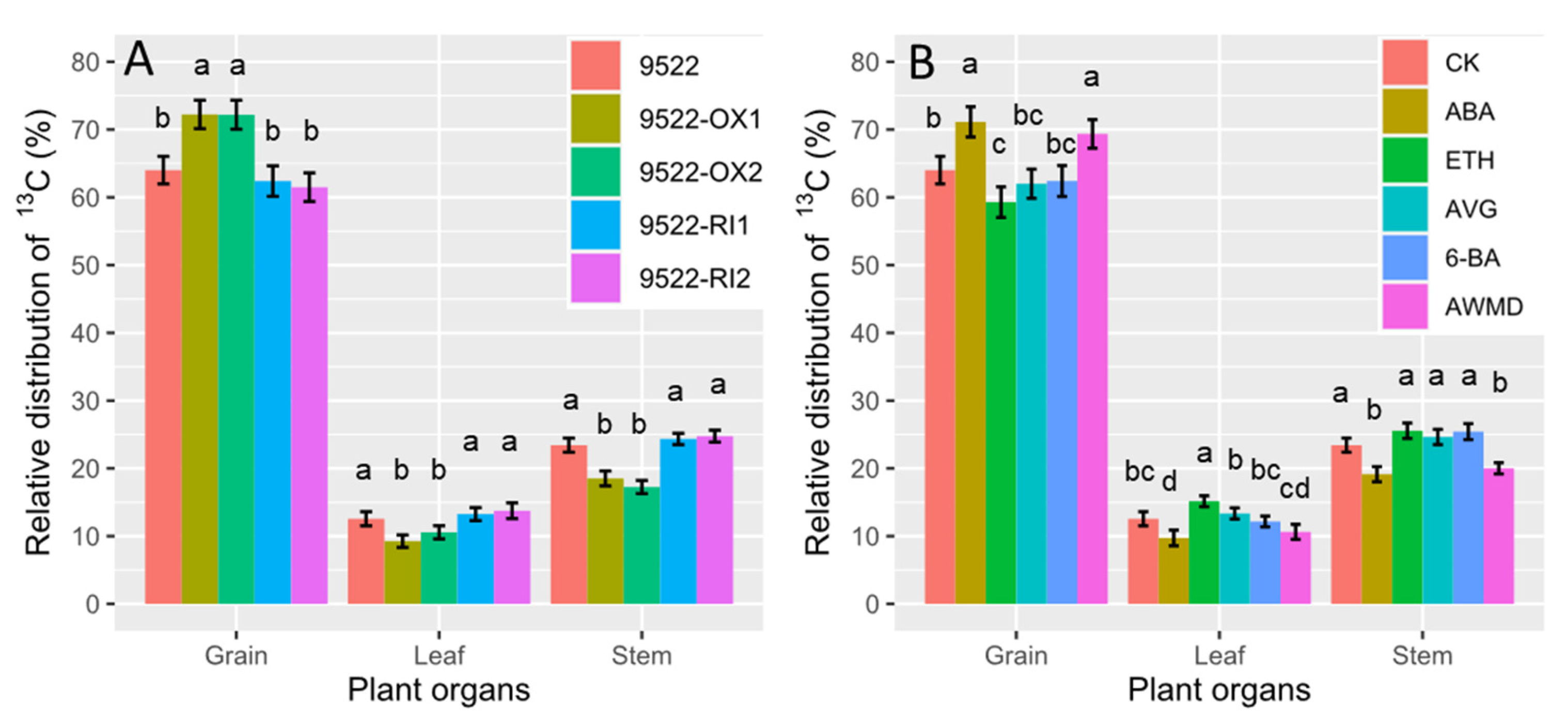
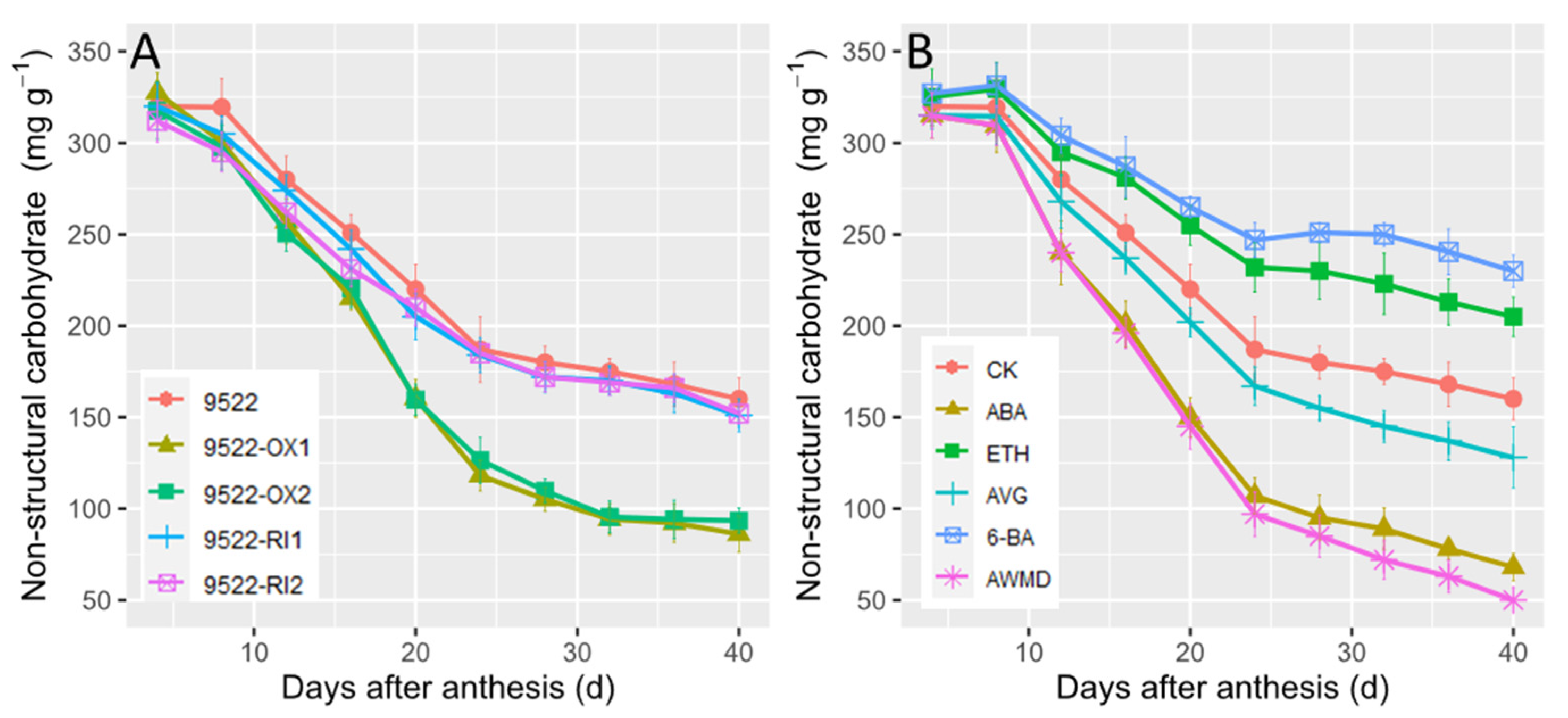
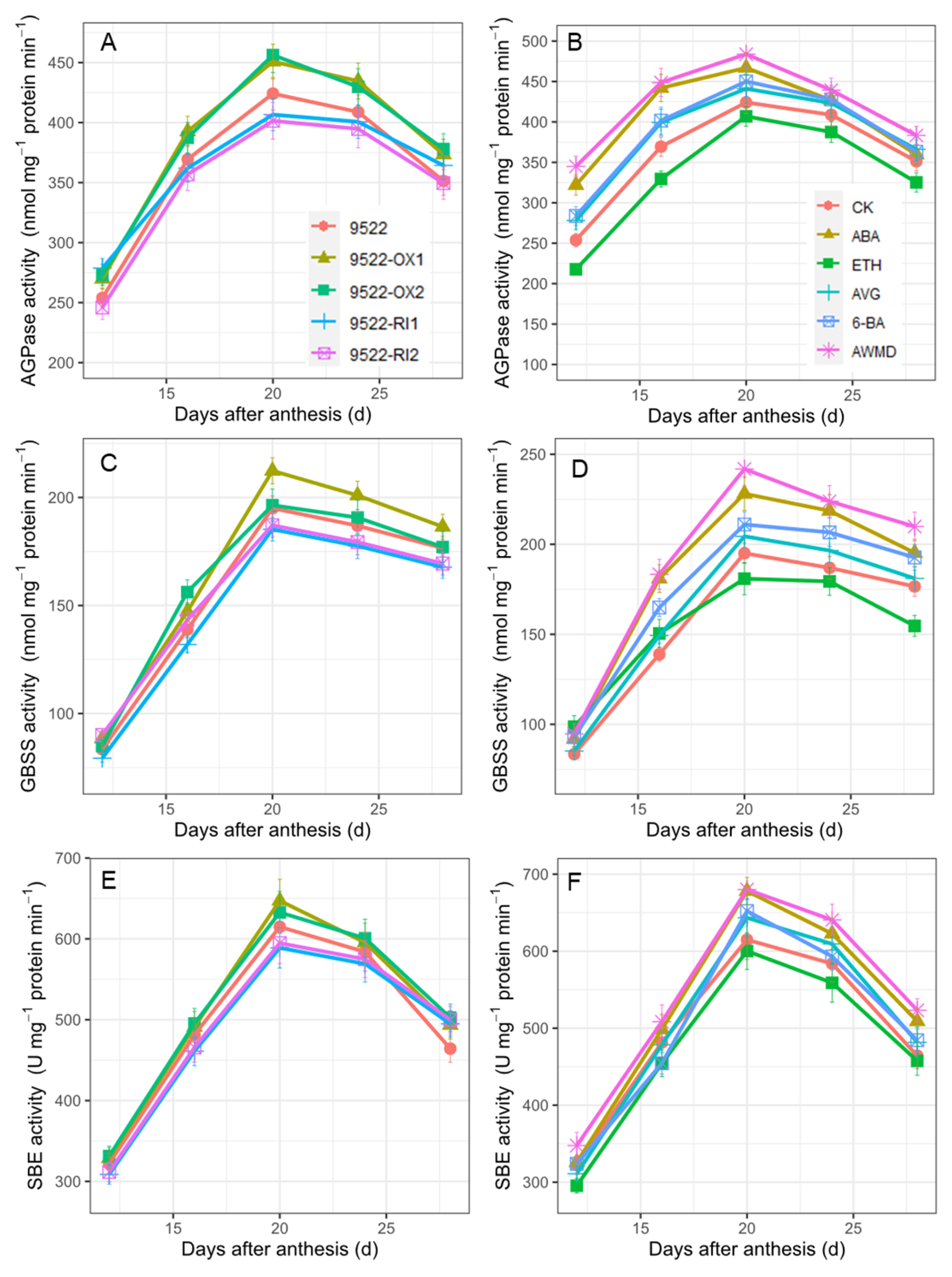
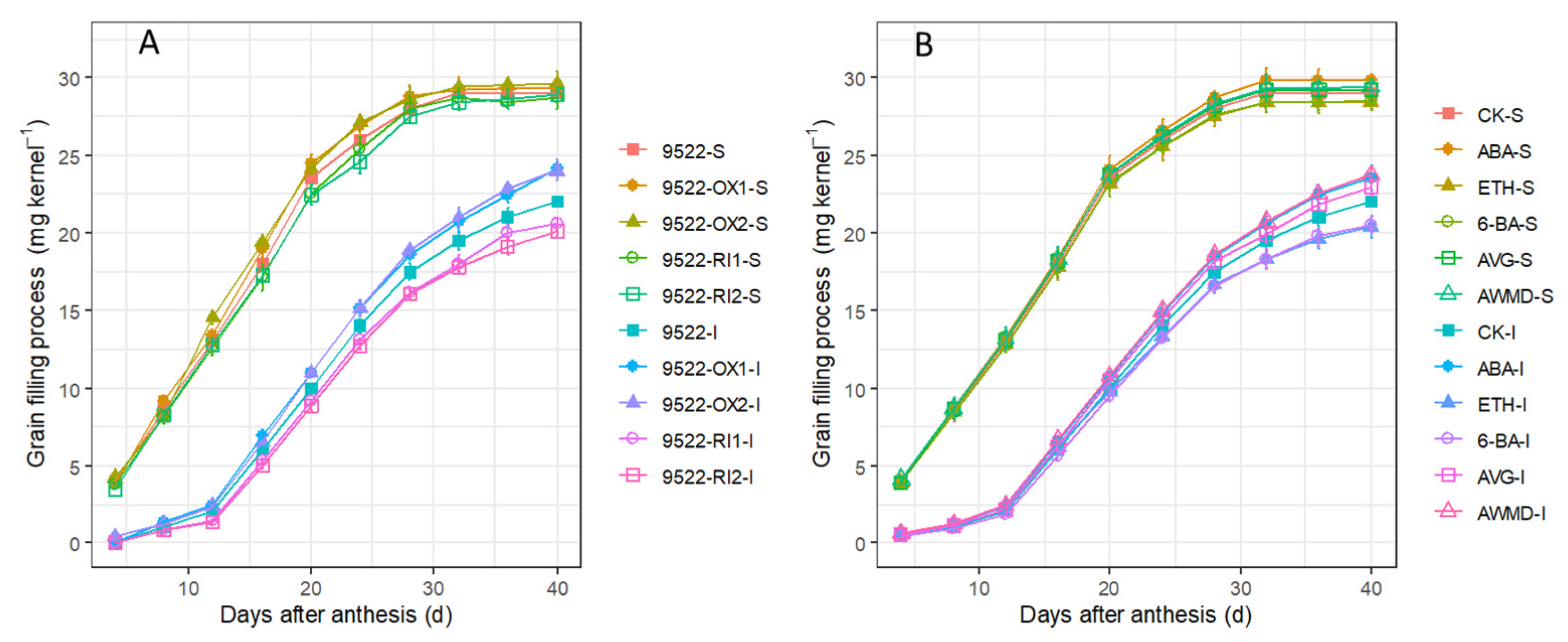
2.3. Grain Filling and Redistribution of Assimilates from Carbon Reserves in Stems
3. Discussion
3.1. The Relationships between “STAY-GREEN” Trait and Crop Production
3.2. Leaf Senescence Needs to Be Finely Tuned to Increase Yields for Cereal Crops
4. Materials and Methods
4.1. Plant Materials
4.2. Plant Growth Environment and Treatment
4.3. SDS-PAGE and Protein Gel Blot Analysis
4.4. 13CO2 Labeling and Sampling
4.5. NSC Measurement, Extraction and Assays of Starch Metabolic Enzymes
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, S.P. Mechanisms of plant response to global atmospheric change. Plant Cell Environ. 2012, 35, 1705–1706. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Approaches to achieve high grain yield and high resource use efficiency in rice. Front. Agric. Sci. Eng. 2015, 2, 115–123. [Google Scholar] [CrossRef]
- Okamura, M.; Arai-Sanoh, Y.; Yoshida, H.; Mukouyama, T.; Adachi, S.; Yabe, S.; Nakagawa, H.; Tsutsumi, K.; Taniguchi, Y.; Kobayashi, N. Characterization of high-yielding rice cultivars with different grain-filling properties to clarify limiting factors for improving grain yield. Field Crops Res. 2018, 219, 139–147. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Patel, R.; Sahu, S.K. Time of flowering affects grain quality and spikelet partitioning within the rice panicle. Funct. Plant Biol. 1993, 20, 231–241. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain-filling problem in ‘super’ rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Gil Nam, H. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence—A genomics approach. Plant Biotechnol. J. 2003, 1, 3–22. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crops Res. 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H.; Yang, J. Photosynthetic properties and potentials for improvement of photosynthesis in pale green leaf rice under high light conditions. Front. Plant Sci. 2017, 8, 1082. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Liao, L.L.; Liu, S.; Nie, M.M.; Li, J.; Zhang, L.D.; Ma, J.F.; Chen, Z.C. Magnesium deficiency triggers SGR-mediated chlorophyll degradation for magnesium remobilization. Plant Physiol. 2019, 18, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Yamatani, H.; Sato, Y.; Masuda, Y.; Kato, Y.; Morita, R.; Fukunaga, K.; Nagamura, Y.; Nishimura, M.; Sakamoto, W.; Tanaka, A.; et al. NYC4, the rice ortholog of Arabidopsis THF1, is involved in the degradation of chlorophyll—Protein complexes during leaf senescence. Plant J. 2013, 74, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, J.; Huang, L.; Leng, Y.; Dai, L.; Rao, Y.; Chen, L.; Wang, Y.; Tu, Z.; Hu, J.; et al. PGL, encoding chlorophyllide a oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice. J. Exp. Bot. 2016, 67, 1297–1310. [Google Scholar] [CrossRef]
- Abdelrahman, M.; EI-sayed, M.; Jogaiah, S.; Burritt, D.J.; Tran, L.S.P. The “STAY-GREEN” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep. 2017, 36, 1009–1025. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef]
- Sultana, N.; Islam, S.; Juhasz, A.; Ma, W. Wheat leaf senescence and its regulatory gene network. Crop J. 2021, 9, 703–717. [Google Scholar] [CrossRef]
- Song, S.; Ge, M.; Wang, W.; Gu, C.; Chen, K.; Zhang, Q.; Yu, Q.; Liu, G.; Jiang, J. BpEIN3.1 represses leaf senescence by inhibiting synthesis of ethylene and abscisic acid in Betula platyphylla. Plant Sci. 2022, 321, 111330. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular mechanism for the regulation of ABA homeostasis during plant development and stress responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Mao, Y.; Struik, P.C.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J. Roles of nitrogen and cytokine signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Hai, N.N.; Chuong, N.N.; Tu, N.H.C.; Kisiala, A.; Hoang, X.L.T.; Thao, N.P. Role and regulation of cytokinins in plant response to drought stress. Plants 2020, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Nat. Ac. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Wang, J.; Wang, Z.; Yang, J.; Zhang, J. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J. Exp. Bot. 2013, 64, 2523–2538. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Q.; Zhang, J. Moderate wetting and drying increases rice yield and reduces water use, grain arsenic level, and methane emission. Crop J. 2017, 5, 151–158. [Google Scholar] [CrossRef]
- Zarei, B.; Naderi, A.; Kamali, M.R.J.; Lack, S.; Modhej, A. Determination of physiological traits related to terminal drought and heat stress tolerance in spring wheat genotypes. Int. J. Agric. Crop Sci. 2013, 5, 2511–2520. [Google Scholar]
- Morita, R.; Sato, Y.; Masuda, Y.; Nishimura, M.; Kusaba, M. Defect in non-yellow coloring 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 2009, 59, 940–952. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef]
- Gan, S.; Amasino, R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 1995, 270, 986–988. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, S. Genetic manipulation of leaf senescence. In Senescence Processes in Plants; Gan, S., Ed.; Blackwell: New Dehli, India, 2007; pp. 304–322. [Google Scholar]
- Kant, S.; Burch, D.; Badenhorst, P.; Palanisamy, R.; Mason, J.; Spangenberg, G. Regulated expression of a cytokinin biosynthesis gene IPT delays leaf senescence and improves yield under rainfed and irrigated conditions in canola (Brassica napus L.). PLoS ONE 2015, 10, e0116349. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Lai, N.; Kisiala, A.B.; Emery, R.N. Isopentenyltransferases as master regulators of crop performance: Their function, manipulation, and genetic potential for stress adaptation and yield improvement. Plant Biotechnol. J. 2021, 19, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A. The importance of grain or kernel number in wheat: A reply to Sinclair and Jamieson. Field Crops Res. 2008, 105, 15–21. [Google Scholar] [CrossRef]
- Sykorova, B.; Kuresova, G.; Daskalova, S.; Trckova, M.; Hoyerova, K.; Raimanova, I.; Motyka, V.; Travnickova, A.; Elliott, M.C.; Kaminek, M. Senescence-induced ectopic expression of the A. tumefaciens ipt gene in wheat delays leaf senescence, increases cytokinin content, nitrate influx, and nitrate reductase activity, but does not affect grain yield. J. Exp. Bot. 2008, 59, 377–387. [Google Scholar] [CrossRef]
- Lin, Y.J.; Cao, M.L.; Xu, C.G.; Chen, H.; Wei, J.; Zhang, Q.F. Cultivating rice with delaying leaf senescence by P-SAG12-IPT gene transformation. Acta Bot. Sin. 2002, 44, 1333–1338. [Google Scholar]
- Liu, L.; Zhou, Y.; Szczerba, M.W.; Li, X.; Lin, Y. Identification and application of a rice senescence-associated promoter. Plant Physiol. 2010, 153, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.H.; He, Y.Q.; Xu, C.G.; Li, X.H.; Zhang, Q. The genetic basis of stay-green in rice analyzed in a population of doubled haploid lines derived from an indica by japonica cross. Theor. Appl. Gen. 2004, 108, 688–698. [Google Scholar] [CrossRef]
- Naruoka, Y.; Sherman, J.D.; Lanning, S.P.; Blake, N.K.; Martin, J.M.; Talbert, L.E. Genetic analysis of green leaf duration in spring wheat. Crop Sci. 2012, 52, 99–109. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, X.; Li, B.; Su, B.; Li, J.; Zhou, D. An improved water-use efficiency for winter wheat grown under reduced irrigation. Field Crops Res. 1998, 59, 91–98. [Google Scholar] [CrossRef]
- Mi, G.; Tang, L.; Zhang, F.; Zhang, J. Carbohydrate storage and utilization during grain filling as regulated by nitrogen application in two wheat cultivars. J. Plant Nutr. 2002, 25, 213–229. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Wang, G.; Qian, X.; Wang, W.; Xu, Y.; Zhang, W.; Zhang, H.; Liu, L.; Wang, Z.; et al. Reducing environmental risk by improving crop management practices at high crop yield levels. Field Crops Res. 2021, 265, 108123. [Google Scholar] [CrossRef]
- Berry, P.M.; Sterling, M.; Spink, J.H.; Baker, C.J.; Sylvester-Bradley, R.; Mooney, S.J.; Tams, A.R.; Ennos, A.R. Understanding and reducing lodging in cereals. Adv. Agron. 2004, 84, 215–269. [Google Scholar]
- Gong, Y.H.; Zhang, J.; Gao, J.F.; Lu, J.Y.; Wang, J.R. Slow export of photoassimilate from stay-green leaves during late grain-filling stage in hybrid winter wheat (Triticum aestivum L.). J. Agron. Crop Sci. 2005, 191, 292–299. [Google Scholar] [CrossRef]
- Yang, J.; Peng, S.; Zhang, Z.; Wang, Z.; Visperas, R.M.; Zhu, Q. Grain and dry matter yields and partitioning of assimilates in japonica/indica hybrid rice. Crop Sci. 2002, 42, 766–772. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Crop management techniques to enhance harvest index in rice. J. Exp. Bot. 2010, 61, 3177–3189. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2021, 10, 13–25. [Google Scholar] [CrossRef]
- Nambara, E.; Van Wees, S. Plant hormone functions and interactions in biological systems. Plant J. 2021, 105, 287–289. [Google Scholar] [CrossRef]
- Boyer, J.S.; Westgate, M.E. Grain yields with limited water. J. Exp. Bot. 2004, 55, 2385–2394. [Google Scholar] [CrossRef]
- Bano, A.; Yasmeen, S. Role of phytohormones under induced drought stress in wheat. Pak. J. Bot. 2010, 42, 2579–2587. [Google Scholar]
- Seiler, C.; Harshavardhan, V.T.; Rajesh, K.; Reddy, P.S.; Strickert, M.; Rolletschek, H.; Scholz, U.; Wobus, U.; Sreenivasulu, N. ABA biosynthesis and degradation contributing to aba homeostasis during barley seed development under control and terminal drought-stress conditions. J. Exp. Bot. 2011, 62, 2615–2632. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, X.G.; Shen, S.; Yin, H.; Zhou, L.L.; Gao, Z.; Lv, X.; Zhou, S. Increasing the abscisic acid level in maize grains induces precocious maturation by accelerating grain filling and dehydration. Plant Growth Regul. 2018, 86, 65–79. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol. 2006, 171, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Zaman, R.; La, V.H.; Park, S.H.; Kim, T.H. Ethephon-induced ethylene enhances protein degradation in source leaves, but its high endogenous level inhibits the development of regenerative organs in Brassica napus. Plants 2021, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.K.; Naik, P.K.; Patel, R. Ethylene inhibitors improve dry matter partitioning and development of late flowering spikelets on rice panicles. Funct. Plant Biol. 2000, 27, 311–323. [Google Scholar] [CrossRef]
- Sharp, R.E. Interaction with ethylene: Changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ. 2002, 25, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Ye, Y.; Wang, Z.; Zhu, Q.; Liu, L. Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant Cell Environ. 2004, 27, 1055–1064. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Liu, K.; Wang, P. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 2005, 57, 149–160. [Google Scholar] [CrossRef]
- Panda, B.B.; Kariali, E.; Panigrahi, R.; Mohapatra, P.K. High ethylene production slackens seed filling in compact panicled rice cultivar. Plant Growth Regul. 2009, 58, 141–151. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J. 2020. Carbohydrate, hormone and enzyme regulations of rice grain filling under post-anthesis soil drying. Environ. Exp. Bot. 2020, 178, 104165. [Google Scholar] [CrossRef]
- Zhu, K.; Ren, W.; Yan, J.; Zhang, Y.; Zhang, W.; Xu, Y.; Wang, Z.; Yang, J. Grain yield and nitrogen use efficiency are increased by exogenous cytokinin application through the improvement in root physiological traits of rice. Plant Growth Regul. 2022, 97, 157–169. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Huang, Z.; Zhu, Q.; Wang, L. Remobilization of carbon reserves is improved by controlled soil-drying during grain filling of wheat. Crop Sci. 2000, 40, 1645–1655. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yuki, K.; Park, S.Y. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Schaffer, A.A.; Petreikov, M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 1997, 113, 739–746. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; v. 4.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 1 January 2020).
- Wickham, H.; Chang, W.; Henry, L. ggplot2: Create elegant data visualisations using the grammar of graphics. 2021. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 1 January 2021).

| Genetic Materials | Treatment | Number of Panicles per m2 | Number of Spikelets per Panicle | Percentage of Filled Grains (%) | 1000-Seed Weight (g) | Yield (t·ha−1) |
|---|---|---|---|---|---|---|
| 9522 | CK | 243.33 ± 4.98 b | 133.53 ± 3.51 ab | 81.33 ± 1.25 b | 27.2 ± 0.15 c | 7.19 ± 0.19 b |
| 9522-OX1 | CK | 238.76 ± 5.17 b | 136.70 ± 3.45 ab | 83.60 ± 2.35 ab | 28.3 ± 0.27 ab | 7.72 ± 0.24 a |
| 9522-OX2 | CK | 243.57 ± 4.05 b | 130.78 ± 4.27 b | 84.50 ± 1.57 a | 28.5 ± 0.25 a | 7.67 ± 0.15 a |
| 9522-RI1 | CK | 255.22 ± 5.12 a | 142.25 ± 5.12 a | 72.53 ± 2.15 d | 26.5 ± 0.12 d | 6.98 ± 0.21 b |
| 9522-RI2 | CK | 254.35 ± 4.65 a | 142.64 ± 4.15 a | 72.52 ± 1.51 d | 26.7 ± 0.26 d | 7.02 ± 0.22 b |
| 9522 | ABA | 240.27 ± 6.87 b | 137.21 ± 4.25 ab | 82.37 ± 2.12 ab | 28.0 ± 0.12 b | 7.60 ± 0.19 a |
| 9522 | ETH | 245.41 ± 4.00 b | 140.08 ± 3.25 a | 78.20 ± 1.52 c | 26.2 ± 0.11 e | 7.04 ± 0.25 b |
| 9522 | 6-BA | 241.96 ± 5.34 b | 135.42 ± 4.42 ab | 81.53 ± 2.14 b | 27.0 ± 0.24 c | 7.22 ± 0.2 b |
| 9522 | AVG | 242.45 ± 7.02 b | 136.00 ± 5.55 ab | 80.35 ± 1.04 bc | 27.3 ± 0.22 c | 7.24 ± 0.14 b |
| 9522 | AWMD | 238.63 ± 6.56 b | 141.20 ± 4.12 a | 82.71 ± 2.71 ab | 28.1 ± 0.29 ab | 7.84 ± 0.27 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, Y.; Yao, Y.; Xu, Z.; Wang, B.; Mao, Y.; Wang, W.; Zhang, W.; Zhang, H.; Liu, L.; Wang, Z.; et al. The Relationships among “STAY-GREEN” Trait, Post-Anthesis Assimilate Remobilization, and Grain Yield in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 13668. https://doi.org/10.3390/ijms232213668
Zang Y, Yao Y, Xu Z, Wang B, Mao Y, Wang W, Zhang W, Zhang H, Liu L, Wang Z, et al. The Relationships among “STAY-GREEN” Trait, Post-Anthesis Assimilate Remobilization, and Grain Yield in Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2022; 23(22):13668. https://doi.org/10.3390/ijms232213668
Chicago/Turabian StyleZang, Yuguang, Yijia Yao, Zheshu Xu, Baoqing Wang, Yiqi Mao, Weilu Wang, Weiyang Zhang, Hao Zhang, Lijun Liu, Zhiqin Wang, and et al. 2022. "The Relationships among “STAY-GREEN” Trait, Post-Anthesis Assimilate Remobilization, and Grain Yield in Rice (Oryza sativa L.)" International Journal of Molecular Sciences 23, no. 22: 13668. https://doi.org/10.3390/ijms232213668
APA StyleZang, Y., Yao, Y., Xu, Z., Wang, B., Mao, Y., Wang, W., Zhang, W., Zhang, H., Liu, L., Wang, Z., Liang, G., Yang, J., Zhou, Y., & Gu, J. (2022). The Relationships among “STAY-GREEN” Trait, Post-Anthesis Assimilate Remobilization, and Grain Yield in Rice (Oryza sativa L.). International Journal of Molecular Sciences, 23(22), 13668. https://doi.org/10.3390/ijms232213668








